Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mustapha Jouiad | -- | 5611 | 2024-01-03 17:07:45 | | | |
| 2 | Rita Xu | -4 word(s) | 5607 | 2024-01-04 03:21:55 | | | | |
| 3 | Rita Xu | Meta information modification | 5607 | 2024-01-05 07:47:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nassereddine, Y.; Benyoussef, M.; Asbani, B.; El Marssi, M.; Jouiad, M. Photocatalytic Proprieties of BiFeO3-Based Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/53382 (accessed on 07 February 2026).
Nassereddine Y, Benyoussef M, Asbani B, El Marssi M, Jouiad M. Photocatalytic Proprieties of BiFeO3-Based Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/53382. Accessed February 07, 2026.
Nassereddine, Yassine, Manal Benyoussef, Bouchra Asbani, Mimoun El Marssi, Mustapha Jouiad. "Photocatalytic Proprieties of BiFeO3-Based Materials" Encyclopedia, https://encyclopedia.pub/entry/53382 (accessed February 07, 2026).
Nassereddine, Y., Benyoussef, M., Asbani, B., El Marssi, M., & Jouiad, M. (2024, January 03). Photocatalytic Proprieties of BiFeO3-Based Materials. In Encyclopedia. https://encyclopedia.pub/entry/53382
Nassereddine, Yassine, et al. "Photocatalytic Proprieties of BiFeO3-Based Materials." Encyclopedia. Web. 03 January, 2024.
Copy Citation
Owing to their remarkable success in photocatalytic applications, multiferroic BiFeO3 and its derivatives have gained a highly promising position as electrode materials for future developments of efficient catalysts. In addition to their appropriate band gaps, these materials exhibit inherent intrinsic polarizations enabling efficient charge carrier separation and their high mobility without the need for additional co-catalysts.
BiFeO3-based materials
substitutions
doping
heterostructures
1. Introduction
The global energy crisis and climate change are driven by the continuously growing world population and industrialization, which are heavily weighing on the future of human well-being and safety [1]. Nowadays, although fossil fuels represent more than 80% of the world’s energy consumption [2], an increased consciousness among policymakers and the population is emerging for taking urgent measures and actions to cope with CO2 footprints. Alternative clean energy sources hold strong potential to overcome environmental issues by limiting the use of fossil fuels. Hydrogen (H2) is a promising energy carrier and green fuel source capable of replacing the energy generated from non-renewable resources such as oil, coal, and natural gas [3][4][5][6][7][8][9][10][11][12][13]. H2 is produced using a variety of methods, including water thermolysis, electrolysis, methane-steam reforming, biofuel reforming, gasification, plasma arc decomposition, and the thermochemical conversion of biomass [14][15][16]. Recently, water splitting using solar irradiation has emerged as a promising process for H2 production, attracting considerable interest in the scientific and industrial communities. Solar-induced water splitting (WS) techniques that are classified include photocatalysis (PC), photoelectrochemical (PEC), and photovoltaic-photoelectrochemical (PV-PEC) systems [17][18][19]. PC systems correspond to a simple and low-cost process in which photocatalyst particles are dispersed in water for H2 production under light irradiation. Nevertheless, PC systems exhibit very low solar-to-H2 efficiency (STH), requiring highly selective materials for separating the produced H2 and O2 gases [20][21][22][23]. PV-PEC systems are very effective for WS owing to their high overall efficiency [24]. However, their high cost and the need for advanced technical expertise constitute two major drawbacks [25]. PEC systems are the most promising techniques for producing H2 in an easy, affordable, and sustainable way [26][27][28]. A typical PEC WS system is composed of semiconducting photoelectrodes, an electrolyte, a counter electrode, and a light source [29]. It features the combination of solar energy and water electrolysis in a single reactor with an STH efficiency of up to 12.4%. In addition, a PEC system does not require gas separation since H2 and O2 are already produced in two spatially distinct compartments [30]. It is worth noting that three essential criteria must be established for an effective and sustainable PEC. First, the semiconductor electrode material must exhibit a suitable band gap (~1.8 eV) [31], which is essential for achieving good band edge alignment concerning water redox potentials. Unfortunately, the best known good WS photocatalyst has a wide band gap energy that restricts light absorption, thus leading to weak photocatalytic performance [32][33]. Second, the semiconductor must be photo-corrosion resistant during its exposure to aqueous solutions and irradiation to avoid the formation of defects and the alteration of its photocatalytic properties while in use, which can lead to lower efficiency and a shorter lifespan [34][35]. Third, the charge transfer and separation in the semiconductor must be favorable and not hindered by the semiconductor’s surface overpotential. Recall that free electrons generated in the conduction band (CB) of the semiconductor (photoanode) will travel to the photocathode to drive the water reduction and hydrogen evolution reaction (HER). Holes generated in the valence band (VB) of the photoanodes will induce an oxygen evolution reaction (OER). Therefore, the ease of charge transfer and the separation of electron holes in the semiconductor material are crucial for improving the overall efficiency of the PEC WS process [36]. To date, several metal oxides have been used as semiconductors in PEC cells for WS, such as TiO2, ZnO, α-Fe2O3, and WO3 [37][38][39][40]. TiO2 is among the most widely used materials owing to its advantageous properties, including high stability and wide band gap (~3.2 eV) [41]. However, its high electron–hole recombination rate, high cost, and low visible light absorption limit its use in PEC-WS cells [42][43].
Recently, oxide perovskite materials (PMs) have attracted great attention for their use in PEC WS owing to their high photocatalytic properties, broadband absorption, low cost, facile synthesis, and well-controlled composition and morphology [44][45]. Moreover, their ferroelectric properties could promote their photocatalytic activity [46][47] as the intrinsic polarization in ferroelectric materials significantly contributes to reducing losses due to electron–hole recombination and back reactions in the system, which will increase the STH efficiency [48]. Additionally, the use of ferroelectric materials with a high dielectric constant can lead to a further increase in the charge separation efficiency, notably enhancing the photocatalytic activity [49]. However, more research is needed to fully understand the relationship between ferroelectric properties and photocatalytic activity.
