Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hang Ruan | -- | 3773 | 2024-01-04 13:08:34 | | | |
| 2 | Sirius Huang | Meta information modification | 3773 | 2024-01-05 03:06:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Weng, S.; Yang, X.; Yu, N.; Wang, P.; Xiong, S.; Ruan, H. ADAR-Mediated Site-Specific RNA Editing in Immune-Related Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/53425 (accessed on 14 January 2026).
Weng S, Yang X, Yu N, Wang P, Xiong S, Ruan H. ADAR-Mediated Site-Specific RNA Editing in Immune-Related Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/53425. Accessed January 14, 2026.
Weng, Shenghui, Xinyi Yang, Nannan Yu, Peng-Cheng Wang, Sidong Xiong, Hang Ruan. "ADAR-Mediated Site-Specific RNA Editing in Immune-Related Disease" Encyclopedia, https://encyclopedia.pub/entry/53425 (accessed January 14, 2026).
Weng, S., Yang, X., Yu, N., Wang, P., Xiong, S., & Ruan, H. (2024, January 04). ADAR-Mediated Site-Specific RNA Editing in Immune-Related Disease. In Encyclopedia. https://encyclopedia.pub/entry/53425
Weng, Shenghui, et al. "ADAR-Mediated Site-Specific RNA Editing in Immune-Related Disease." Encyclopedia. Web. 04 January, 2024.
Copy Citation
ADAR (Adenosine Deaminases Acting on RNA) proteins are a group of enzymes that play a vital role in RNA editing by converting adenosine to inosine in RNAs. This process is a frequent post-transcriptional event observed in metazoan transcripts. Recent studies indicate widespread dysregulation of ADAR-mediated RNA editing across many immune-related diseases, such as human cancer.
ADAR
RNA editing
immune-related disease
1. Introduction
ADAR (Adenosine Deaminases Acting on RNA) is a deaminase family specifically targeting adenosine in the double-stranded RNAs [1], which replaces an amino group of adenosines with a keto group, thus contributing to the adenosine to inosine (A-I) conversion. Inosine is further recognized as guanosine (G), which has a similar structure to inosine. This conversion forms a wobble RNA loop or leads to A-G RNA substitutions during further processes [2][3][4].
ADARs are presumed to originate in the early ancestors of metazoans because they are conserved in most major phyla of extant metazoans. A-G substitutions are far more dominant in these species than the other 11 possible nucleotide substitution types [5]. ADAR-mediated A-I RNA editing sites (RESs) are abundant in eukaryotes and mainly in the nascent nuclear mRNA [6]. Across 16 representative metazoan species, ADAR-mediated RNA editing is generally conserved [7], and these post-transcriptional RNA editing events could be considered the hallmark of the metazoan transcriptional regulation [8][9]. In mice and humans, large-scale A-I RNA editing sites are observed in the non-coding regions, while a few editing sites occur in the coding region, such as conserved recoding sites of FlnA, CyFip2, Blcap, and IGFBP7 [10]. In Bilateria, the ADAR-edited recoding sites tend to accumulate in neural and cytoskeleton genes [5].
Abnormal expression of ADAR has been detected in numerous diseases, such as multiple autoimmune diseases and human cancers, and the abnormal expression of ADAR1/2 was positively correlated with the degree of RNA editing [11]. Apart from the pathogenetic global changes in editing levels, many specific RNA editing sites play opposite roles at various stages of cancers; some are strongly associated with high aggressiveness and poor tumor prognosis [12][13][14][15], while some RESs are related to tumor suppression [16][17]. In addition to non-synonymous substitutions on coding sequences [18], there are other types of pathogenic editing sites, such as RESs on 3′ untranslated region (UTR) [19], intron [13], and microRNA (miRNA) [20]. RES is also associated with anti-tumor drug resistance [18]. In the context of human genetics, many editing quantitative trait loci (edQTLs) were significantly enriched in genome-wide association study (GWAS) signals for autoimmune and immune-mediated diseases. RNA editing sites near these edQTLs often exhibited a reduced editing state in disease-associated samples, which might activate the melanoma differentiation-associated gene (MDA5) pathway-mediated interferon response [21][22]. Furthermore, RESs are also believed to contribute to the pathogenesis of other immune-related neurodegenerative diseases, including schizophrenia [23].
To further study the functions of ADAR-mediated RNA editing, the broad spectrum of RNA editing sites is necessary. Developing computational tools that efficiently predict RNA editing sites across the transcriptome is now plausible using high-throughput sequencing technology [24][25][26]. Large-scale RNA editing sites have been identified in both human tissues and animal models, thus providing ample opportunity to explore site-specific editing functions [27][28][29].
Recently, there has been growing interest in recruiting endogenous ADAR for in vivo engineered site-directed RNA editing (SDRE) [30][31]. Exogenous ADAR was also employed to operate precise editing. Along with A-I editing, engineered C-U editing has also been developed [32]. Based on the understanding of the recognition and regulation mechanism of ADAR enzymes, various ADAR recruitment methods have been adopted to improve the efficiency of SDRE, including adding hairpin structures, R/G motifs, or introducing Cas13 proteins to guide RNAs [33].
2. ADAR Proteins as Pattern Recognizers in Immune-Related Pathways
2.1. ADAR Proteins Are Cross-Species Conserved Pattern Recognizers
In mammals, ADAR enzymes have three main subtypes: ADAR1, ADAR2, and ADAR3 [34]. ADAR1 and ADAR2 are expressed in multiple organs, while ADAR3 is mainly expressed in the brain [9]. ADAR1 is widely expressed almost throughout the human body except for skeletal muscle. Although ADAR1 and ADAR2 are expressed in most tissues, the identified edited pre-mRNA typically encodes receptors in the central nervous system [35]. ADAR2 exhibits exceptionally high expression in the central nervous system. It is thought to be primarily responsible for site-specific editing of adenosine in shorter RNA hairpins of central nervous system transcripts [36]. In contrast to ADAR2, which is located mainly in the nucleus, the p150 isoform of ADAR1 can shuttle between the nucleus and cytoplasm by binding to transportin-1 or exportin-5 [8][35][37][38][39]. The translocation of p110 was not as regular as p150 but was observed under stress to block the stress-induced Staufen1-mediated mRNA degradation [40][41]. Intracellular localization analyses of ADAR revealed that ADAR1 and ADAR2 were dynamically moving in nuclei in vivo. The transient sequestration of ADAR1 and ADAR2 in the corners of the nucleus may be caused by the rich endogenous dsRNA structures from the small nucleoli RNA in these corners. In contrast, ADAR homologous protein ADAT (primarily edited tRNA), which lacks a dsRNA binding domain, has not been observed to accumulate dynamically in the nucleus [38]. To activate the A-I RNA editing activity, the homodimerization of ADAR1 and ADAR2 is required [42]. Mammalian ADAR3 is catalytically inactive and acts predominantly as a dsRNA binding protein. ADAR3 is a brain-specific protein and might work as an inhibitor to regulate the process of ADAR1/2-mediated RNA editing [43][44]. According to some studies, the expression of ADAR1 or ADAR2 alone did not correlate with the editing level. In contrast, the combined value of ADAR1 + ADAR2-ADAR3 was positively correlated with the editing level of RNA [45].
The ADAR enzymes exhibit a regional preference to catalyze specific sites by recognizing nearby sequences, as confirmed in Drosophila and humans [46][47][48]. ADAR1 and ADAR2 catalyze their preferred characteristic dsRNAs [45][49][50]. There is no significant difference between ADAR1 and ADAR2 in the global editing sites or regional preference [50]. The values fluctuated slightly between studies, but introns or 3′UTR always cover the most editing sites, and 5′UTR or coding sequences account for only a tiny fraction [50][51]. In humans, the RNA editing sites (more than 80%) are dominantly located in repeat regions, such as short interspersed nuclear elements (SINE), long interspersed nuclear elements (LINE), long terminal repeats (LTR), etc. Only a tiny fraction of RNA editing sites happened on exons. Globally, most of these sites occur in primate-specific, small 140–300 bp Alu elements—a class of repeating SINE (short interspersed nuclear element) inverse elements. The Alu element covers the most editing sites relative to other repetitive elements in humans. Alu was observed mainly in introns and 3′UTRs, which are regions of the RNA molecule that are not translated into protein sequences but have regulatory functions [52][53][54][55]. This shows that ADAR1 primarily edited the Alu element in mRNA transcribed by RNA polymerase II, but not the putative pol-III-transcribed Alu elements [55]. In addition, although both ADAR1 and ADAR2 enzymes target dsRNA hairpins and there are some overlapping editing substrates between ADAR1 and ADAR2 in cells expressing both of these two enzymes, ADAR1 is particularly biased towards catalyzing around 300-base-long hairpins formed from paired inverted copies of Alu elements in the pre-mRNA [10]. ADAR1 has also been proven to contribute to hyper-editing in the repeat element, and ADAR2 tends to be responsible for non-repetitive coding sites [45], which may be induced by the formation of the sense–antisense structure on these non-repeat regions [5][56].
