Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiang Wang | -- | 2765 | 2024-01-04 21:16:29 | | | |
| 2 | Jessie Wu | Meta information modification | 2765 | 2024-01-05 04:39:01 | | | | |
| 3 | Jessie Wu | + 1 word(s) | 2766 | 2024-01-05 04:41:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kusumoputro, S.; Au, C.; Lam, K.H.; Park, N.; Hyun, A.; Kusumoputro, E.; Xia, T.; Wang, X. Nanoparticle Features That Impact Liver-Targeting and Tolerogenic Effects. Encyclopedia. Available online: https://encyclopedia.pub/entry/53450 (accessed on 08 February 2026).
Kusumoputro S, Au C, Lam KH, Park N, Hyun A, Kusumoputro E, et al. Nanoparticle Features That Impact Liver-Targeting and Tolerogenic Effects. Encyclopedia. Available at: https://encyclopedia.pub/entry/53450. Accessed February 08, 2026.
Kusumoputro, Sydney, Christian Au, Katie H. Lam, Nathaniel Park, Austin Hyun, Emily Kusumoputro, Tian Xia, Xiang Wang. "Nanoparticle Features That Impact Liver-Targeting and Tolerogenic Effects" Encyclopedia, https://encyclopedia.pub/entry/53450 (accessed February 08, 2026).
Kusumoputro, S., Au, C., Lam, K.H., Park, N., Hyun, A., Kusumoputro, E., Xia, T., & Wang, X. (2024, January 04). Nanoparticle Features That Impact Liver-Targeting and Tolerogenic Effects. In Encyclopedia. https://encyclopedia.pub/entry/53450
Kusumoputro, Sydney, et al. "Nanoparticle Features That Impact Liver-Targeting and Tolerogenic Effects." Encyclopedia. Web. 04 January, 2024.
Copy Citation
Liver-targeting nanoparticles have emerged as a promising platform for the induction of immune tolerance by taking advantage of the liver’s unique tolerogenic properties and nanoparticles’ physicochemical flexibility. Such an approach provides a versatile solution to the treatment of a diversity of immunologic diseases.
immune tolerance
liver-targeting nanoparticles
autoimmune diseases
allergies
1. Introduction
The synthetic versatility of nanoparticles and the opportunity to fine-tune their physicochemical properties have implored their use as novel platforms for antigen-specific immune tolerance induction. In this regard, some nanoparticles have been implemented to manipulate antigen presentation pathways while others directly interfere with the function or elimination of antigen-specific immunological signaling [1][2]. Nanoparticles’ ability to enter cells, such as phagocytic cells, also makes them an attractive solution to the hydrophilicity barrier [3]. In these cases, there is a range of physical parameters (e.g., size, shape, surface charge, and composition, etc.) and functional modifications (e.g., surface modifications and payload encapsulation, etc.) that can be bioengineered to provide different advantages, as illustrated in Figure 1. Additionally, since most intravenously administered nanoparticles show natural liver accumulation, they are intrinsic liver-targeting platforms for immunomodulation [4]. Engineering to achieve passive targeting, active targeting, and therapeutic delivery can, therefore, be integrated into nanoparticle designs for the development of liver-targeting tolerance induction nanoplatforms.