Nowadays, the increasing global need for water resources coupled with their dwindling availability has emerged as a significant global concern [50]. While recycling wastewater has been suggested as a solution to address water scarcity, the presence of harmful organic substances like pesticides, phenols, and organic dyes in wastewater has raised multiple concerns regarding its environmental impact [51]. Practical solutions and strategies have been adopted to achieve more sustainable water resources. Since solar energy is one of the most accessible renewable energy sources, it makes sense to use it in combating waste degradation by synthesizing materials that can be put to direct use [52]. The development of an innovative technology, known as waste degradation through photocatalysis, is currently underway to address the issue of harmful pollutants. This process involves harnessing the power of light to initiate a chemical reaction in a photocatalyst material resulting in the conversion of these pollutants into less toxic or non-toxic substances. When exposed to light, electron–hole pairs are generated by the photocatalyst, which can serve as potent oxidizing or reducing agents. These electron–hole pairs can subsequently undergo reactions with water or oxygen molecules, leading to the creation of highly reactive oxygen species (ROS) such as hydroxyl radicals (•OH), superoxide radicals (•O2−), and hydrogen peroxide (H2O2) [53]. These ROS can be employed to facilitate the breakdown of organic and inorganic pollutants in waste materials, ultimately transforming them into simpler and less harmful compounds. Nonetheless, a recent development has sparked significant interest in a novel set of materials categorized within the perovskite structure class, particularly for their potential applications in photodegradation [54].
For instance, PbTiO3 is regarded as a potential material for photocatalytic applications due to its promising properties since its internal electric field could ensure an effective charge separation and prevent electron–hole recombination [55]. However, lead is a toxic element with known environmental effects [56]. In this sense, lead-free BiFeO3 (BFO) could be considered a potential multiferroic material with a high spontaneous polarization value of P~90 μC·cm−2 [57][58]. BFO has been widely used in various applications, including organics degradation, air purification, and H2 production (i.e., as a photoanode) [59][60][61]. In addition, BFO exhibits a high absorption coefficient in the visible region and relative stability under photocatalytic conditions [62]. Yet, the band alignment of BFO needs to be tuned to the water redox potentials to increase the STH [63]. To enhance the photocatalytic activity of BFO, several strategies were employed, such as doping/co-doping, size control, surface modification, co-catalysts, and heterostructures [64][65][66][67][68].
2. BFO-Based Materials Photocatalytic Applications
2.1. Degradation of Organic Pollutants
Recently, TiO2 and ZnO have been extensively exploited for their various photocatalytic applications. However, their ability to absorb in the UV region (which comprises only 10% of the total sun radiation) and the challenge of their removal after treatment have hindered their usage as photocatalysts [69]. Therefore, developing a suitable catalyst working in the visible region is a currently pressing need. BFO, a room-temperature multiferroic material, constitutes an attractive candidate owing to its activity in the visible region and its magnetic behavior favoring an easy removal of the photocatalyst after treatment. In addition, its photocatalytic activity under visible light becomes prominent due to its narrow band gap of 2.1–2.7 eV, which is particularly important because visible light energy occupies about 48% of the total solar energy [70].
A large majority of earth metals, such as Gd, La, Nd, Dy, Er, and Sm, have been introduced as dopants into BFO nanostructures to investigate their photocatalytic properties [71][72][73][74][75]. This route has proved to be relatively more successful due to the 4f electron configurations of rare earth metals that facilitate the abruption of photogenerated electron–hole pairs. The band gap of some bismuth photocatalysts has been shown to decrease with rare earth element doping, which might increase the photocatalytic activities. It should be noted that substituting Bi3+ cations with rare earth ions that have smaller ionic radii than Bi3+ (1.03 Å), such as Dy3+ (0.912 Å), Gd3+ (0.938 Å), or Sm3+ (0.958 Å), is requisite in order to cause significant structural distortions in the BFO lattice for improved photocatalytic properties. The photocatalytic activities of Gd-doped (10%) BFO were found to considerably enhance its photocatalytic performance under simulated solar irradiation. Findings show that 10% Gd BFO photocatalyst degradation rates reach 80% and 79% for ciprofloxacin and levofloxacin, respectively [76]. Similar results reported the degradation activity of Gd-doped BFO photocatalysts for decomposing methylene blue and rhodamine B under visible light irradiation. It was found that Gd-doped (10%) BFO photocatalysts exhibit much higher photocatalytic activity than pure BFO. Gd-doped BFO decomposes 94% of methylene blue after 240 min and 94% of rhodamine B after 120 min [71]. The distinctive photocatalytic efficiency of Gd-doped (10%) BFO can be largely attributed to its excellent morphology and good crystallinity that facilitate improved light absorption and the effective separation of photogenerated charge carriers. These results illustrate the excellent photocatalytic activity of Gd-doped (10%) BFO, which can be employed in various applications related to environmental remediation. Considerably enhanced photocatalytic activity was also obtained by adding La doping to BFO nanoparticles [72]. In this case, approximately 87% higher degradation of the methylene blue was observed after 70 min under visible-light illumination. La-doped BFO presents better photocatalytic activity compared to undoped BFO nanoparticles, which could be ascribed to the increase in the recombination rate of holes and electrons in doped samples or to band gap variations. In a recent study, Dy-doped BFO was found to induce a high photocatalytic degradation of methylene blue (92%) achieved after 240 min under visible-light irradiation [73], which could be attributed to the reduced band gap energy and ferroelectric properties. Increasing Dy concentrations up to 15% mediated a charge transfer process through band bending in this composition that was associated with enhanced electrical domains. Likewise, Er-doped BFO was used as a photocatalyst for the photocatalytic removal of tetracycline hydrochloride (TC) under visible light [74]. The photocatalytic activities of Er-doped BFO for TC removal were much higher than those for