2.2. Immune-Related Pathways Mediated by ADARs
ADAR1 is mainly present as two isoforms, ADAR1 p150 and ADAR1 p110, which are named based on their molecular weight (Figure 1). ADAR1 p150 is 150 Da in size and is crucial for protecting mammals against viral infections. It serves as a major pattern recognition protein. It is induced by interferon [57], thus emphasizing its importance in the body’s immune response against viruses. Both the p150 and p110 isoforms can be found in the cytoplasm. ADAR1 p110 is a shorter isoform with a 110 Da size. These two ADAR1 isoforms are generated from alternate splicing. They share a double-strand RNA binding domain (dsRBD) and a deaminase [58]. The double-strand RNA binding domain (dsRBD) shared by ADAR1 p150, p110, and ADAR2, primarily recognizes common right-handed dsRNA (A-RNA) conformations. Type-I-Interferon-inducible p150 has an additional Zα (Z-RNA binding domain, ZBD) at the N-terminal compared to p110. Thus, p150 could bind endogenous Z-RNA to avoid activating ZBP1 and downstream RIPK3-mediated necroptosis by recognizing unbound Z-RNA (Figure 1A), thus mediating the innate immune system activation caused by immunogenetic Z-RNA [59][60][61][62][63]. It has been discovered that ZBP1 plays a crucial role in sensing Z-RNA during influenza A virus infection. This activation of ZBP1 leads to hyperinflammation and necroptosis, which are some of the main ways the body fights off viral infections [64]. When ADAR1 cannot prevent the binding of ZBP1 to Z-form RNA through editing or binding, activated ZBP1 activates downstream Receptor Interacting Serine/Threonine Kinase 3 (RIPK3)-mediated necroptosis. The ZBD of ADAR1 p150 is primarily bound to endogenous left-handed dsRNA (Z-RNA) enriched in the 3′UTR of the interferon-stimulated genes (ISGs) [59]. ADAR1 p150, unlike ADAR1 p110 and ADAR2, can recognize Z-RNAs and inhibit downstream immune responses by independently binding (Figure 1). Overexpressed ZBD could rescue the phenotype caused by the lack of a catalysis domain. In diseases, aberrant ADAR1 p150 may be involved in ZBP1-mediated cell necrosis. Activation of the ZBP1 pathway may also be inhibited by p150, thus representing a potential treatment option in cold tumors [59]. Different Alu families contain a conserved Z-forming sequence [65]. The Z-forming sequence is prone to forming Z-DNA conformations and could be recognized by the ZBD of ADAR1 p150 [65][66][67]. Different Alu families contain a conserved Z-forming sequence. The Z-forming sequence is prone to forming Z-DNA conformations and could be recognized by the ZBD of ADAR1 p150 [66]. In addition, p150’s extra RNA binding domain allows for higher binding ability than p110, resulting in more editing sites [51]. Except for the Z-forming sequence, other RNA modifications can potentially affect the binding between ADAR and Alu, such as 2′-O-methyl and N6-methyl adenosine marks. Because these modifications share the same substrate with ADAR, exogenous double-stranded RNA, they may have a competing relationship with ADAR-mediated RNA editing and play similar roles in regulating MDA5-mediated immune pathways [68][69].
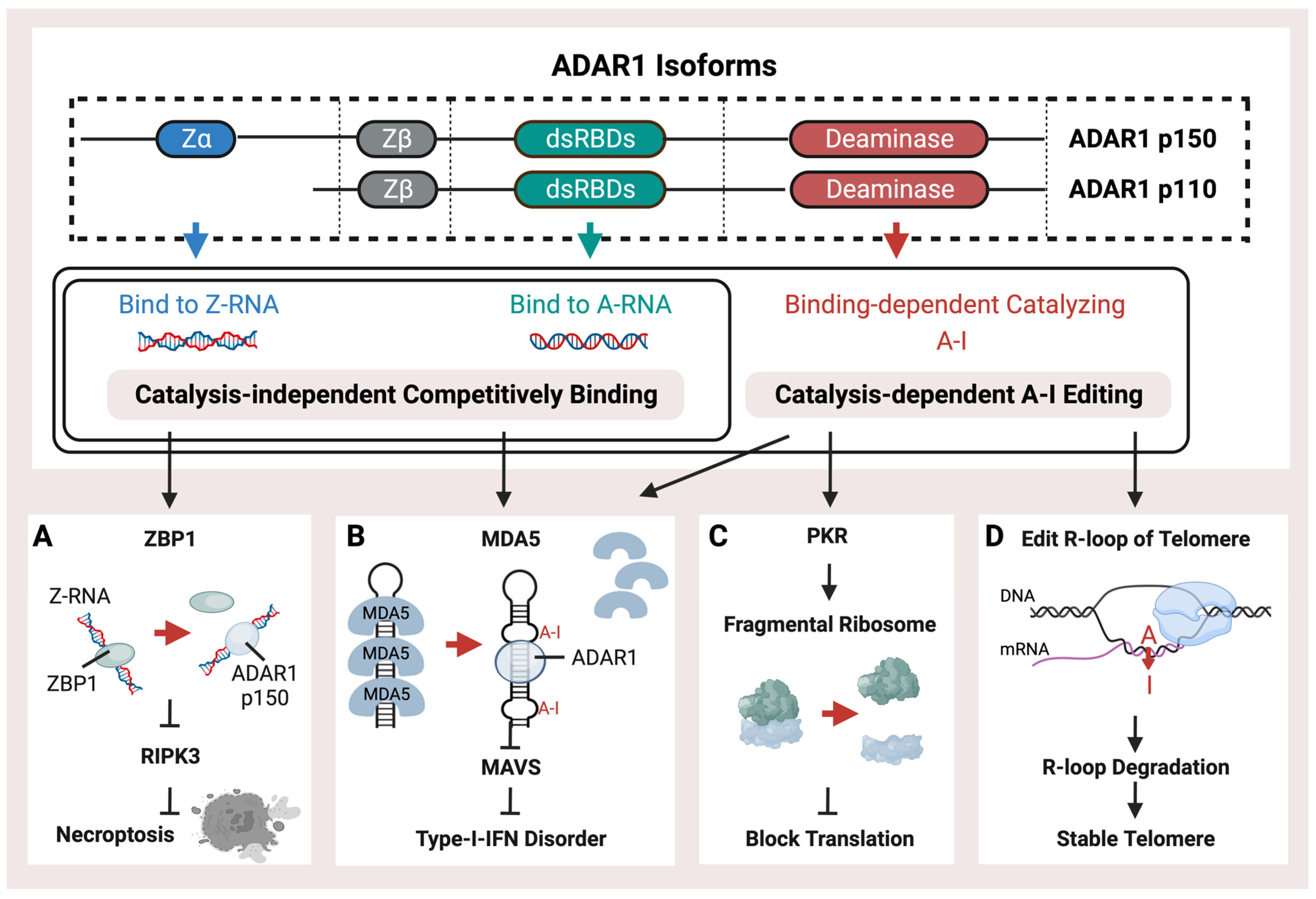
Figure 1. Schematic representation of the structure and functions of ADAR1. ADAR1 isoforms, p110 and p150, possess a right-handed A-RNA bindable double-strand RNA binding domain (dsRBD) and a catalysis domain (deaminase). p150 also contains an additional left-hand Z-RNA binding domain (Z-alpha). ADAR1 performs catalysis-independent competitive binding or catalysis-dependent A-I RNA editing through its distinct binding and catalysis domains. ADAR1 has multiple pathways of operation, (A) including binding with endogenous Z-RNA to inhibit RIPK3-induced necroptosis and to block the activation of ZBP1. (B) Additionally, ADAR1 prevents immunogenetic dsRNA from inducing MDA5-mediated Type-I interferon disorder by either binding or editing to impede the recognition of dsRNA. (C) Moreover, ADAR1-mediated RES inhibits the PKR pathway, thus facilitating translation shutdown. (D) Finally, ADAR1 catalyzes the R-loop to promote its degradation and stabilize the telomere.
ADAR-mediated RNA editing is a crucial mechanism in human cells that plays a key role in fighting against viral infections. This mechanism helps to differentiate between endogenous and exogenous double-stranded RNA, which is essential for the immune system to recognize and respond effectively to viral threats. Editing of dsRNA through co-transcriptional ADAR-mediated A-I editing prevents downstream Type-I-Interferon activation of MDA5 (Figure 1B) by creating wobble A-I base pairs so that MDA5 cannot recognize endogenous dsRNA [51][54][70]. ADAR is critical during mice’s embryonic development to maintain hematopoiesis [71][72]. The mice lacking ADAR1 died at the embryonic stage with wide apoptosis in multiple tissues, fetal liver disintegration, and defective hematopoiesis, and mice with homozygous ADAR1 p150 deletion exhibit a similarly lethal phenotype [73][74][75]. The MDA5 pathway induced by loss of ADAR1 function is associated with the interferon (IFN) disorder of developing AGS [76][77].