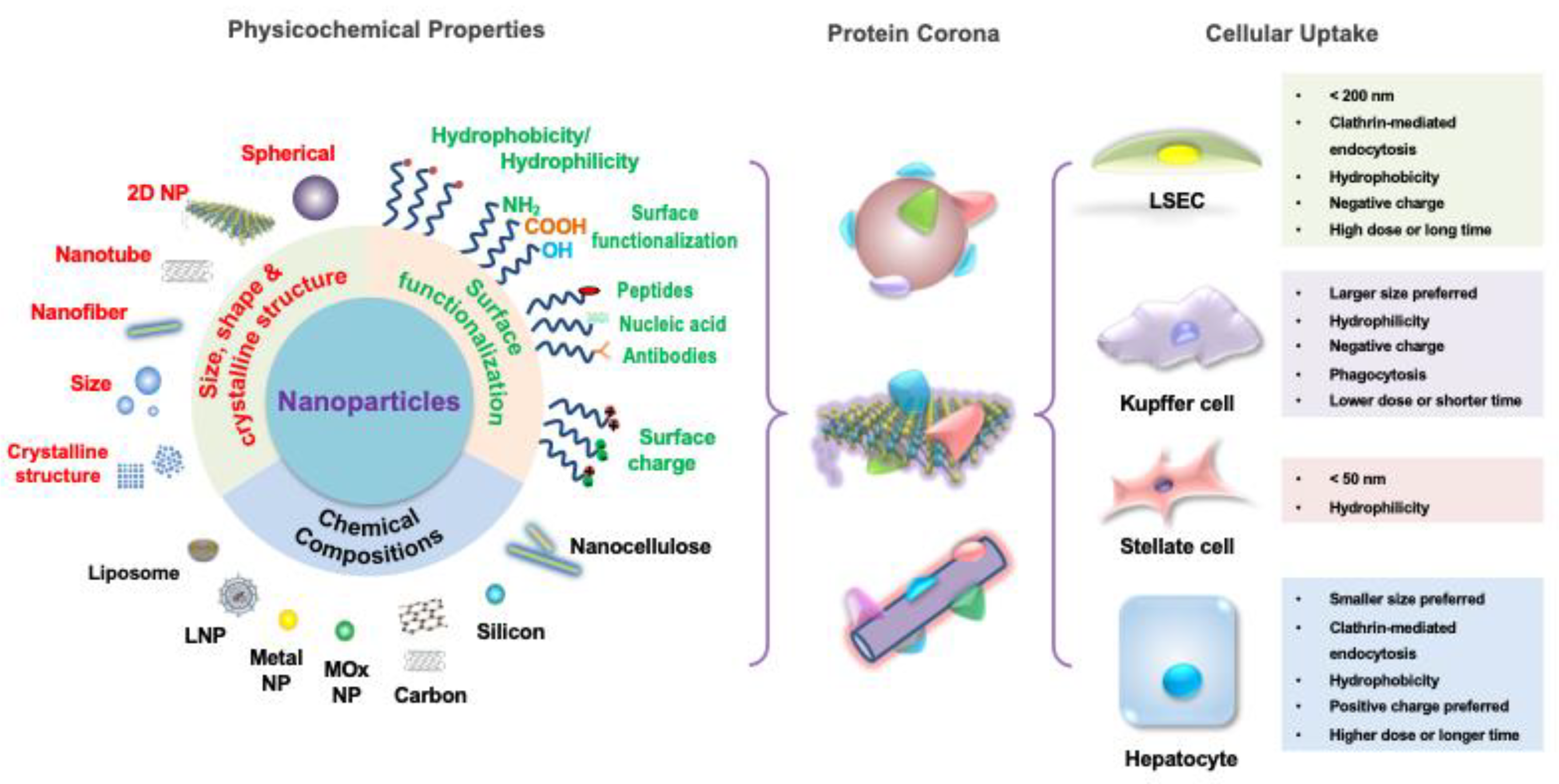
Figure 1. Nanoparticles (NP) features and designs for hepatic cellular targeting. The different features of nanoparticles individually contribute to the particles’ unique biochemical activity but also act synergistically to target the different tolerogenic hepatic cells. The composition of the nanoparticle can be of inorganic materials, such as metals, metal oxides, carbons or silicon, or organic materials, such as lipids or polymers, which influences their tolerance induction capability. Nanoparticle size, shape, and crystallography regulate its biodistribution, cellular internalization, and cellular clearance. Surface functionalization influencing hydrophilicity, valence, ligand-specific binding ability, and biomimicry impacts cell-particle interactions with targeting, activating, and inhibiting effects. Thus, by selecting the appropriate combination of features, a diversity of nanoparticles that target the hepatic cells according to each one’s unique cellular uptake preferences can be designed.
2. Size and Shape
The evolution of the immune system to sense and capture particulates, such as microbes and microbial products, on the nanometer scale initially entreats the use of synthetic nanoparticles for tolerance induction [5][6]. Therefore, when considering immune system targeting, and in particular liver targeting, particle size and shape are fundamental design parameters. They are especially important when considering “passive targeting” of the liver, or the manipulation of physical parameters to influence nanoparticle pharmacodynamics and targeting. Size and shape will not only affect kinetics regarding bloodstream circulation, cellular clearance, and intracellular transport but also contribute to nanoparticles’ biodistribution and cellular internalization [7].
In terms of biokinetics, it has been observed that the smaller the hydrodynamic diameter of the nanoparticle, the more widely distributed throughout the body the nanoparticle becomes [8]. This has been attributed to smaller particles being less prone to marginalize in normal laminar blood flow, having a higher propensity to accumulate within all types of cells, and having decreased cellular clearance [6][7]. Multiple studies also support this observation, with the convergence of intravenously delivered nanoparticle size towards an ideal diameter range of 30–300 nm for maximum particle circulation [7]. Likewise, it has been observed that as particle size increases, there is an increased accumulation in the liver and spleen, both of which have sinusoidal basal lamina allowing for permeability and the former of which is the largest cardiac-output-receiving organ at rest [9].