BFO, where Er-3%-doped BFO samples achieved the highest photocatalytic TC-degradation efficiency of 75.8% after 180 min (~2.8 times higher than that of the BFO samples). The Er-3%-doped BFO photoelectrode manifested higher photocurrent intensity compared with BFO photoelectrodes, implying a much more efficient charge separation and a transfer with a longer charge lifespan of the photoinduced carriers, thus improving the photocatalytic performance. Notably, Er is a popular rare earth element for doping semiconductor photocatalysts owing to its unique transitions of Er intra-f electrons that to the sensitization of the photocatalyst to visible light. Chen et al. reported an enhancement in photocatalytic activity of Nd-doped BFO with the increase in Nd-doping concentrations when x = 0.2 (59% after 120 min) [75]. However, the photocatalytic activity was found to decrease with the further increase of the Nd-dopant concentration. The maximum photocatalytic activity of x = 0.2 was ascribed to the anomalously high dielectric constant at the morphotropic phase boundary, enlarging the width of the space-charge region. This phenomenon results from the increase in the defect sites in the lattice, which enhances the charge separation and reduces electron/hole–pair recombination rates. Nonetheless, higher doping concentrations produce more defect sites that convert to recombination centers. Another study shows that the photocatalytic activity of Sm-doped BFO was significantly affected by the Sm-doping content [77]. Compared to pure BFO, the Sm-doped BFO samples exhibited much higher photocatalytic activity, which was attributed to the enhanced visible-light absorption and the efficient separation of photogenerated electrons and holes derived from Sm-dopant trapping level. Moreover, the visible-light photodegradation of organic dyes using BFO doped with Ba, Mn, Co and Pb metal ions was studied. Soltani and Lee reported a complete photocatalytic degradation of toluene and benzene with 91% and 81% reductions after 50 min under visible-light irradiation for Ba-doped BFO [78]. The BFO nanoparticles doped with Ba exhibited a low band gap energy, high specific surface area, and high ferromagnetic properties, all contributing to the improvement of the photocatalytic performance. The findings showed that Ba-doped BFO exhibits a decreasing band gap energy with reduced O2 vacancies, which is related to the lattice distortion of the Ba-doped BFO nanoparticles. In fact, the growth of the particles is restricted, leading to an increasing specific surface area and a significant improvement of the photocatalytic activity.
Photocatalysis has been reported for the degradation of AR-85 under visible-light irradiation using Mn-doped (10%) BFO photocatalysts [69]. The photocatalytic activity was demonstrated at 100% degradation of the dye in only 50 min after light exposure, whereas the degradation time required for the undoped bismuth ferrite was much longer. Mn-doped (10%) BFO led to a decrease in particle size, while the band gap gradually decreased from 2.2 eV to 1.97 eV with an increasing Mn content. The greater photocatalytic activity in Mn-doped BFO compared with pristine BFO is associated with the efficient separation and migration of photogenerated charge carriers and the decreased recombination probability of electron/hole pairs derived from the Mn ion doping. In another study, the effect of co-doping on the B-site of BFO was investigated [79]. A remarkable photocatalytic performance was observed for co-doped BFO with a degradation rate of 93.79% after 2 h under light exposure. The results indicate that co-doping promoted the effective charge separation of the catalyst to enhance photocatalytic behavior, which was attributed to the reduction in the crystal size and the creation of O2 vacancies in the system due to co-doping. Hence, co-doping improves the position of BFO as a promising candidate for environmental remediation applications. Jaffari et al. reported the effect of a Pd-doped BFO catalyst for the degradation of malachite green dye and phenol from waste water [80]. Particularly, the 2 wt% Pd-BFO exhibited the best photoactivity (95.7% degradation) compared with pure BFO (72.3% degradation). The enhanced photoactivity could be credited to the appropriate Pd contents that enhanced the e−-trapping capacity, which was helpful in the generation and transmission of e−/h+ pairs. The charge carrier generation and separation/transfer are key factors in the photocatalytic process. Furthermore, the separation/transfer of e−/h+ pairs using Pd-doped BFO photocatalysts were investigated under the on/off circulation of 105 W of visible light using transient photocurrent measurements. Pd-BFO possessed the highest current intensity of 2.59 μA, which was 1.6 times higher than that of pure BFO. These results explicitly revealed that the loaded metallic Pd on the BFO surface would highly expedite the generation and separation/transfer of charge carriers, which validated the improved photocatalytic ability of Pd-doped BFO to degrade organic pollutants.
Meanwhile, the co-substitution of the BFO structure in both the A- and B-sites with (La, Se), (Ce, Ni), (Nd, Ni), and (Ba, Ca) have been used to improve the photocatalytic activity and visible light response of the material compared with the bulk BFO material. The substitution of elements at the A-site can help suppress bismuth volatilization, while the substitution of transition metals at the B-site can reduce the Fe valence fluctuations. These changes in the elemental composition and oxidation state can result in improved photocatalytic activity, greater stability, and longer lifespan for BFO photocatalysts. These doping strategies have been widely investigated, offering great potential for developing even more efficient BFO photocatalysts. In this context, the co-substitution of La in place of Bi as well as Se in place of Fe was studied to control the recombination and enhance the number of delocalized electrons [81]. The photodegradation activity of La- and Se-co-doped BFO was investigated under visible-light irradiation using Congo red as a model dye in an aqueous solution. The developed material exhibited excellent photocatalytic activities for model dye, catalyzing more than 90% of the dye in the first 30 min of exposure to visible light. Higher dye degradation activities for La- and Se-co-doped BFO can be attributed to the complete phase transition from rhombohedral to orthorhombic, which provides a favorable band gap (1.77 eV) and binding energies for the enhanced catalysis of dye species. The lower band gap provided easy electron availability upon exposure to incident radiation, while the sheet-type morphology ensured larger contact between the surface of the catalyst and the adsorbing species, resulting in an enhanced synergistic response and higher catalytic activities.