It has been demonstrated that mice can tolerate the loss of global A-I editing when the type-I IFN-induced pathway is blocked by MDA5 inhibition, thus indicating that ADAR1-mediated RNA editing sites are not crucial for the development and homeostasis of mice [78][79]. As a result, the MDA5-dependent interferon pathway, induced by unedited Alu elements, could be the main feature of ADAR and probably plays a significant role in interferon-related diseases. Both loss of function (LOF) mutations on ZBD or dsRBD of ADAR1 have been identified in AGS patients [76][80]. The catalysis-independent competitive binding of ADAR proteins against MDA5 may also achieve the same effect as the A-I editing (Figure 1B) [81]. In addition to the MDA5 activated by un-catalyzed dsRNA, the Z-RNA binding ability could also inhibit the MDA5 pathway, and the LOF mutation, such as P154A or W197A on ZBD of ADAR1, could activate the MDA5 pathway without decreasing the global RNA editing level [82][83]. Meanwhile, other dsRNA sensors, such as OAS and RIG-I, acted as the immune activators when the ADAR editing was insufficient [84][85].
During the Type-I-IFN response, the Protein Kinase R (PKR) pathway (Figure 1C) could also be activated to block translation by the endogenous dsRNA through the fragmentation of the ribosome. Knockdown of ADAR1 caused differentiated human neuronal progenitor cells to exhibit both MDA5-activated interferon upregulation and PKR activation, accompanied by cell death [55]. Furthermore, the ADAR1 p110 isoform has been discovered to possess a distinct function of preserving genomic stability through the catalyzation of R-loop structures (Figure 1D). These structures represent a stable form of the RNA/DNA hybrid on telomeres, which may sometimes occur when newly synthesized RNA molecules fail to detach from their template DNA immediately after transcription [86].
2.3. Regulatory Functions of Site-Specific dsRNA
RNA editing sites are predominantly found in intronic, intergenic, and 3′UTR regions, with only a few in exons. These sites are known to affect RNA splicing and may also have an impact on miRNA-mediated transcript regulation, as well as altering the mRNA sequence, thus ultimately changing protein functions [9].
2.3.1. Recoding RNA Editing
Recoding editing sites (Figure 2A) were widely found in diseases with cis [48] or trans [87] regulators. In cancers, RESs that result in non-synonymous substitution of codons could introduce proteomic diversities [88] to affect cancer cell proliferation, migration, and invasion, such as RES in the coding region of COPA. RES could also create neoantigens in cancer cells to promote the activation of the immune system [17].
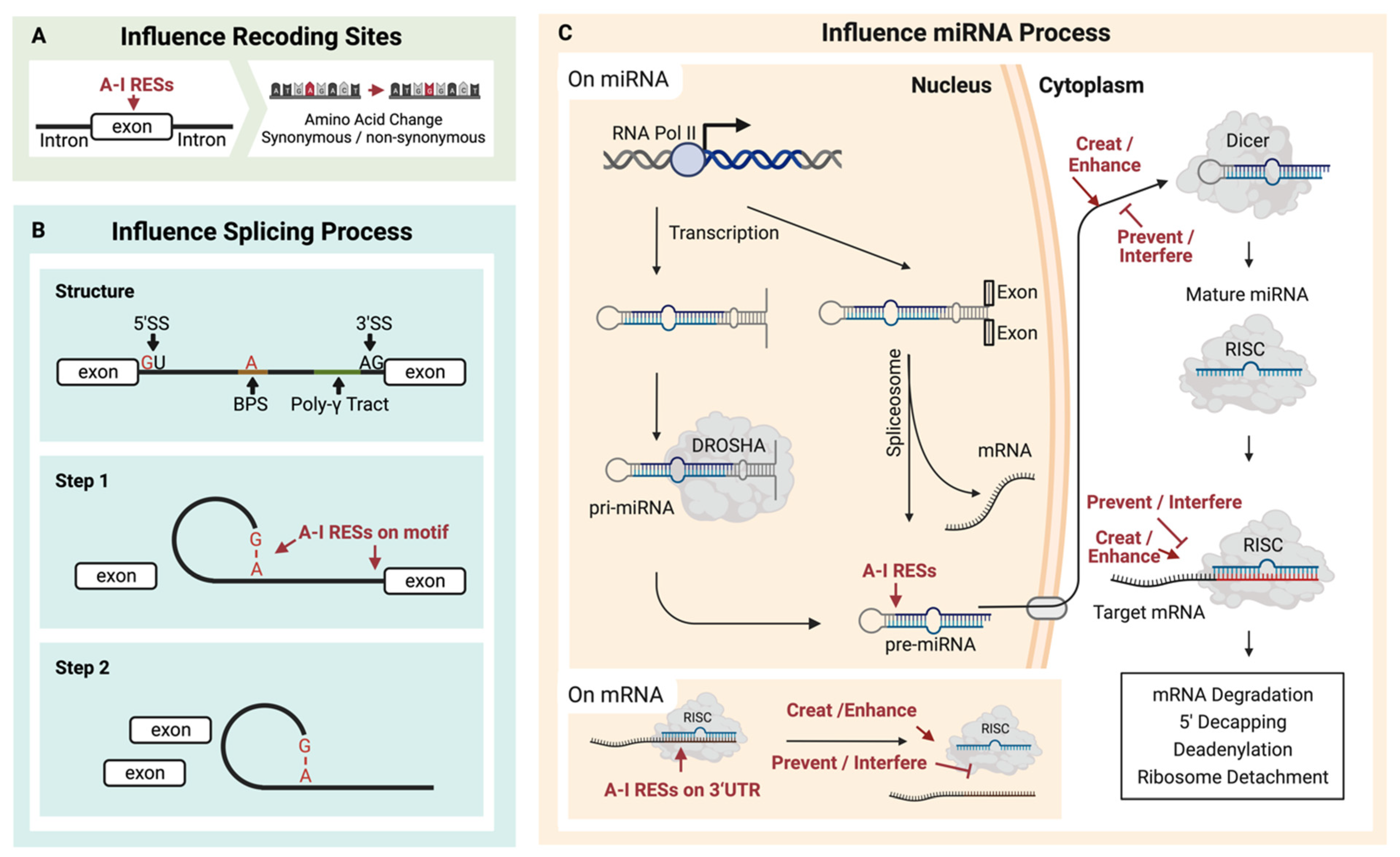
Figure 2. Multiple contributions can result from A-I RNA editing sites (RESs). (A) RESs located on exons may cause amino acid recoding. (B) RESs located on splicing motifs may change splicing events, and (C) RESs on pre-miRNA may affect the formation of miRNA and consequently impact downstream mRNA regulation induced by miRNA.
2.3.2. RNA Editing Influences RNA Splicing
ADAR-mediated A-I conversion could alter RNA splicing sites (Figure 2B). As a functional splicing element, the 5′-donor sequence is mostly conserved as GU, while the 3′-acceptor sequence is predominantly AG [89]. Additionally, the splicing element branch point sequence (BPS) contains an adenosine residue that will accept a guanosine residue at the 5′ end of the intron [89][90]. Because the inosine could work as guanosine, these elements might be destroyed [91] or created [92][93] by A-I RNA editing in pre-mRNA. As a result, the editing of pre-mRNA might contribute to multiple effects, such as exon skipping or novel exon [94]. Except for directly editing these elements, other relationships between A-I RNA editing and splicing should exist [95]. Kapoor et al. found that some splicing perturbations were provided independently of the editing process through physical interaction with the splicing machinery [96].
Knocking out ADAR1 and ADAR2 in mice experiments caused significant changes in splicing [96]. Most splicing modulation events are associated with ADAR1, while ADAR2 is one of two subforms of catalytic RNA-editing ADAR. These two editing enzymes are not replaceable, especially at some specific sites. For example, intron 42, which was retained in Filamine A, has been proven to be a specific target for ADAR2 editing, with little association with ADAR1 editing [97].
RNA editing can create alternative splicing isoforms that can be oncogenic. One example is ADAR1 editing, which can affect splicing through the edited site, while ADAR2 binding can prevent U2AF65 from entering the 3′ splice site and subsequently blocking the splicing [15]. Splicing changes regulated by ADARs have been reported not only as a by-product of ADARs editing but also in relation to tumorigenesis. The dysregulation of RNA editing and altered splicing has been reported in breast cancer, B-cell lymphoma, and other cancers [98][99][100]. Alternative splicing could also be tumor-suppressive. In cancer cells, the protoplasts of CCDC15 (including exon 9) were oncogenic, and ADAR acted as a tumor inhibitor by influencing the growth of cancer cells through binding or catalytic editing, which makes the exon skip [15].