Specific liver-residing cells also display particle size preferences due to the impact of size on cellular internalization rates and mechanisms. For example, LSECs have been shown to engulf particles with a diameter of up to 200 nm due to their internalization method of receptor-mediated endocytosis [10][11]. This upper size limit is shown to facilitate intracellular accumulation in late endosomal and lysosomal compartments, an important mechanism for immunomodulation, within other non-phagocytic cells [11]. On the other hand, Kupffer cells, which can also utilize receptor-mediated endocytosis, preferentially uptake particles > 200 nm in the liver due to their phagocytic activity [11][12]. This creates a well-controlled dichotomy that makes up most of the liver’s scavenger function. To access other liver cells, such as hepatocytes and HSCs, penetration through the liver sinusoidal fenestrae via the space of Dissé may require minimization of nanoparticle size, ideally to <100 nm [13]. However, it has been shown that nanoparticles up to 400 nm have been able to extravasate into the space of Dissé with a proper lipid composition of negatively charged phosphatidylserine [14].
Regarding shape, spherical nanoparticles are the most common particle shape and are shown to have some preferential accumulation in the lungs, liver, and spleen [7]. Most studies have additionally shown increased phagocytosis of spherical nanoparticles by macrophages compared to elongated ones [15]. PEG-bl-PPS micelle nanoparticles, for example, were shown to preferentially target liver phagocytes compared to more elongated fillomicelle nanoparticles [16]. Contrastingly, however, cylindrical silicon-based nanoparticles showed nearly two times higher accumulation in the liver than their spherical counterparts [17]. Therefore, while there is a strong impact of size and shape on liver targeting and biodistribution, more research needs to be conducted to discern their specific targeting impact and the varying influence different nanoparticle compositions may have on such impact. Furthermore, nanoparticle size may dictate the contribution of shape towards cellular accumulation, as seen with rod-shaped nanoparticles, which have shown higher uptake efficiencies within a certain range of aspect ratios and lengths (e.g., 200–300 nm for nanocellulose NPs) [18][19].
When discussing size and shape, it is also important to note their contribution to toxicity. A review of the literature suggests that particles smaller than 10 nm may exhibit toxic effects due to inefficient cellular clearance and prolonged accumulation while those larger than 1000 nm may exhibit toxicity secondary to capillary obstruction [7]. Similarly, certain shapes, such as needle-like particles, have been shown to damage lysosomal membranes, inducing NLRP3 inflammasome activation and instigating inflammatory responses due to characteristics such as their rigidity and propensity to shed toxic ions [19][20]. These overarching safety parameters must be an important consideration during nanoparticle design.
3. Surface Charge
Another important physical parameter that contributes to the nanoparticle passive targeting of the liver, especially at the cellular level, is surface charge. While cationic nanoparticles have shown greater safety and biocompatibility concerns due to their strong membrane permeability and binding of negatively charged DNA, deviations towards either a positive or negative surface charge have shown significant effects on particle–cell interactions [21][22]. Highly cationic or anionic nanoparticles, for example, adsorb a significant amount of serum protein, resulting in aggregation and increased uptake by macrophages in vitro [23]. Kupffer cells and LSECs have also been shown to preferentially bind and clear negatively charged nanoparticles through interactions with scavenger receptors, such as stabilin-1 and stabilin-2 [24][25]. Conversely, hepatocytes demonstrate significant uptake of positively charged NPs over negatively charged ones [25]. The desired zeta potential for enhanced and targeted membrane interactions can be achieved either through material selection of choice, as in the case of many ionizable lipids, or chemical modifications [26]. However, it is important to note that protein binding to, e.g., albumin, formation of protein coronas, and interactions with phagocytes can change the synthetically given surface charge in vivo and subsequently influence liver cellular uptake [15][27]. Therefore, despite the role that electrostatic binding plays in liver uptake, the interplay is complex between the nanoparticles’ different characteristics that ultimately determine their activity.
4. Composition
Another advantage of nanoparticles is the diversity of materials from which they can be manufactured to optimize their desired function and payload compatibility. Four broad categories of materials used for nanoparticle synthesis include metals, lipids, carbon-based materials, as well as synthetic and natural polymers [1].