It was reported that the co-substitution of Ce and Ni enormously impacts the photocatalytic efficiency of undoped BFO [82], which is maximized with the increase of co-doping levels. The best photocatalytic methylene blue and rhodamine B degradation efficiency were estimated at 93.29% and 96.05% after 90 min for Ce- and Ni-co-substitution BFO. The results suggest that the photocatalyst activity depends on the quantity of photon energy absorbed by the catalyst and the extent of the pollutant’s adsorption on the photocatalyst’s surface. The bandgap of Ce- and Ni-co-doped BFO is smaller than pristine bismuth ferrite, which helps absorb more energy than pure BFO. Meanwhile, the adsorption of pollutants on the photocatalyst surface is high for Ce–Ni-co-substitution BFO due to its larger porosity and raised surface area, which reveals a remarkable photocatalytic activity. The bandgap of undoped bismuth ferrite would be effectively reduced from 2.10 eV to 1.85 eV, which provides large photocatalytic efficiency under irradiation using various wavelengths of light. Depending on these experimental findings, the enhanced photocatalytic efficiency of Ce–Ni-co-substitution BFO would be ascribed to the raised optical absorption, the successful separation, and then the migration of photo-produced charge carriers with the reduced recombination feasibility of electron–hole pair findings from the co-substitution influence [82].
Photocatalytic activities of (Nd, Ni)-co-doped BFO nanoparticles are determined through the degradation of methylene blue dye under visible light and H2O2 [83]. After 90 min reaction time, the degradation of MB is improved for (Nd, Ni)-co-doped BFO (93% degradation). A similar result was found for (Ba and Ca)-co-doped BFO [72]. Its photodegradation efficiency was found to be 93% after 90 min performed in the conditions of pH value 2 and with the addition of 0.5 mL H2O2. Basically, the efficiency of the photocatalysts depends on the nature of doping, which affects factors like the crystallite size, morphology, surface area, band gap (Figure 1a), and photo-induced electron–hole separation efficiency of the catalyst. Figure 1b summarizes the degradation time and efficiency of the BFO-doped elements.
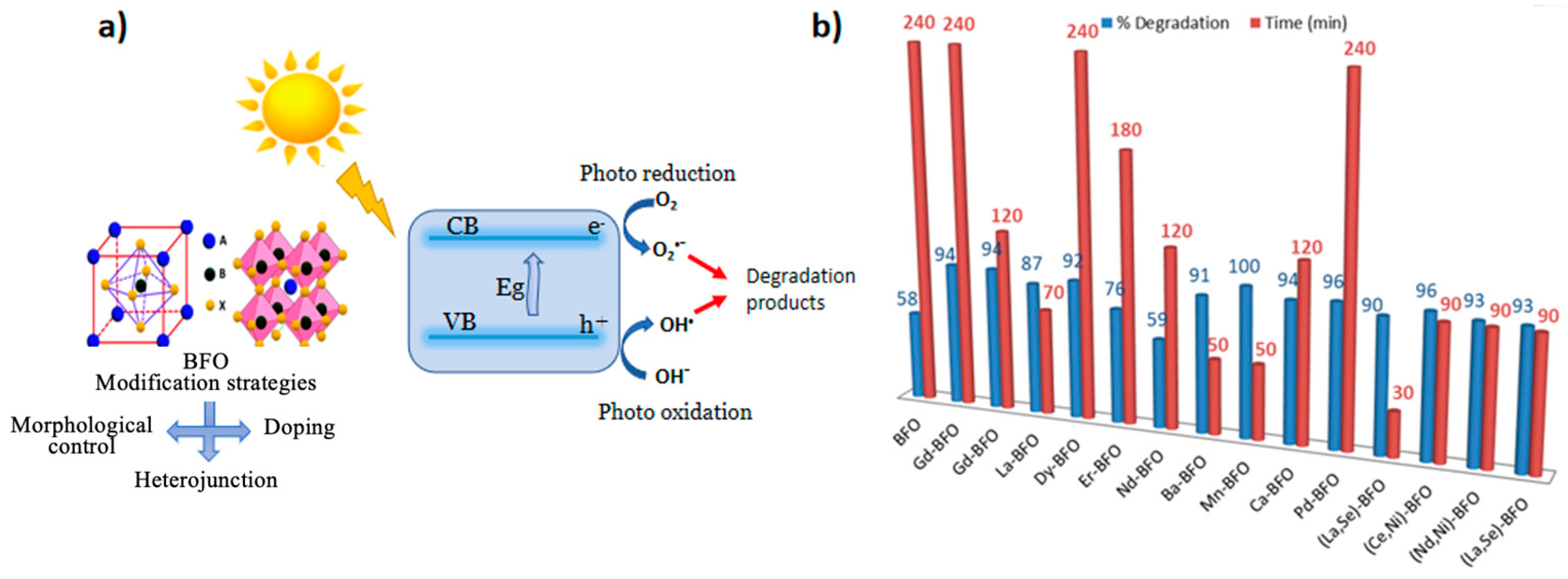
Figure 1. (a) Photocatalytic degradation of dye using doped BFO catalyst and (b) its degradation time and degradation efficiency.
The crucial process of removing harmful pollutants from the environment is facilitated by the photodegradation of organic dyes. A vital role in this process is played by BFO-based heterostructures due to their unique properties, such as high photocatalytic activity and excellent stability. Visible light is effectively absorbed through these heterostructures and the electrons generated during the process react with the dye molecules, leading to their degradation. Furthermore, the synthesis of BFO-based heterostructures can be easily accomplished using simple methods, making them a cost-effective and sustainable solution for environmental remediation. The photodegradation of organic dyes using BFO-based heterojunctions has been studied extensively in recent years; various organic dyes have been subjected to this process, including methylene blue or orange, rhodamine B, and Congo red. Several factors should be considered when selecting an organic dye for photodegradation with a BFO heterostructure. These include the properties of the dye itself, such as its absorption spectrum and chemical stability, as well as the specific conditions of the photodegradation process, such as the light source and the presence of any co-catalysts. Ultimately, the choice of dye will depend on balancing these factors to achieve optimal performance and efficiency in the photodegradation process.