RNA editing and splicing exhibit a mutually regulatory relationship. RNA editing precedes splicing in most transcripts [93]. In some cases, splicing regulates editing by affecting the conformation of splicing transcripts. For example, the osmosensitive cation channel TMEM63b has interdependent exon four skipping and an ADAR2-mediated recoding site in exon 20, Gln to Arg. Wu et al. found that if exon four is retained, it destroys the hairpin structure in exon 20, which is prone to ADAR2 action [101]. In addition, ADAR-mediated binding or catalytically regulated splicing may be flexibly regulated in homeostasis. A major splicing factor, SRSF9, inhibits specific editing on novel exons. SRSF9 is a major splicing factor that has been found to inhibit specific editing of some exons in the brain, such as voltage-gated calcium channel CaV1.3. The physical interaction between SRSF9 and ADAR2 could decrease the editing function by preventing the dimerization of ADAR2. In the two RNA recognition domains of SRSF9 (RRM1 or RRM2), RRM2 is mainly involved in the interaction between ARSF9 and ADAR2 [102][103].
2.3.3. RNA Editing Regulates miRNA Binding
MicroRNAs are short RNAs of about 22 nucleotides that regulate gene expression [104]. The much longer miRNA precursors (pre-miRNAs) contain dsRNA and are potential substrates for A-I RNA editing (Figure 2C). Around 20% of miRNA precursors are edited in the adult brain [105]. It has been reported that several miRNA RESs conserved in ten different human tissues may increase the diversity of miRNA targets and regulate miRNA function [106]. RES on microRNA affects the processing of the miRNA. ADAR1 could form protein complexes with endoribonuclease Dicer. The catalysis-inactivated heterodimer promotes Dicer to recognize miRNA precursors, thereby increasing the miRNA processing to produce more miRNA. Meanwhile, the interaction between ADAR1 and Dicer could promote miR-155-5P maturation and further inhibit adipogenesis [107]. The catalysis-activated homodimers (ADAR1-ADAR1) edited microRNA to block the recognition and cleaving by the miRNA process enzyme, Dicer or Drosha, and thus reduce the miRNA process. In addition, the influence comes from protein–protein interaction. The RES on the miRNA could affect the multiple miRNA process stages, such as the recognition by the Dicer–DGCR8 complex, which has been proved for several miRNA precursors of pri-miR-142 [108], pri-miR-33, pri-miR-133a2, and pri-miR-379 [105]. In contrast, RES enhances the Drosha cleavage for pri-miR-197 and pri-miR-203, also reported by Kawahara et al. In addition, in human melanocytes, RES inhibited the Drosha on pri-miR-455 at the +2 and +17 positions, further decreasing miR-455-5p levels. The suppression of ADAR1 is linked to an increase in miR-455-5p, thus leading to a decrease in cytoplasmic polyadenylation element-binding protein 1 (CPEB1), a tumor suppressor. This association may be a contributing factor to melanoma metastasis [109]. Except for Drosha, RES also affects Dicer cleavage to inhibit miRNA expressions, such as pri-miR-151 [110] and pre-let-7g [105]. Additionally, RES on the precursor of miR-BART6-5p of the DNA virus, Epstein–Barr virus (EBV), can disturb the binding to AGO2-containing RISC, thus inhibiting this miRNA and blocking the specific binding to human Dicer mRNA [111].
A substitution in a single site on the pre-miRNA seed sequence can change the target mRNA, just like the RES in miR-376 alters the target specificity [112]. This can be significant in embryo development [113]. ADAR1 regulates miRNA-targeted mRNA by editing it, thus preventing miR-155 from binding to MDM2 3′ UTR. This results in the non-suppression of MDM2 and no activation of downstream p53 [14].
2.4. The Upstream Regulators of RNA Editing
Besides studying the functions of specific RNA editing sites, many studies aim to understand the global regulation of ADAR activity. RNA editing is known to introduce diversity in the transcriptome and proteome, but ensuring that its activity is regulated in living organisms is essential. Studies have shown that the efficiency and specificity of edited sites can be controlled. Proteins that bind to ADAR can affect its activity. These include all DZF-domain-containing proteins, such as ILF3, which can negatively regulate RNA editing [87].
The RNA editing site shows some specific primary sequence preferences. The 5′ neighbor of the sites mostly influences the catalysis of ADAR1 and ADAR2 [114][115][116]. The 3′ neighbor affects ADAR2 more. Thus, ADAR2 prefers certain trinucleotides, such as UAU, AAG, UAG, and AAU [115]. Based on the primary sequence, by learning the structural characteristics of the nearby sequence, there is the possibility of predicting the RESs [116]. Stabilizing the hairpin covering the RES influences the editing efficiency, and the decreased hairpin stabilization could reduce the editing frequency [46]. The cis-regulation is conserved in Drosophila species. Also, the earlier RESs in the species’ evolutionary tree tend to have higher editing efficiency, and these sites are enriched in neuronal genes [117]. The secondary structure of the substrate (hairpin or sense–antisense structure) could interfere with the access of the enzyme, and the binding of the enzyme would, in turn, open around ten base pairs for further catalysis [1]. In addition, tertiary structure in vivo could influence RNA editing efficiency [118]. Moreover, it is plausible that remote sequence structures may affect RNA editing events [119]. Quantitative trait loci analysis in Drosophila melanogaster showed that functional edQTLs may work by changing the secondary structure and affecting nearby RNA editing levels [1]. In addition, only a tiny fraction of dsRNAs must be edited to change their immunogenic secondary structure [120][121]. Sun and colleagues showed that immunogenic hairpins have shorter loops between stems than other dsRNAs [121].
In addition, other types of RNA modifications may affect ADAR-mediated RNA editing. The deamination activity of ADAR2 is sensitive to RNA modification at the 2′-carbon ribose. Methylation on 2′-OH would lead to a significant decrease in deamination efficiency, while 2′-deoxyadenosine and 2′-deoxy-2′-fluoroadenosine would not change the rate much [122]. The 2′-O-methylation on the ribose of eukaryote mRNAs 5′ cap could, similarly to ADAR-mediated self/non-self dsRNA, distinguish and avoid coronavirus by activating the MDA5-mediated IFN pathway [68].
The RNA editing sites often cluster closely in specific genome regions, especially in aging or diseased samples. This phenomenon is referred to as hyper-editing. Hyper-editing regions exist in the aging human brain, and these hyper-editing sites remove the loops in hairpins and make the dsRNA structure more stable [123]. Hyper-editing also occurs in the 3′UTR of MDM2 mRNA, and these RESs block the related miRNA, miR-155, and binding, which stabilizes the MDM2 mRNA and is finally involved in promoting the malignant progenitor propagation [14]. Emerging RES hotspots were further found in cancers. Hyper-editing sites block the recognition between miR-200b and the 3′UTR of ZEB1/ZEB2 mRNA, thus reducing the inhibition of ZEB1/ZEB2 to promote cell invasion and migration. Also, the hyper-edited miR-200b could target new mRNA, such as LIFR, a metastasis suppressor [124].
References
- Ramaswami, G.; Deng, P.; Zhang, R.; Anna Carbone, M.; Mackay, T.F.C.; Billy Li, J. Genetic Mapping Uncovers Cis-Regulatory Landscape of RNA Editing. Nat. Commun. 2015, 6, 8194.
- Nishikura, K. Editor Meets Silencer: Crosstalk between RNA Editing and RNA Interference. Nat. Rev. Mol. Cell Biol. 2006, 7, 919–931.
- Jin, Y.; Tian, N.; Cao, J.; Liang, J.; Yang, Z.; Lv, J. RNA Editing and Alternative Splicing of the Insect nAChR Subunit Alpha6 Transcript: Evolutionary Conservation, Divergence and Regulation. BMC Evol. Biol. 2007, 7, 98.
- Alon, S.; Mor, E.; Vigneault, F.; Church, G.M.; Locatelli, F.; Galeano, F.; Gallo, A.; Shomron, N.; Eisenberg, E. Systematic Identification of Edited microRNAs in the Human Brain. Genome Res. 2012, 22, 1533–1540.
- Zhang, P.; Zhu, Y.; Guo, Q.; Li, J.; Zhan, X.; Yu, H.; Xie, N.; Tan, H.; Lundholm, N.; Garcia-Cuetos, L.; et al. On the Origin and Evolution of RNA Editing in Metazoans. Cell Rep. 2023, 42, 112112.
- Knoop, V. When You Can’t Trust the DNA: RNA Editing Changes Transcript Sequences. Cell. Mol. Life Sci. 2011, 68, 567–586.
- Duan, Y.; Tang, X.; Lu, J. Evolutionary Driving Forces of A-to-I Editing in Metazoans. WIREs RNA 2022, 13, e1666.
- Nishikura, K. A-to-I Editing of Coding and Non-Coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016, 17, 83–96.
- Eisenberg, E.; Levanon, E.Y. A-to-I RNA Editing—Immune Protector and Transcriptome Diversifier. Nat. Rev. Genet. 2018, 19, 473–490.
- Riedmann, E.M.; Schopoff, S.; Hartner, J.C.; Jantsch, M.F. Specificity of ADAR-Mediated RNA Editing in Newly Identified Targets. RNA 2008, 14, 1110–1118.