Metal and metal-oxide nanoparticles have long been implemented for theranostics and have recently been designed to conjugate target ligands, antigens, and immunomodulators on their surfaces. A few examples for the treatment of autoimmune diseases, such as experimental autoimmune encephalomyelitis (EAE) and type I diabetes (T1D), include iron oxide NPs, which have been popularly conjugated to high-avidity MHC peptide ligands, and gold NPs, modified with T-cell epitopes and 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester [28][29][30]. Gold and silicon nanoparticles, amongst others, have also been designed for various therapeutic applications [31]. However, the typical requirement for payloads to be conjugated to the nanoparticle metal surface may severely limit the types of molecules and, thus, applications of such particles [1]. Innocuous antigens and/or their epitopes, for example, may be difficult to implement with such metal or metal-oxide nanoparticles for antigen-specific tolerance. Additionally, while the stability of these particles can be highly favorable depending on the application, their lack of biodegradability has been demonstrated to increase tissue accumulation, creating toxicity and safety concerns [1][32].
Lipid-based nanoparticles (LNPs) are another popular nanoparticle of choice and have been clinically used for a variety of FDA-approved applications, ranging from ONPATTRO® polyneuropathy therapy to the mRNA-1274 (Moderna, Cambridge, MA, USA) and BNT162b2 (Pfizer-BioNTech, New York, NY, USA) COVID-19 vaccines [33][34]. One reason for this is their high degree of biocompatibility and their reduced risk of producing inflammatory responses due to lipids’ status as a natural component of the cell membrane [7]. Another advantage is the ability of liposomal NPs to encapsulate hydrophobic molecules in their lipid bilayer, hydrophilic molecules in their aqueous core, or both simultaneously, giving them compatibility with diverse payloads. Conversely, other lipid-containing NPs incorporate hydrophobic molecules into the lipid construct before mixing with the aqueous phase for NP synthesis [35]. LNPs have been shown to deliver not only small molecules, such as anti-cancer drugs and chemotherapy agents [36][37], but also, more recently, nucleic acids. LNPs encapsulated with siRNA targeting ApoB [38], PCSK9 [39], PLK1 [40], VEGF, and KSP [38] expression are just a few examples, along with those delivering self-amplifying RNA (saRNA) [41] and mRNA [36][37][38][39][40][41]. The enhanced flexibility for payloads of diverse chemical properties also allows opportunities for the co-delivery of molecules, such as a combination of antigenic material with tolerogenic agents (e.g., suppressive cytokines and broad immunosuppressants) [42]. Additionally, other LNPs have been surface-modified with engineered peptides and whole membrane proteins, providing further avenues for their possible use as antigen-specific immune tolerance therapeutics [43]. Finally, a low-shear extrusion manufacturing method can minimize the protein denaturation risk during encapsulation. However, payload release kinetics may be difficult to fine-tune with liposomes [44].
Liposomes may also experience a slight propensity to targeting. This has been partially attributed to their cholesterol-dependent membrane fluidity that enables the clustering of surface molecules upon interaction with the target cell [1]. As such, there has recently been research and development into passively and actively targeting LNPs in the liver through engineering their physicochemical properties and taking advantage of surface modifications by targeting ligands [45]. Lipid-coated calcium phosphate NPs, for example, have been extensively studied in their targeting of hepatocytes and HSCs to deliver siRNA [46]. The successful use of LNPs in ONPATTRO, the first FDA-approved liver-targeting nanomedicine, in 2018 validates such widespread usage and further development of LNPs for liver targeting [33].
Carbon-based NPs, such as carbon nanotubes, fullerenes, graphene and graphene oxides, have mostly been implemented to promote or suppress immune responses as adjuvants, showing various results in antigen-based immunotherapy (AIT), with carbon nanotubes worsening systemic allergic reactions and carbon allotropes reducing anaphylaxis [46][47]. Conclusively, however, observations of high levels of toxicity and detrimental health effects have been attributed to their failure to degrade naturally [48]. Therefore, extensive investigation into their persistence, bioaccumulation, and toxicity in the human body would be further required before implementation.
Finally, natural and synthetic polymers have been popularly used for therapeutic NP manufacturing, notably in the field of tolerance induction [47]. Their synthetic diversity over their physicochemical properties and their proclivity for surface modifications, such as receptor ligands, make them apt to target specific liver-residing cells [47][48]. Therapeutically, their encapsulation of antigens provides a physical protective barrier from the human body environment and possible IgE binding-causing anaphylaxis [47]. Their long shelf-life is another important factor for clinical usage [7].
Polymeric nanoparticles can also be split into non-degradable NPs and biodegradable NPs. Non-degradable NPs that have been researched for immune modulation mainly consist of dendrimers, such as allergen-encapsulating poly(ethylene glycol) (PEG), and benefit from their design flexibility, multivalent surfaces for ligand surface modification, and enhanced solubility and bioavailability [47][48].