Nanocomposites of BiFeO3-GdFeO3 (BFO-GFO) heterostructures were synthesized for the first time utilizing the sol–gel technique and investigated for dye degradation [84]. According to Tauc plots, the band gap energies of BiFeO3-GdFeO3 were found to be 1.8 eV, while 2.0 eV and 2.3 eV were determined for BFO and GdFeO3, respectively [84]. The findings showed that when methylene blue was exposed to pure GdFeO3 for 9 h, its degradation was limited, indicating that GdFeO3 has a restricted photocatalytic activity under visible light. On the one hand, the decreased photodegradation efficiency of GdFeO3 was linked to its high band gap potential, inadequate absorption in the UV light range, and less-than-optimal photoelectric conversion. On the other hand, BFO was able to break down as much as 76% of the methylene blue after 9 h of irradiation. Remarkably, a high photodegradation efficiency of 98% for methylene blue was achieved for the BiFeO3-GdFeO3 composite after 9 h of irradiation. The improved photocatalytic performance of the BiFeO3-GdFeO3 composite could potentially be attributed to the formation of a heterojunction, hypothesized to induce the generation of photogenerated electron–hole pairs, resulting in an elevation in photocatalytic activity. Furthermore, the lowering of band gap values has been instrumental in enhancing its photodegradation efficiency by allowing improved visible-light absorption [84]. Xu et al. reported the rational design of Ag/BiFeO3 fibrous heterostructures using an electrospinning process, as illustrated in Figure 2a, with the aim of coupling piezoelectric and plasmonic effects (Figure 2b) to modulate the separation and migration of photogenerated charge carriers. It was demonstrated through PFM testing that the piezoelectric feature of 29.3 pm at −7.53 V was exhibited using the Ag2/BFO hybrid. Furthermore, when the ultrasound was introduced, the photocatalytic degradation rate of MO and MB over Ag2/BFO reached 96% and 95% within 100 min, respectively. The significant improvement in the photocatalytic activity was attributed to the synergistic effect of the piezoelectricity and LSPR, wherein the piezoelectric field within the BFO was found to further promote the directional migration and separation of photogenerated charge carriers induced through the LSPR effect of Ag NPs on the surface [85]. Through a combination of hydrothermal and post-impregnation techniques, the CuO/BFO composite with a p–n heterojunction structure was fabricated. In the context of the photocatalytic evaluation of methylorange degradation under visible light, it was observed that an optimal photocatalytic degradation efficiency of up to 50% was attained when the loading content of CuO was set at 15%, surpassing that of pure BFO and CuO by more than threefold. Furthermore, following five cycles of photodegradation of methyl orange, no significant loss of photocatalytic activity in CuO/BFO was observed, confirming its stability and long-term reusability [86]. In another piece of research, a BFO/MoS2 nanocomposite was successfully synthesized using a combination of the sol–gel procedure for BFO and the hydrothermal method for MoS2 [87]. The as-prepared BFO/MoS2 nanocomposite demonstrated a remarkable performance in the visible-light photo-decolorization of RhB. The photocatalytic experiments indicated that an impressive removal rate of approximately 89% of rhodamine B is achieved through the nanocomposite (50% BFO/50% MoS2 Wt) within 200 min. under visible-light irradiation. This exceptional photocatalytic activity can be ascribed to the highly efficient separation of photogenerated electron–hole pairs. Furthermore, the high activity is maintained by the BFO/MoS2 nanocomposite, even after undergoing three photoreaction cycles, and can be easily separated and collected using an external magnetic field [87]. In another study, a facile ultrasonic/hydrothermal route was employed to synthesize the BFO/BVO p–n junction, resulting in a significant improvement in the performance of n-type BVO and p-type BFO for the photocatalytic degradation of tetracycline (TC) and the photoelectrochemical (PEC) water splitting process [88]. Notably, the photodegradation of TC using BVO and BFO was found highly dependent on the pH level, while that using BFO/BVO exhibited pH-independent behavior. The introduction of BFO/BVO p–n junction nanostructures led to a significant improvement in TC photocatalytic degradation, achieving removal rates of 84% and 95% at pH 6.7 and 9.5, respectively, as compared with 31% and 22% with BFO alone. Moreover, an increase from 37% with BVO to 84% with the BFO/BVO p–n junction at pH = 2.5 was demonstrated [88].