- Han, L.; Diao, L.; Yu, S.; Xu, X.; Li, J.; Zhang, R.; Yang, Y.; Werner, H.M.J.; Eterovic, A.K.; Yuan, Y.; et al. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell 2015, 28, 515–528.
- Galore-Haskel, G.; Nemlich, Y.; Greenberg, E.; Ashkenazi, S.; Hakim, M.; Itzhaki, O.; Shoshani, N.; Shapira-Fromer, R.; Ben-Ami, E.; Ofek, E.; et al. A Novel Immune Resistance Mechanism of Melanoma Cells Controlled by the ADAR1 Enzyme. Oncotarget 2015, 6, 28999–29015.
- Amin, E.M.; Liu, Y.; Deng, S.; Tan, K.S.; Chudgar, N.; Mayo, M.W.; Sanchez-Vega, F.; Adusumilli, P.S.; Schultz, N.; Jones, D.R. The RNA-Editing Enzyme ADAR Promotes Lung Adenocarcinoma Migration and Invasion by Stabilizing FAK. Sci. Signal. 2017, 10, eaah3941.
- Jiang, Q.; Isquith, J.; Zipeto, M.A.; Diep, R.H.; Pham, J.; Delos Santos, N.; Reynoso, E.; Chau, J.; Leu, H.; Lazzari, E.; et al. Hyper-Editing of Cell-Cycle Regulatory and Tumor Suppressor RNA Promotes Malignant Progenitor Propagation. Cancer Cell 2019, 35, 81–94.e7.
- Tang, S.J.; Shen, H.; An, O.; Hong, H.; Li, J.; Song, Y.; Han, J.; Tay, D.J.T.; Ng, V.H.E.; Bellido Molias, F.; et al. Cis- and Trans-Regulations of Pre-mRNA Splicing by RNA Editing Enzymes Influence Cancer Development. Nat. Commun. 2020, 11, 799.
- Velazquez-Torres, G.; Shoshan, E.; Ivan, C.; Huang, L.; Fuentes-Mattei, E.; Paret, H.; Kim, S.J.; Rodriguez-Aguayo, C.; Xie, V.; Brooks, D.; et al. A-to-I miR-378a-3p Editing Can Prevent Melanoma Progression via Regulation of PARVA Expression. Nat. Commun. 2018, 9, 461.
- Zhang, M.; Fritsche, J.; Roszik, J.; Williams, L.J.; Peng, X.; Chiu, Y.; Tsou, C.-C.; Hoffgaard, F.; Goldfinger, V.; Schoor, O.; et al. RNA Editing Derived Epitopes Function as Cancer Antigens to Elicit Immune Responses. Nat. Commun. 2018, 9, 3919.
- Teoh, P.J.; An, O.; Chung, T.-H.; Chooi, J.Y.; Toh, S.H.M.; Fan, S.; Wang, W.; Koh, B.T.H.; Fullwood, M.J.; Ooi, M.G.; et al. Aberrant Hyperediting of the Myeloma Transcriptome by ADAR1 Confers Oncogenicity and Is a Marker of Poor Prognosis. Blood 2018, 132, 1304–1317.
- Jiang, L.; Hao, Y.; Shao, C.; Wu, Q.; Prager, B.C.; Gimple, R.C.; Sulli, G.; Kim, L.J.; Zhang, G.; Qiu, Z.; et al. ADAR1-Mediated RNA Editing Links Ganglioside Catabolism to Glioblastoma Stem Cell Maintenance. J. Clin. Investig. 2022, 132, e143397.
- Nemlich, Y.; Greenberg, E.; Ortenberg, R.; Besser, M.J.; Barshack, I.; Jacob-Hirsch, J.; Jacoby, E.; Eyal, E.; Rivkin, L.; Prieto, V.G.; et al. MicroRNA-Mediated Loss of ADAR1 in Metastatic Melanoma Promotes Tumor Growth. J. Clin. Investig. 2013, 123, 2703–2718.
- Zhang, H.; Fu, Q.; Shi, X.; Pan, Z.; Yang, W.; Huang, Z.; Tang, T.; He, X.; Zhang, R. Human A-to-I RNA Editing SNP Loci Are Enriched in GWAS Signals for Autoimmune Diseases and under Balancing Selection. Genome Biol. 2020, 21, 288.
- Li, Q.; Gloudemans, M.J.; Geisinger, J.M.; Fan, B.; Aguet, F.; Sun, T.; Ramaswami, G.; Li, Y.I.; Ma, J.-B.; Pritchard, J.K.; et al. RNA Editing Underlies Genetic Risk of Common Inflammatory Diseases. Nature 2022, 608, 569–577.
- Choudhury, M.; Fu, T.; Amoah, K.; Jun, H.-I.; Chan, T.W.; Park, S.; Walker, D.W.; Bahn, J.H.; Xiao, X. Widespread RNA Hypoediting in Schizophrenia and Its Relevance to Mitochondrial Function. Sci. Adv. 2023, 9, eade9997.
- Flati, T.; Gioiosa, S.; Spallanzani, N.; Tagliaferri, I.; Diroma, M.A.; Pesole, G.; Chillemi, G.; Picardi, E.; Castrignanò, T. HPC-REDItools: A Novel HPC-Aware Tool for Improved Large Scale RNA-Editing Analysis. BMC Bioinform. 2020, 21, 353.
- Liu, Z.; Quinones-Valdez, G.; Fu, T.; Choudhury, M.; Reese, F.; Mortazavi, A.; Xiao, X. L-GIREMI Uncovers RNA Editing Sites in Long-Read RNA-Seq. Bioinformatics 2022. preprint.
- Chen, L.; Ou, L.; Jing, X.; Kong, Y.; Xie, B.; Zhang, N.; Shi, H.; Qin, H.; Li, X.; Hao, P. DeepEdit: Single-Molecule Detection and Phasing of A-to-I RNA Editing Events Using Nanopore Direct RNA Sequencing. Genome Biol. 2023, 24, 75.
- Solomon, O.; Eyal, E.; Amariglio, N.; Unger, R.; Rechavi, G. e23D: Database and Visualization of A-to-I RNA Editing Sites Mapped to 3D Protein Structures. Bioinformatics 2016, 32, 2213–2215.
- Picardi, E.; D’Erchia, A.M.; Lo Giudice, C.; Pesole, G. REDIportal: A Comprehensive Database of A-to-I RNA Editing Events in Humans. Nucleic Acids Res. 2017, 45, D750–D757.
- Ruan, H.; Li, Q.; Liu, Y.; Liu, Y.; Lussier, C.; Diao, L.; Han, L. GPEdit: The Genetic and Pharmacogenomic Landscape of A-to-I RNA Editing in Cancers. Nucleic Acids Res. 2022, 50, D1231–D1237.
- Reautschnig, P.; Wahn, N.; Wettengel, J.; Schulz, A.E.; Latifi, N.; Vogel, P.; Kang, T.-W.; Pfeiffer, L.S.; Zarges, C.; Naumann, U.; et al. CLUSTER Guide RNAs Enable Precise and Efficient RNA Editing with Endogenous ADAR Enzymes in vivo. Nat. Biotechnol. 2022, 40, 759–768.
- Kaseniit, K.E.; Katz, N.; Kolber, N.S.; Call, C.C.; Wengier, D.L.; Cody, W.B.; Sattely, E.S.; Gao, X.J. Modular, Programmable RNA Sensing Using ADAR Editing in Living Cells. Nat. Biotechnol. 2023, 41, 482–487.
- Abudayyeh, O.O.; Gootenberg, J.S.; Franklin, B.; Koob, J.; Kellner, M.J.; Ladha, A.; Joung, J.; Kirchgatterer, P.; Cox, D.B.T.; Zhang, F. A Cytosine Deaminase for Programmable Single-Base RNA Editing. Science 2019, 365, 382–386.
- Merkle, T.; Merz, S.; Reautschnig, P.; Blaha, A.; Li, Q.; Vogel, P.; Wettengel, J.; Li, J.B.; Stafforst, T. Precise RNA Editing by Recruiting Endogenous ADARs with Antisense Oligonucleotides. Nat. Biotechnol. 2019, 37, 133–138.
- Savva, Y.A.; Rieder, L.E.; Reenan, R.A. The ADAR Protein Family. Genome Biol. 2012, 13, 252.
- Emeson, R.B.; Singh, M. Adenosine-to-Inosine RNA Editing: Substrates and Consequences. In RNA Editing; Oxford University Press: Oxford, UK, 2001; pp. 109–138.
- Sinigaglia, K.; Wiatrek, D.; Khan, A.; Michalik, D.; Sambrani, N.; Sedmík, J.; Vukić, D.; O’Connell, M.A.; Keegan, L.P. ADAR RNA Editing in Innate Immune Response Phasing, in Circadian Clocks and in Sleep. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2019, 1862, 356–369.