Biodegradable polymers, on the other hand, provide a safe, biocompatible material for nanoparticles, many of which have already been approved in medical devices and drugs. Furthermore, the choice of polymer and composition can strongly affect the payload release rate [49][50]. Natural biodegradable polymers, such as chitosan, alginate, carrageenan, albumin, gelatin, collagen, and mannan, have been implemented as allergen carriers for relatively quick antigen release while synthetic biodegradable polymers, such as polylactic acid (PLA), poly lactic-co-glycolic acid (PLGA), poly(methyl methacrylate), polycaprolactone, poly(alkyl cyanoacrylates), and copolymers, have enabled extended release over days or weeks [47]. The selection of material, thus, endows a fine-tuned control over the release rate that may be necessary for AIT therapies requiring a short or long treatment period. Amongst these, PLGA is the most utilized and extensively studied NP material for tolerance induction due to its strong biocompatibility, biodegradability, nontoxicity, and FDA approval for drug delivery [51][52]. These have been demonstrated in tolerance towards EAE, rheumatoid arthritis, T1D, etc. [51]. Overall, while the unique advantages and disadvantages of different manufacturing materials must be carefully chosen for the desired tolerance applications, the diverse selection provides a wide array of modifications and opportunities for tolerance induction.
5. Ligands and Modifications
As previously discussed, modifications and ligand conjugations to nanoparticles, regardless of composition, can be important features in the targeting design (targeting ligands) and therapeutic action (alloantigens, activating and blocking ligands, biomimicry, and therapeutic cargo) of nanoparticles. Targeting ligands, such as mannose, ApoB peptide (RLYRKRGLK with GCC tag), and hyaluronic acid, amongst others, are vital for directing nanoparticles toward desired liver-residing cell populations [53]. This is especially important to increase their potency and safety [46]. Alloantigens, including ovalbumin (OVA), myelin oligodendrocyte glycoprotein (MOG), and proteolipid protein (PLP), will also be discussed as they can be either loaded or surface conjugated as the payloads to be delivered for AIT and autoimmune treatments [54]. Possible conjugations to nanoparticles, however, expand beyond these.
Activating ligands, for example, have also been accessorized onto NPs to target tolerogenic receptors. Gold NPs conjugated with aryl hydrocarbon receptor (AHR) ligands have been shown to induce FoxP3+ and IL-10+ regulatory T cells (Tregs) and Th17 differentiation [30][55]. Similar ligands have also demonstrated effector cell killing effects; polymeric latex bead NPs conjugated to Fas receptor monoclonal antibodies deleted antigen-specific cytotoxic T cells and LNPs displaying CD22 glycan ligands and antigens caused the apoptosis of human B cells [56]. Meanwhile, NPs decorated with blocking ligands have shown anergic and “sponge”-like results. The addition of antigen peptide-bound MHC I or MHC II on NPs without costimulatory signals, for example, has converted CD8+ T cells into regulatory, anergic phenotypes and also increased the production of Tregs, IL-10, and regulatory B cells [28][29]. Likewise, the adornment of macrophage membrane antigens TNFR2, CD36, and CCR2 onto chitosan NPs has caused the sequestration of the pro-inflammatory cytokines TNF-alpha and IL-1β [57]. The latter nanoplatform, which mimicked the macrophage membrane’s anti-inflammatory characteristics, is one example of the rapidly dividing research on biomimetic NPs that take advantage of numerous modifications.
A major advantage to utilizing NPs for tolerance in transplantation, autoimmunity, and allergies is that they can simultaneously functionalize a set of antigens or antibodies onto the surface of the same NP to achieve biomimicry. Surface modifications with such proteins derived from biological sources endow nanoparticles with complex cellular functions, such as adhesion, targeting, and signaling, while maintaining loading abilities [5]. To capitalize on this, NPs have been engineered to mimic a diversity of biologics, such as leukocytes, chromatin, dead cells, and platelets [1][28][58][59]. Leukosomes have been designed to conjugate leukocyte ligands, including LFA-1, CD45, CD47, PSLG-1, Mac-1, etc., to increase the expression of IL-10 and TGF-β genes for immunomodulation [7][58]. Alternatively, DNA-protein nanocomplexes mimicking chromatin’s tolerogenic function to induce the tolerogenic phenotype in APCs have induced tolerance towards its OVA and keyhole limpet hemocyanin (KLH) cargos [59]. This tolerogenic APC phenotype can be further taken advantage of by other nanoparticles that use dead-cell peptide conjugates to mimic dying cells with the purpose of inducing apoptosis and antigen-specific tolerance [1]. Liposomes decorated with phosphatidylserine, for example, have been shown to bind macrophage PS-specific scavenger receptors, promoting tolerogenic phenotypes for hemophilia A and Pompe disease treatment [60][61].