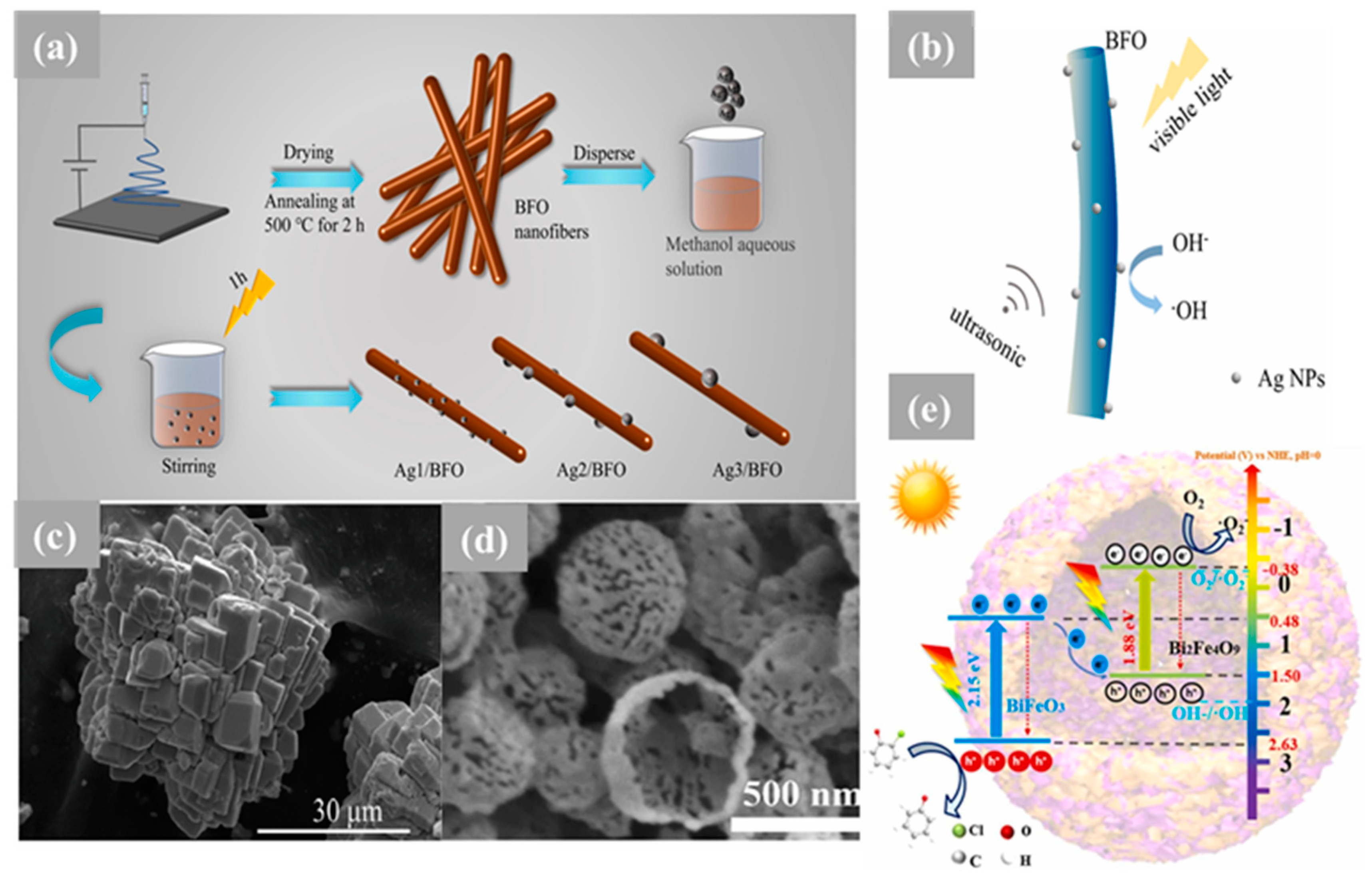
Figure 2. (a) Illustration of the synthesis procedure of the pure BFO nanofibers and Ag/BFO composites. (b) Schematic illustration of the synergy of plasmonic and piezotronic effects. (c) SEM image of BiFeO3-Ns. (d) SEM image of BiFeO3/Bi2Fe4O9 nanospheres. (e) S-scheme of the BiFeO3/Bi2Fe4O9 heterojunction hollow nanospheres with an Fe–O channel for enhancing charge separation to achieve high-efficiency photocatalytic o-chlorophenol degradation.
To enhance visible-light adsorption and photocatalytic activity, a modified BFO/rGO nanocomposite was fabricated via sol–gel process by controlling heat treatment parameters and rGO% [89]. When compared with BFO, BGO exhibits a narrower band gap energy of 1.8 eV, a lower rate of charge carrier recombination, and stronger magnetic characteristics. The highest photocatalytic activity at the optimum concentration was demonstrated by BGO with 1 wt% rGO in the range of photocatalysts prepared (1, 5, 10, and 20 wt% rGO), leading to MB degradation under visible light of up to 98% after 5 h [89]. Wang et al. reported a sonocatalytic removal of tetracycline using an S-scheme Cu2O/BFO heterojunction. BFO was synthesized through a simple solvothermal method, while Cu2O/BFO was fabricated through a co-precipitation method. The formation of heterojunctions between BFO and Cu2O was proved using photoluminescence (PL) spectroscopy, showing a low intensity in the case of BFO/Cu2O compared with pure BFO, which effectively inhibits the carrier recombination and improves the charge transfer efficiency. Superior sonocatalytic oxidation of TET is exhibited through CBF-3, with a degradation efficiency of TET reaching 98.0% under optimal conditions, such as a 1 g·L−1 of CBF-3 composite, a 20 mg·L−1 TET solution, a US irradiation power of 500 W, and a US irradiation time of 5 h [90].
2.2. Solar Water Splitting
The photocatalytic water splitting process is the conversion of solar energy into chemical energy used to drive the production of H2 and O2. This process is achieved when the photocatalyst absorbs natural solar light (i.e., sunlight) while dispersed in water and then electron–hole pairs migrate to the surface of the photocatalyst to generate and produce H2 and O2 [91]. The photocatalytic dissociation of water has many advantages, such as being suited to splitting water of a nearly neutral pH in a one-step process without the need for an applied external bias. However, unassisted overall water splitting under a single-absorber photocatalytic process must achieve the following two conditions: (1) the valence and conduction gap edges of this photocatalyst must astride across the water oxidation (redox) and proton reduction and potentials; (2) this photocatalyst must possess an adequate narrow bandgap to absorb a majority of the solar spectrum [59]. Considering the potential of water splitting, the lowest energy of the absorbed photon must be larger than 1.23 eV to trigger this reaction. In view of the energy requirements set by H2O reduction and oxidation potentials of the conduction band and valence band levels, the optimal band gap of the semiconductor for efficient H2 production is about 2.0 eV [70]. Therefore, developing a new photocatalytic material with an adequate band gap that can directly split water into H2 under visible-light irradiation is essential for H2 production. BFO is an interesting multiferroic material for energy-related applications, especially H2 generation, through photocatalytic water splitting due to its small band gap (~2.2 eV) [92], good carrier transport properties, and large absorption of visible light extending up to 750 nm.