- Eckmann, C.R.; Neunteufl, A.; Pfaffstetter, L.; Jantsch, M.F. The Human But Not the Xenopus RNA-Editing Enzyme ADAR1 Has an Atypical Nuclear Localization Signal and Displays the Characteristics of a Shuttling Protein. Mol. Biol. Cell 2001, 12, 1911–1924.
- Desterro, J.M.P.; Keegan, L.P.; Lafarga, M.; Berciano, M.T.; O’Connell, M.; Carmo-Fonseca, M. Dynamic Association of RNA-Editing Enzymes with the Nucleolus. J. Cell Sci. 2003, 116, 1805–1818.
- Fritz, J.; Strehblow, A.; Taschner, A.; Schopoff, S.; Pasierbek, P.; Jantsch, M.F. RNA-Regulated Interaction of Transportin-1 and Exportin-5 with the Double-Stranded RNA-Binding Domain Regulates Nucleocytoplasmic Shuttling of ADAR1. Mol. Cell. Biol. 2009, 29, 1487–1497.
- Kim, Y.K.; Furic, L.; DesGroseillers, L.; Maquat, L.E. Mammalian Staufen1 Recruits Upf1 to Specific mRNA 3′UTRs so as to Elicit mRNA Decay. Cell 2005, 120, 195–208.
- Sakurai, M.; Shiromoto, Y.; Ota, H.; Song, C.; Kossenkov, A.V.; Wickramasinghe, J.; Showe, L.C.; Skordalakes, E.; Tang, H.-Y.; Speicher, D.W.; et al. ADAR1 Controls Apoptosis of Stressed Cells by Inhibiting Staufen1-Mediated mRNA Decay. Nat. Struct. Mol. Biol. 2017, 24, 534–543.
- Cho, D.-S.C.; Yang, W.; Lee, J.T.; Shiekhattar, R.; Murray, J.M.; Nishikura, K. Requirement of Dimerization for RNA Editing Activity of Adenosine Deaminases Acting on RNA. J. Biol. Chem. 2003, 278, 17093–17102.
- Chen, C.-X.; Cho, D.-S.C.; Wang, Q.; Lai, F.; Carter, K.C.; Nishikura, K. A Third Member of the RNA-Specific Adenosine Deaminase Gene Family, ADAR3, Contains Both Single- and Double-Stranded RNA Binding Domains. RNA 2000, 6, 755–767.
- Oakes, E.; Anderson, A.; Cohen-Gadol, A.; Hundley, H.A. Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-mRNA Inhibits RNA Editing in Glioblastoma. J. Biol. Chem. 2017, 292, 4326–4335.
- Tan, M.H.; Li, Q.; Shanmugam, R.; Piskol, R.; Kohler, J.; Young, A.N.; Liu, K.I.; Zhang, R.; Ramaswami, G.; Ariyoshi, K.; et al. Dynamic Landscape and Regulation of RNA Editing in Mammals. Nature 2017, 550, 249–254.
- Sapiro, A.L.; Deng, P.; Zhang, R.; Li, J.B. Cis Regulatory Effects on A-to-I RNA Editing in Related Drosophila Species. Cell Rep. 2015, 11, 697–703.
- Duan, Y.; Dou, S.; Luo, S.; Zhang, H.; Lu, J. Adaptation of A-to-I RNA Editing in Drosophila. PLoS Genet. 2017, 13, e1006648.
- Park, E.; Guo, J.; Shen, S.; Demirdjian, L.; Wu, Y.N.; Lin, L.; Xing, Y. Population and Allelic Variation of A-to-I RNA Editing in Human Transcriptomes. Genome Biol. 2017, 18, 143.
- Cruz, P.H.C.; Kato, Y.; Nakahama, T.; Shibuya, T.; Kawahara, Y. A Comparative Analysis among ADAR Mutant Mice Reveals Site-Specific Regulation of RNA Editing. Mol. Biol. 2019, preprint.
- Licht, K.; Kapoor, U.; Amman, F.; Picardi, E.; Martin, D.; Bajad, P.; Jantsch, M.F. A High Resolution A-to-I Editing Map in the Mouse Identifies Editing Events Controlled by Pre-mRNA Splicing. Genome Res. 2019, 29, 1453–1463.
- Pestal, K.; Funk, C.C.; Snyder, J.M.; Price, N.D.; Treuting, P.M.; Stetson, D.B. Isoforms of RNA-Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5-Driven Autoimmunity and Multi-Organ Development. Immunity 2015, 43, 933–944.
- Kawahara, Y.; Nishikura, K. Extensive Adenosine-to-Inosine Editing Detected in Alu Repeats of Antisense RNAs Reveals Scarcity of Sense-Antisense Duplex Formation. FEBS Lett. 2006, 580, 2301–2305.
- Ramaswami, G.; Li, J.B. RADAR: A Rigorously Annotated Database of A-to-I RNA Editing. Nucleic Acids Res. 2014, 42, D109–D113.
- Ahmad, S.; Mu, X.; Yang, F.; Greenwald, E.; Park, J.W.; Jacob, E.; Zhang, C.-Z.; Hur, S. Breaching Self-Tolerance to Alu Duplex RNA Underlies MDA5-Mediated Inflammation. Cell 2018, 172, 797–810.e13.
- Chung, H.; Calis, J.J.A.; Wu, X.; Sun, T.; Yu, Y.; Sarbanes, S.L.; Dao Thi, V.L.; Shilvock, A.R.; Hoffmann, H.-H.; Rosenberg, B.R.; et al. Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell 2018, 172, 811–824.e14.
- Walkley, C.R.; Li, J.B. Rewriting the Transcriptome: Adenosine-to-Inosine RNA Editing by ADARs. Genome Biol. 2017, 18, 205.
- Samuel, C.E. Adenosine Deaminases Acting on RNA (ADARs) Are Both Antiviral and Proviral. Virology 2011, 411, 180–193.
- Yang, J.-H.; Nie, Y.; Zhao, Q.; Su, Y.; Pypaert, M.; Su, H.; Rabinovici, R. Intracellular Localization of Differentially Regulated RNA-Specific Adenosine Deaminase Isoforms in Inflammation. J. Biol. Chem. 2003, 278, 45833–45842.
- Zhang, T.; Yin, C.; Fedorov, A.; Qiao, L.; Bao, H.; Beknazarov, N.; Wang, S.; Gautam, A.; Williams, R.M.; Crawford, J.C.; et al. ADAR1 Masks the Cancer Immunotherapeutic Promise of ZBP1-Driven Necroptosis. Nature 2022, 606, 594–602.
- Jiao, H.; Wachsmuth, L.; Kumari, S.; Schwarzer, R.; Lin, J.; Eren, R.O.; Fisher, A.; Lane, R.; Young, G.R.; Kassiotis, G.; et al. Z-Nucleic-Acid Sensing Triggers ZBP1-Dependent Necroptosis and Inflammation. Nature 2020, 580, 391–395.
- Tang, Q.; Rigby, R.E.; Young, G.R.; Hvidt, A.K.; Davis, T.; Tan, T.K.; Bridgeman, A.; Townsend, A.R.; Kassiotis, G.; Rehwinkel, J. Adenosine-to-Inosine Editing of Endogenous Z-Form RNA by the Deaminase ADAR1 Prevents Spontaneous MAVS-Dependent Type I Interferon Responses. Immunity 2021, 54, 1961–1975.e5.
- De Reuver, R.; Dierick, E.; Wiernicki, B.; Staes, K.; Seys, L.; De Meester, E.; Muyldermans, T.; Botzki, A.; Lambrecht, B.N.; Van Nieuwerburgh, F.; et al. ADAR1 Interaction with Z-RNA Promotes Editing of Endogenous Double-Stranded RNA and Prevents MDA5-Dependent Immune Activation. Cell Rep. 2021, 36, 109500.
- Jiao, H.; Wachsmuth, L.; Wolf, S.; Lohmann, J.; Nagata, M.; Kaya, G.G.; Oikonomou, N.; Kondylis, V.; Rogg, M.; Diebold, M.; et al. ADAR1 Averts Fatal Type I Interferon Induction by ZBP1. Nature 2022, 607, 776–783.
- Zhang, T.; Yin, C.; Boyd, D.F.; Quarato, G.; Ingram, J.P.; Shubina, M.; Ragan, K.B.; Ishizuka, T.; Crawford, J.C.; Tummers, B.; et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell 2020, 180, 1115–1129.e13.
- Herbert, A. Z-DNA and Z-RNA in Human Disease. Commun. Biol. 2019, 2, 7.
- Herbert, A. To “Z” or Not to “Z”: Z-RNA, Self-Recognition, and the MDA5 Helicase. PLoS Genet. 2021, 17, e1009513.
- Nichols, P.J.; Bevers, S.; Henen, M.; Kieft, J.S.; Vicens, Q.; Vögeli, B. Recognition of Non-CpG Repeats in Alu and Ribosomal RNAs by the Z-RNA Binding Domain of ADAR1 Induces A-Z Junctions. Nat. Commun. 2021, 12, 793.