Since cargo is another important feature of NP design for therapeutic effects, it is also important to note that NPs have co-administered protein antigens and immunosuppressive drugs, such as NF-kB and mTOR inhibitors [1]. Immunomodulatory cytokines have likewise been loaded into and conjugated onto nanoparticles. TGF-β, for example, was conjugated onto antigen-loaded PLGA NPs to improve their tolerance induction efficacy [62].
Overall, the ability to fine-tune NPs’ physicochemical properties of size, shape, and surface charge; pick from a wide selection of materials; and diversely modify ligands and payload positions NPs as a strong platform for liver-targeting tolerance induction.
References
- Kishimoto, T.K.; Maldonado, R.A. Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Front. Immunol. 2018, 9, 230.
- Xu, X.; Xia, T. Recent Advances in Site-Specific Lipid Nanoparticles for mRNA Delivery. ACS Nanosci. Au 2023, 3, 192–203.
- Barus, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243.
- Ernst, L.; Casais, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991.
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990.
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Gupta, A.S. Influence of particle size and shape on their margination and wall adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 32, 15350–15364.
- Thorp, E.B.; Boada, C.; Jarbath, C.; Luo, X. Nanoparticle Platforms for Antigen-Specific Immune Tolerance. Front. Immunol. 2020, 11, 945.
- Van Haute, D.; Berlin, J.M. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv. 2017, 8, 763–774.
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Scháffler, M.; Takenaka, S.; Móller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416.
- Jacobs, F.; Wisse, E.; De Geest, B. The Role of Liver Sinusoidal Cells in Hepatocyte-Directed Gene Transfer. Am. J. Pathol. 2010, 176, 14–21.
- Li, R.; Otieza, A.; Sørensen, K.K.; McCourt, P.; Olsen, R.; Smedsrød, B.; Svistounov, D. Role of liver sunosoidal endothelial cells and stabilins in elimination of oxidized low-density lipoproteins. Am. J. Gastrointest. Liver Physiol. 2011, 300, G71–G81.
- Tian, Z.; Xu, L.; Chen, Q.; Feng, R.; Lu, H.; Tan, H.; Kang, J.; Wang, Y.; Yan, H. Treatment of Surgical Brain Injury by Immune Tolerance Induced by Peripheral Intravenous Injection of Biotargeting Nanoparticles Loaded with Brain Antigens. Front. Immunol. 2019, 10, 743.
- Wisse, E.; de Zanger, R.B.; Charels, K.; van der Smissen, P.; McCuskey, R.S. The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of disse. Hepatology 1985, 5, 683–692.
- Daemen, T.; Velinova, M.; Regts, J.; de Jager, M.; Kalicharan, R.; Donga, J.; van der Want, J.J.L.; Scherphof, G.L. Different intrahepatic distribution of phosphatidylglycerol and phosphatidylserine liposomes in the rat. Hepatology 2003, 26, 416–423.
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghanderhari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423.
- Yi, S.; Allen, S.D.; Lu, Y.G.; Ouyang, B.Z.; Li, X.; Augsornworawat, P.; Thorp, E.B.; Scott, E.A. Tailoring Nanostructure Morphology for Enhanced Targeting of Dendritic Cells in Atherosclerosis. ACS Nano 2016, 10, 11290–11303.
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327.
- Li, J.; Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liu, X.; Liao, Y.P.; Ma, T.; Meng, H.; Xia, T. Nanocellulose Length Determines the Differential Cytotoxic Effects and Inflammatory Responses in Macrophages and Hepatocytes. Small 2021, 17, 2102545.
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liao, Y.P.; Lu, J.; Li, L.; Liu, X.; Kim, K.; Ahmed, A.; et al. The Crystallinity and Aspect Ratio of Cellulose Nanomaterials Determine Their Pro-Inflammatory and Immune Adjuvant Effects In Vitro and In Vivo. Small 2019, 15, 1901642.
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 103–1052.
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446.
- Malek, M.; Curtis, I.S.; MacCormack, T.J.; Meli, M.V. Charged and Neutral Au Nanoparticles Interact Differently with Langmuir Film-Based Synthetic Membranes: Implications for Nanoparticle Uptake and Membrane Protein Activity. ACS Appl. Nano Mater. 2020, 3, 9276–9284.
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147.
- Arias-Alpizar, G.; Koch, B.; Hamelmann, N.M.; Neustrup, M.A.; Paulusse, J.M.J.; Jiskoot, W.; Kros, A.; Bussmann, J. Stabilin-1 is required for endothelial clearance of small anionic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102395.