Through the systemic investigation of the Sr-doping level of BFO, it is found that the HER enhancement originates from the improvement of ferromagnetism of Sr-doped BFO without the obvious scarification of ferroelectricity at room temperature [92]. The H2 evolution of Sm-5%-doped BFO has also been elucidated recently [93]. The rate of H2 production has been found to be 6.54 μmol·h−1·cm−2. The improved photocatalytic activity of Sm-5%-doped BFO has been explained based on the effect of doping, better solar spectral response, hindering the recombination loss of photo-generated charge carriers, and fast and facile charge transport.
To enhance the photocatalytic dissociation of water splitting, a new perovskite material has been reported by doping Gd in place of Bi and Co in place of Fe for H2 production through the photoelectrochemical splitting of water [94]. The doping levels lead to the band gap engineering from 2.23 eV to 1.77 eV, as shown in Figure 3. This band gap lowering improves the photocatalytic response of the resulting materials. The highest H2 production rate of 74.57 mmol·h−1·cm−2 has been found for Gd- and Co-co-doped BFO possessing the lowest band gap of 1.77 eV, with a maximum photo-conversion efficiency of 2.29%. Thus, the higher rate of H2 production and better photo-conversion efficiency of Co-co-doped BFO is due to its better solar spectral response.
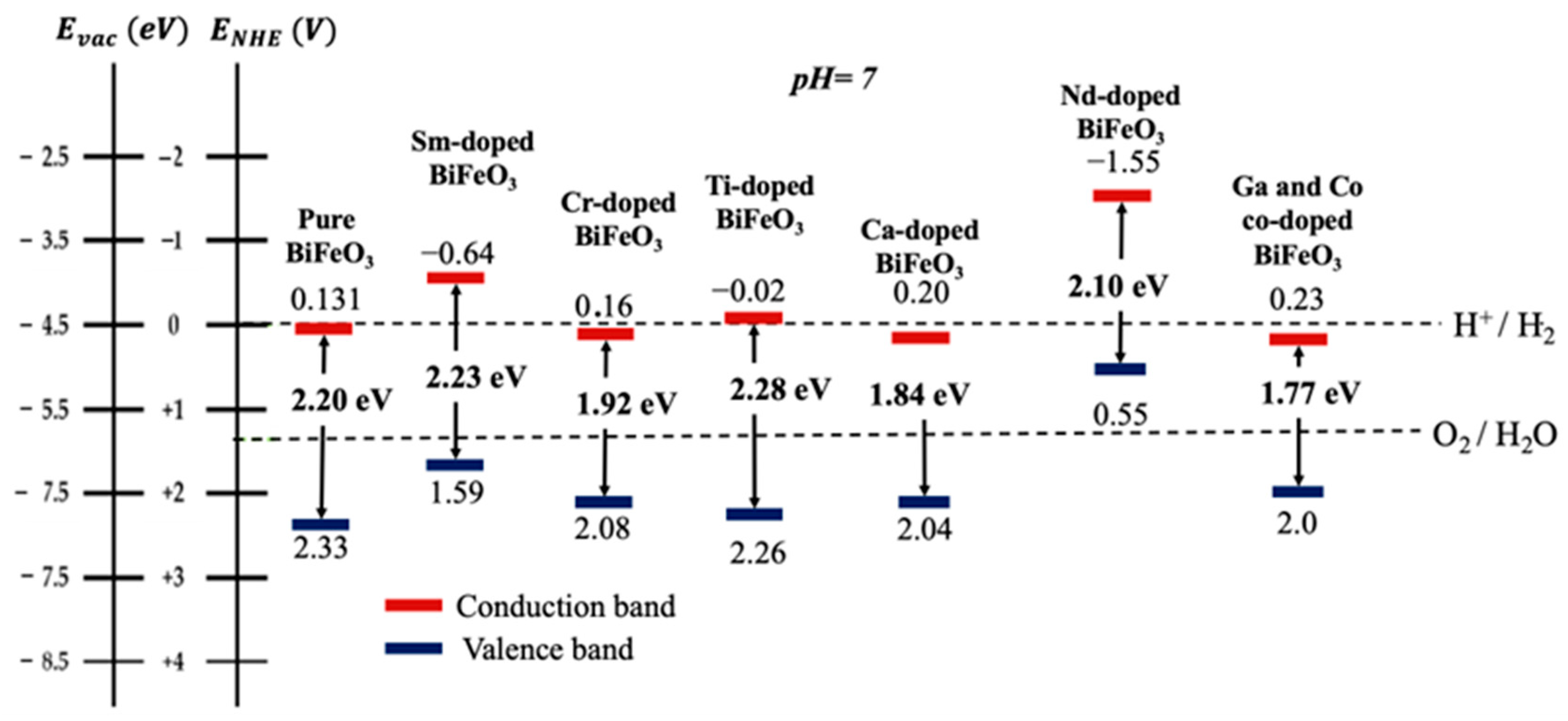
Figure 3. Band gap positions with respect to valence and conduction band at pH = 7 of pure BFO, Sm-doped BFO, Cr-doped BFO, Ti-doped BFO, Ca-doped BFO, Nd-doped BFO, Ga- and Co-co-doped BFO.