- Züst, R.; Cervantes-Barragan, L.; Habjan, M.; Maier, R.; Neuman, B.W.; Ziebuhr, J.; Szretter, K.J.; Baker, S.C.; Barchet, W.; Diamond, M.S.; et al. Ribose 2′-O-Methylation Provides a Molecular Signature for the Distinction of Self and Non-Self mRNA Dependent on the RNA Sensor Mda5. Nat. Immunol. 2011, 12, 137–143.
- Shulman, Z.; Stern-Ginossar, N. The RNA Modification N6-Methyladenosine as a Novel Regulator of the Immune System. Nat. Immunol. 2020, 21, 501–512.
- Mannion, N.M.; Greenwood, S.M.; Young, R.; Cox, S.; Brindle, J.; Read, D.; Nellåker, C.; Vesely, C.; Ponting, C.P.; McLaughlin, P.J.; et al. The RNA-Editing Enzyme ADAR1 Controls Innate Immune Responses to RNA. Cell Rep. 2014, 9, 1482–1494.
- Hartner, J.C.; Walkley, C.R.; Lu, J.; Orkin, S.H. ADAR1 Is Essential for the Maintenance of Hematopoiesis and Suppression of Interferon Signaling. Nat. Immunol. 2009, 10, 109–115.
- Liddicoat, B.J.; Hartner, J.C.; Piskol, R.; Ramaswami, G.; Chalk, A.M.; Kingsley, P.D.; Sankaran, V.G.; Wall, M.; Purton, L.E.; Seeburg, P.H.; et al. Adenosine-to-Inosine RNA Editing by ADAR1 Is Essential for Normal Murine Erythropoiesis. Exp. Hematol. 2016, 44, 947–963.
- Hartner, J.C.; Schmittwolf, C.; Kispert, A.; Müller, A.M.; Higuchi, M.; Seeburg, P.H. Liver Disintegration in the Mouse Embryo Caused by Deficiency in the RNA-Editing Enzyme ADAR1. J. Biol. Chem. 2004, 279, 4894–4902.
- Wang, Q.; Miyakoda, M.; Yang, W.; Khillan, J.; Stachura, D.L.; Weiss, M.J.; Nishikura, K. Stress-Induced Apoptosis Associated with Null Mutation of ADAR1 RNA Editing Deaminase Gene. J. Biol. Chem. 2004, 279, 4952–4961.
- Ward, S.V.; George, C.X.; Welch, M.J.; Liou, L.-Y.; Hahm, B.; Lewicki, H.; De La Torre, J.C.; Samuel, C.E.; Oldstone, M.B. RNA Editing Enzyme Adenosine Deaminase Is a Restriction Factor for Controlling Measles Virus Replication That Also Is Required for Embryogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 331–336.
- Rice, G.I.; Kasher, P.R.; Forte, G.M.A.; Mannion, N.M.; Greenwood, S.M.; Szynkiewicz, M.; Dickerson, J.E.; Bhaskar, S.S.; Zampini, M.; Briggs, T.A.; et al. Mutations in ADAR1 Cause Aicardi-Goutières Syndrome Associated with a Type I Interferon Signature. Nat. Genet. 2012, 44, 1243–1248.
- Rice, G.I.; Del Toro Duany, Y.; Jenkinson, E.M.; Forte, G.M.A.; Anderson, B.H.; Ariaudo, G.; Bader-Meunier, B.; Baildam, E.M.; Battini, R.; Beresford, M.W.; et al. Gain-of-Function Mutations in IFIH1 Cause a Spectrum of Human Disease Phenotypes Associated with Upregulated Type I Interferon Signaling. Nat. Genet. 2014, 46, 503–509.
- Liddicoat, B.J.; Piskol, R.; Chalk, A.M.; Ramaswami, G.; Higuchi, M.; Hartner, J.C.; Li, J.B.; Seeburg, P.H.; Walkley, C.R. RNA Editing by ADAR1 Prevents MDA5 Sensing of Endogenous dsRNA as Nonself. Science 2015, 349, 1115–1120.
- Heraud-Farlow, J.E.; Chalk, A.M.; Linder, S.E.; Li, Q.; Taylor, S.; White, J.M.; Pang, L.; Liddicoat, B.J.; Gupte, A.; Li, J.B.; et al. Protein Recoding by ADAR1-Mediated RNA Editing Is Not Essential for Normal Development and Homeostasis. Genome Biol. 2017, 18, 166.
- Herbert, A. Mendelian Disease Caused by Variants Affecting Recognition of Z-DNA and Z-RNA by the Zα Domain of the Double-Stranded RNA Editing Enzyme ADAR. Eur. J. Hum. Genet. 2020, 28, 114–117.
- Heale, B.S.E.; Keegan, L.P.; McGurk, L.; Michlewski, G.; Brindle, J.; Stanton, C.M.; Caceres, J.F.; O’Connell, M.A. Editing Independent Effects of ADARs on the miRNA/siRNA Pathways. EMBO J. 2009, 28, 3145–3156.
- Guo, X.; Liu, S.; Sheng, Y.; Zenati, M.; Billiar, T.; Herbert, A.; Wang, Q. ADAR1 Zα Domain P195A Mutation Activates the MDA5-Dependent RNA-Sensing Signaling Pathway in Brain without Decreasing Overall RNA Editing. Cell Rep. 2023, 42, 112733.
- Nakahama, T.; Kato, Y.; Shibuya, T.; Inoue, M.; Kim, J.I.; Vongpipatana, T.; Todo, H.; Xing, Y.; Kawahara, Y. Mutations in the Adenosine Deaminase ADAR1 That Prevent Endogenous Z-RNA Binding Induce Aicardi-Goutières-Syndrome-like Encephalopathy. Immunity 2021, 54, 1976–1988.e7.
- Li, Y.; Banerjee, S.; Goldstein, S.A.; Dong, B.; Gaughan, C.; Rath, S.; Donovan, J.; Korennykh, A.; Silverman, R.H.; Weiss, S.R. Ribonuclease L Mediates the Cell-Lethal Phenotype of Double-Stranded RNA Editing Enzyme ADAR1 Deficiency in a Human Cell Line. eLife 2017, 6, e25687.
- Lamers, M.M.; Van Den Hoogen, B.G.; Haagmans, B.L. ADAR1: “Editor-in-Chief” of Cytoplasmic Innate Immunity. Front. Immunol. 2019, 10, 1763.
- Shiromoto, Y.; Sakurai, M.; Minakuchi, M.; Ariyoshi, K.; Nishikura, K. ADAR1 RNA Editing Enzyme Regulates R-Loop Formation and Genome Stability at Telomeres in Cancer Cells. Nat. Commun. 2021, 12, 1654.
- Freund, E.C.; Sapiro, A.L.; Li, Q.; Linder, S.; Moresco, J.J.; Yates, J.R.; Li, J.B. Unbiased Identification of Trans Regulators of ADAR and A-to-I RNA Editing. Cell Rep. 2020, 31, 107656.
- Peng, X.; Xu, X.; Wang, Y.; Hawke, D.H.; Yu, S.; Han, L.; Zhou, Z.; Mojumdar, K.; Jeong, K.J.; Labrie, M.; et al. A-to-I RNA Editing Contributes to Proteomic Diversity in Cancer. Cancer Cell 2018, 33, 817–828.e7.
- William Roy, S.; Gilbert, W. The Evolution of Spliceosomal Introns: Patterns, Puzzles and Progress. Nat. Rev. Genet. 2006, 7, 211–221.
- Kadri, N.K.; Mapel, X.M.; Pausch, H. The Intronic Branch Point Sequence Is under Strong Evolutionary Constraint in the Bovine and Human Genome. Commun. Biol. 2021, 4, 1206.
- Beghini, A.; Ripamonti, C.B.; Peterlongo, P.; Roversi, G.; Cairoli, R.; Morra, E.; Larizza, L. RNA Hyperediting and Alternative Splicing of Hematopoietic Cell Phosphatase (PTPN6) Gene in Acute Myeloid Leukemia. Hum. Mol. Genet. 2000, 9, 2297–2304.
- Rueter, S.M.; Dawson, T.R.; Emeson, R.B. Regulation of Alternative Splicing by RNA Editing. Nature 1999, 399, 75–80.
- Hsiao, Y.-H.E.; Bahn, J.H.; Yang, Y.; Lin, X.; Tran, S.; Yang, E.-W.; Quinones-Valdez, G.; Xiao, X. RNA Editing in Nascent RNA Affects Pre-mRNA Splicing. Genome Res. 2018, 28, 812–823.
- Goncharov, A.O.; Shender, V.O.; Kuznetsova, K.G.; Kliuchnikova, A.A.; Moshkovskii, S.A. Interplay between A-to-I Editing and Splicing of RNA: A Potential Point of Application for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 5240.
- Solomon, O.; Oren, S.; Safran, M.; Deshet-Unger, N.; Akiva, P.; Jacob-Hirsch, J.; Cesarkas, K.; Kabesa, R.; Amariglio, N.; Unger, R.; et al. Global Regulation of Alternative Splicing by Adenosine Deaminase Acting on RNA (ADAR). RNA 2013, 19, 591–604.