- Cheng, S.H.; Li, F.C.; Souris, J.S.; Yang, C.S.; Tseng, F.G.; Lee, H.S.; Chen, C.T.; Dong, C.Y.; Lo, L.W. Visualizing Dynamics of Sub-Hepatic Distribution of Nanoparticles Using Intravital Multiphoton Fluorescence Microscopy. ACS Nano 2012, 6, 4122–4131.
- Bouchie, A. First microRNA mimic enters clinic. Nat. Biotechnol. 2013, 31, 577.
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, 1801451.
- Tsai, S.; Shameli, A.; Yamanouchi, J.; Clemente-Casares, X.; Wang, J.; Serra, P.; Yang, Y.; Medarova, Z.; Moore, A.; Santamaria, P. Reversal of Autoimmunity by Boosting Memory-like Autoregulatory T Cells. Immunity 2010, 32, 568–580.
- Clemente-Casares, X.; Blanco, J.; Ambalavanan, P.; Yamanouchi, J.; Singha, S.; Fandos, C. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016, 530, 434–440.
- Yeste, A.; Takenaka, M.C.; Mascanfroni, I.D.; Nadeau, M.; Kenison, J.E.; Patel, B.; Tukpah, A.; Babon, J.A.B.; Denicola, M.; Kent, S.C.; et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal. 2016, 9, ra-61.
- Tieu, T.; Alba, M.; Elnathan, R.; Cifuentes-Rius, A.; Voelcker, N.H. Advances in Porous Silicon-Based Nanomaterials for Diagnostic and Therapeutic Applications. Adv. Therap. 2018, 2, 1800095.
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, X.; Li, J.; Liu, Q.; Liao, Y.P.; Li, L.; Nel, A.E.; Xia, T. Mechanistic Differences in Cell Death Responses to Metal-Based Engineered Nanomaterials in Kupffer Cells and Hepatocytes. Small 2020, 16, 2000528.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087.
- Shoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586.
- Böttger, R.; Pauli, G.; Chao, P.H.; Al Fayez, N.; Hohenwarter, L.; Li, S.D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154–155, 79–101.
- James, N.D.; Coker, R.J.; Tomlinson, D.; Harris, J.R.W.; Gompels, M.; Pinching, A.J.; Stewart, J.S.W. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. 1994, 6, 294–296.
- Burnett, J.C.; Rossi, J.J.; Tiemann, K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol. J. 2011, 6, 1130–1146.
- Frank-Kamenetsky, M.; Grefhorst, A.; Anderson, N.N.; Racie, T.S.; Bramlage, B.; Akinc, A.; Butler, D.; Charisse, K.; Dorkin, R.; Fan, Y.; et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA 2008, 105, 11915–11920.
- El Dika, I.; Lim, H.Y.; Yong, W.P.; Lin, C.C.; Yoon, J.H.; Modiano, M.; Freilich, B.; Choic, H.J.; Chao, T.Y.; Kelley, R.K. An Open-Label, Multicenter, Phase I, Dose Escalation Study with Phase II Expansion Cohort to Determine the Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Intravenous TKM-080301 in Subjects with Advanced Hepatocellular Carcinoma. Oncologist 2019, 24, 747-e218.
- Zhou, J.; Li, H.; Xia, X.; Herrera, A.; Pollock, N.; Reebye, V.; Sodergren, M.H.; Dorman, S.; Littman, B.H.; Doogan, D.; et al. Anti-inflammatory Activity of MTL-CEBPA, a Small Activating RNA Drug, in LPS-Stimulated Monocytes and Humanized Mice. Mol. Ther. 2019, 27, 999–1016.
- Fenton, O.S.; Kauffman, K.J.; McClellan, R.L.; Appel, E.A.; Dorkin, J.R.; Tibbitt, M.W.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Bioinspired Alkenyl Amino Alcohol Ionizable Lipid Materials for Highly Potent In Vivo mRNA Delivery. Adv. Mater. 2016, 28, 2939–2943.
- Capini, C.; Jaturanpinyo, M.; Chang, H.I.; Mutalik, S.; McNally, A.; Street, S.; Steptoe, R.; O’Sullivan, B.; Davies, N.; Thomas, R. Antigen-Specific Suppression of Inflammatory Arthritis Using Liposomes. J. Immunol. 2009, 182, 3556–3565.
- Martinez, J.O.; Molinaro, R.; Hartman, K.A.; Boada, C.; Sukhovershin, R.; De Rosa, E.; Kirui, D.; Zhang, S.; Evangelopoulos, M.; Carter, A.M.; et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics 2018, 8, 1131–1145.