- Kapoor, U.; Licht, K.; Amman, F.; Jakobi, T.; Martin, D.; Dieterich, C.; Jantsch, M.F. ADAR-Deficiency Perturbs the Global Splicing Landscape in Mouse Tissues. Genome Res. 2020, 30, 1107–1118.
- Jain, M.; Weber, A.; Maly, K.; Manjaly, G.; Deek, J.; Tsvyetkova, O.; Stulić, M.; Toca-Herrera, J.L.; Jantsch, M.F. A-to-I RNA Editing of Filamin A Regulates Cellular Adhesion, Migration and Mechanical Properties. FEBS J. 2022, 289, 4580–4601.
- Shapiro, I.M.; Cheng, A.W.; Flytzanis, N.C.; Balsamo, M.; Condeelis, J.S.; Oktay, M.H.; Burge, C.B.; Gertler, F.B. An EMT-Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype. PLoS Genet. 2011, 7, e1002218.
- Anczuków, O.; Akerman, M.; Cléry, A.; Wu, J.; Shen, C.; Shirole, N.H.; Raimer, A.; Sun, S.; Jensen, M.A.; Hua, Y.; et al. SRSF1-Regulated Alternative Splicing in Breast Cancer. Mol. Cell 2015, 60, 105–117.
- Quentmeier, H.; Pommerenke, C.; Bernhart, S.H.; Dirks, W.G.; Hauer, V.; Hoffmann, S.; Nagel, S.; Siebert, R.; Uphoff, C.C.; Zaborski, M.; et al. RBFOX2 and Alternative Splicing in B-Cell Lymphoma. Blood Cancer J. 2018, 8, 77.
- Wu, D.; Zang, Y.-Y.; Shi, Y.-Y.; Ye, C.; Cai, W.-M.; Tang, X.-H.; Zhao, L.; Liu, Y.; Gan, Z.; Chen, G.; et al. Distant Coupling between RNA Editing and Alternative Splicing of the Osmosensitive Cation Channel Tmem63b. J. Biol. Chem. 2020, 295, 18199–18212.
- Huang, H.; Kapeli, K.; Jin, W.; Wong, Y.P.; Arumugam, T.V.; Koh, J.H.; Srimasorn, S.; Mallilankaraman, K.; Chua, J.J.E.; Yeo, G.W.; et al. Tissue-Selective Restriction of RNA Editing of CaV1.3 by Splicing Factor SRSF9. Nucleic Acids Res. 2018, 46, 7323–7338.
- Shanmugam, R.; Zhang, F.; Srinivasan, H.; Charles Richard, J.L.; Liu, K.I.; Zhang, X.; Woo, C.W.A.; Chua, Z.H.M.; Buschdorf, J.P.; Meaney, M.J.; et al. SRSF9 Selectively Represses ADAR2-Mediated Editing of Brain-Specific Sites in Primates. Nucleic Acids Res. 2018, 46, 7379–7395.
- Kim, V.N. MicroRNA Biogenesis: Coordinated Cropping and Dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385.
- Kawahara, Y.; Megraw, M.; Kreider, E.; Iizasa, H.; Valente, L.; Hatzigeorgiou, A.G.; Nishikura, K. Frequency and Fate of microRNA Editing in Human Brain. Nucleic Acids Res. 2008, 36, 5270–5280.
- Blow, M.; Grocock, R.; Van Dongen, S.; Enright, A.; Dicks, E.; Futreal, P.A.; Wooster, R.; Stratton, M. RNA Editing of Human microRNAs. Genome Biol. 2006, 7, R27.
- Yu, Z.; Luo, R.; Li, Y.; Li, X.; Yang, Z.; Peng, J.; Huang, K. ADAR1 Inhibits Adipogenesis and Obesity by Interacting with Dicer to Promote the Maturation of miR-155-5P. J. Cell Sci. 2022, 135, jcs259333.
- Yang, W.; Chendrimada, T.P.; Wang, Q.; Higuchi, M.; Seeburg, P.H.; Shiekhattar, R.; Nishikura, K. Modulation of microRNA Processing and Expression through RNA Editing by ADAR Deaminases. Nat. Struct. Mol. Biol. 2006, 13, 13–21.
- Shoshan, E.; Mobley, A.K.; Braeuer, R.R.; Kamiya, T.; Huang, L.; Vasquez, M.E.; Salameh, A.; Lee, H.J.; Kim, S.J.; Ivan, C.; et al. Reduced Adenosine-to-Inosine miR-455-5p Editing Promotes Melanoma Growth and Metastasis. Nat. Cell Biol. 2015, 17, 311–321.
- Kawahara, Y.; Zinshteyn, B.; Chendrimada, T.P.; Shiekhattar, R.; Nishikura, K. RNA Editing of the microRNA-151 Precursor Blocks Cleavage by the Dicer-TRBP Complex. EMBO Rep. 2007, 8, 763–769.
- Iizasa, H.; Wulff, B.-E.; Alla, N.R.; Maragkakis, M.; Megraw, M.; Hatzigeorgiou, A.; Iwakiri, D.; Takada, K.; Wiedmer, A.; Showe, L.; et al. Editing of Epstein-Barr Virus-Encoded BART6 microRNAs Controls Their Dicer Targeting and Consequently Affects Viral Latency. J. Biol. Chem. 2010, 285, 33358–33370.
- Kawahara, Y.; Zinshteyn, B.; Sethupathy, P.; Iizasa, H.; Hatzigeorgiou, A.G.; Nishikura, K. Redirection of Silencing Targets by Adenosine-to-Inosine Editing of miRNAs. Science 2007, 315, 1137–1140.
- Ekdahl, Y.; Farahani, H.S.; Behm, M.; Lagergren, J.; Öhman, M. A-to-I Editing of microRNAs in the Mammalian Brain Increases during Development. Genome Res. 2012, 22, 1477–1487.
- Polson, A.G.; Bass, B.L. Preferential Selection of Adenosines for Modification by Double-Stranded RNA Adenosine Deaminase. EMBO J. 1994, 13, 5701–5711.
- Lehmann, K.A.; Bass, B.L. Double-Stranded RNA Adenosine Deaminases ADAR1 and ADAR2 Have Overlapping Specificities. Biochemistry 2000, 39, 12875–12884.
- Eggington, J.M.; Greene, T.; Bass, B.L. Predicting Sites of ADAR Editing in Double-Stranded RNA. Nat. Commun. 2011, 2, 319.
- Zhang, R.; Deng, P.; Jacobson, D.; Li, J.B. Evolutionary Analysis Reveals Regulatory and Functional Landscape of Coding and Non-Coding RNA Editing. PLoS Genet. 2017, 13, e1006563.
- Rieder, L.E.; Staber, C.J.; Hoopengardner, B.; Reenan, R.A. Tertiary Structural Elements Determine the Extent and Specificity of Messenger RNA Editing. Nat. Commun. 2013, 4, 2232.
- Daniel, C.; Venø, M.T.; Ekdahl, Y.; Kjems, J.; Öhman, M. A Distant Cis Acting Intronic Element Induces Site-Selective RNA Editing. Nucleic Acids Res. 2012, 40, 9876–9886.
- Kim, J.I.; Nakahama, T.; Yamasaki, R.; Costa Cruz, P.H.; Vongpipatana, T.; Inoue, M.; Kanou, N.; Xing, Y.; Todo, H.; Shibuya, T.; et al. RNA Editing at a Limited Number of Sites Is Sufficient to Prevent MDA5 Activation in the Mouse Brain. PLoS Genet. 2021, 17, e1009516.
- Sun, T.; Li, Q.; Geisinger, J.M.; Hu, S.-B.; Fan, B.; Su, S.; Tsui, W.; Guo, H.; Ma, J.; Li, J.B. A Small Subset of Cytosolic dsRNAs Must Be Edited by ADAR1 to Evade MDA5-Mediated Autoimmunity. Genetics 2022, preprint.
- Yi-Brunozzi, H.Y.; Easterwood, L.M.; Kamilar, G.M.; Beal, P.A. Synthetic Substrate Analogs for the RNA-Editing Adenosine Deaminase ADAR-2. Nucleic Acids Res. 1999, 27, 2912–2917.
- Cuddleston, W.H.; Fan, X.; Sloofman, L.; Liang, L.; Mossotto, E.; Moore, K.; Zipkowitz, S.; Wang, M.; Zhang, B.; Wang, J.; et al. Spatiotemporal and Genetic Regulation of A-to-I Editing throughout Human Brain Development. Cell Rep. 2022, 41, 111585.
- Wang, Y.; Xu, X.; Yu, S.; Jeong, K.J.; Zhou, Z.; Han, L.; Tsang, Y.H.; Li, J.; Chen, H.; Mangala, L.S.; et al. Systematic Characterization of A-to-I RNA Editing Hotspots in microRNAs across Human Cancers. Genome Res. 2017, 27, 1112–1125.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