- Carambia, A.; Gottwick, C.; Schwinge, D.; Stein, S.; Digigow, R.; Şeleci, M.; Mungalpara, D.; Heine, M.; Schuran, F.A.; Corban, C.; et al. Nanoparticle-mediated targeting of autoantigen peptide to cross-presenting liver sinusoidal endothelial cells protects from CD8 T-cell-driven autoimmune cholangitis. Immunology 2021, 162, 452–463.
- Hsu, C.Y.; Chen, C.H.; Aljuffali, I.A.; Dai, Y.S.; Fang, J.Y. Nanovesicle delivery to the liver via retinol binding protein and platelet-derived growth factor receptors: How targeting ligands affect biodistribution. Nanomedicine 2017, 12, 317–331.
- Di Gioacchino, M.; Petrarca, C.; Gatta, A.; Scarano, G.; Farinelli, A.; Della Valle, L.; Lumaca, A.; Del Biondo, P.; Paganelli, R.; Di Giampaolo, L. Nanoparticle-based immunotherapy: State of the art and future perspectives. Expert Rev. Clin. Immunol. 2020, 16, 513–525.
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokathlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146.
- Yang, K.W.; Li, X.R.; Yang, Z.L.; Li, P.Z.; Wang, F.; Liu, Y. Novel polyion complex micelles for liver-targeted delivery of diammonium glycyrrhizinate: In vitro and in vivo characterization. J. Biomed. Mater. Res. Part A 2008, 88, 140–148.
- Pohlit, H.; Bellinghausen, I.; Schömer, M.; Heydenreich, B.; Saloga, J.; Frey, H. Biodegradable pH-Sensitive Poly (ethylene glycol) Nanocarriers for Allergen Encapsulation and Controlled Release. Biomacromolecules 2015, 16, 3103–3111.
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591.
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for Next-Generation Tolerogenic Vaccines against Autoimmune Diseases. Int. J. Mol. Sci. 2019, 201, 204.
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18.
- Liu, Q.; Wang, X.; Liu, X.; Liao, Y.; Chang, C.; Mei, K.; Jiang, J.; Tseng, S.; Gochman, G.; Huang, M. Antigen- and Epitope-Delivering Nanoparticles Targeting Liver Induce Comparable Immunotolerance in Allergic Airway Disease and Anaphylaxis as Nanoparticle-Delivering Pharmaceuticals. ACS Nano 2021, 15, 1608–1626.
- Feng, X.; Liu, J.; Xu, W.; Li, G.; Ding, J. Tackling autoimmunity with nanomedicines. Nanomedicine 2020, 15, 1585–1597.
- Yeste, A.; Nadeau, M.; Burns, E.J.; Weiner, H.L.; Quintana, F.J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2012, 109, 11270–11275.
- Macauley, M.S.; Pfrengle, F.; Rademacher, C.; Nycholat, C.M.; Gale, A.J.; von Drygalski, A.; Paulson, J.C. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Investig. 2013, 123, 3074–3083.
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622.
- Molinaro, R.; Pastó, A.; Corbo, C.; Taraballi, F.; Giordano, F.; Martinex, J.O.; Zhao, P.; Wang, X.; Zinger, A.; Boada, C.; et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale 2019, 11, 13576–13586.
- Li, B.; Yuan, Z.; McMullen, P.; Xie, J.; Jain, P.; Hung, H.; Xu, S.; Zhang, P.; Lin, X.; Wu, K.; et al. A Chromatin-Mimetic Nanomedicine for Therapeutic Tolerance Induction. ACS Nano 2018, 12, 12004–12014.
- Ramakrishnan, R.; Balu-Iyer, S.V. Effect of Biophysical Properties of Phosphatidylserine Particle on Immune Tolerance Induction Toward Factor VIII in a Hemophilia A Mouse Model. J. Pharm. Sci. 2016, 105, 3039–3045.
- Schneider, J.L.; Balu-Iyer, S.V. Phosphatidylserine converts immunogenic recombinant human acid alpha-glucosidase to a tolerogenic form in a mouse model of Pompe disease. J. Pharm. Sci. 2016, 105, 3097–3104.
- Casey, L.M.; Pearson, R.M.; Hughes, K.R.; Liu, J.M.H.; Rose, J.A.; North, M.G.; Wang, L.Z.; Lei, M.; Miller, S.D.; Shea, L.D. Conjugation of transforming growth factor-β to antigen-loaded poly(lactide-co-glycolide) nanoparticles enhances efficiency of antigen-specific tolerance. Bioconjug. Chem. 2018, 29, 813–823.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
05 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