The construction of heterojunctions is deemed a prospective methodology for the development of innovative photocatalysts for solar water splitting with outstanding performance. It involves combining different semiconductor materials with unequal band structures and suitable band alignment to form a junction interface that can promote the separation of photogenerated electron–hole pairs, leading to enhanced photocatalytic activity. WO3/BiFeO3 n-p heterojunction films were prepared using the sol–gel spin coating method [95]. Using 2-methoxyethanol as a solvent and DEA as an additive, the best BFO phase has been obtained with regard to impurity phases, micro-structural morphology, and photocurrents. The photocurrent exhibited through the WO3/BFO n-p heterojunction (35.2 mA⋅cm−2) shows a significant improvement over the photocurrents of neat WO3 (6.5 mA⋅cm−2) and BFO (17.5 mA⋅cm−2) thin films (Figure 4a) [95]. In another study, a facile ultrasonic/hydrothermal route was employed to synthesize the BFO/BVO p–n junction, resulting in a significant improvement in the performance of n-type BVO and p-type BFO for the photoelectrochemical (PEC) water splitting process [88]. The BFO/BVO nanostructures exhibited a favorable photocurrent density of 0.36 mA⋅cm−2 under UV–vis light and 0.23 mA⋅cm−2 under visible light at 1.0 V vs. Ag/AgCl [88]. In addition, a simple sol–gel process was used to synthesize a single-phase BFO film on a TiO2 photoanode to enhance photoelectrochemical (PEC) water splitting efficiency. The controllable thickness of the BFO films facilitated the induction of a significant ferroelectric polarization under bias voltage, thereby effectively adjusting the electric band bending at the BFO/TiO2 interface. As a result of this approach, the photocurrent density achieved using the BFO-5/TiO2 photoanode reached an impressive value of 11.25 mA⋅cm−2, surpassing that of bare TiO2 by over 20-fold. Furthermore, when the BFO-5/TiO2 photoanode was positively poled, it demonstrated a remarkable photocurrent density of 28.75 mA⋅cm−2 at 1.5 V vs. SCE under AM 1.5G illumination [96].
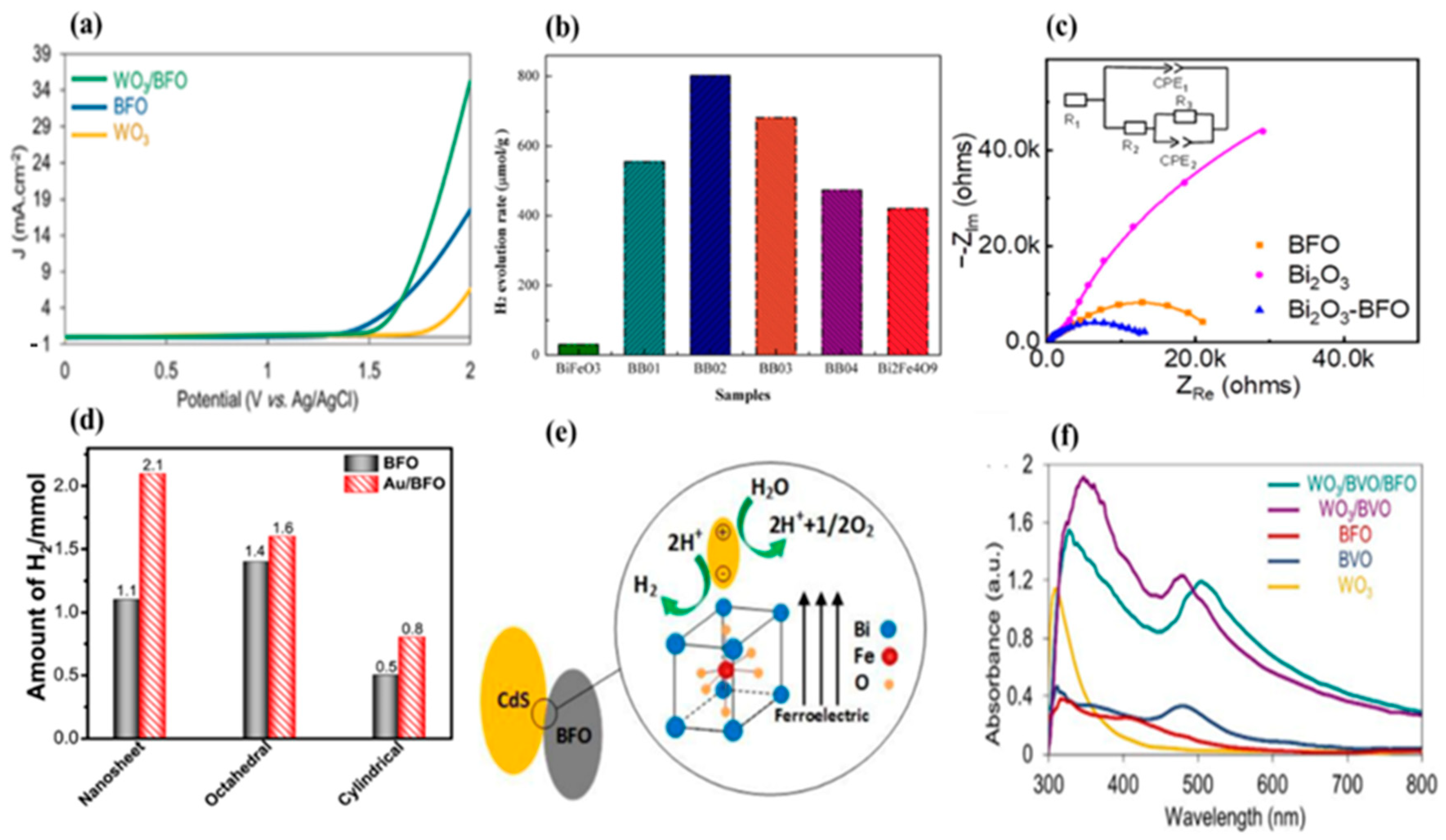
Figure 4. (a) LSV curves of WO3, BFO, and WO3/BFO photoanodes. (b) Stable hydrogen evolution from water using BiFeO3, Bi2Fe4O9, and BiFeO3/Bi2Fe4O9 heterojunction nanofibers under visible-light irradiation (λ > 420 nm). (c) Photoelectrochemical impedance spectra (PEIS) of BFO, Bi2O3, and Bi2O3/BFO films. (d) Comparative H2 generation data after 2 h visible-light irradiation using the catalysts of BFO nanosheets (BFO-Ns), a BFO octahedron (BFO-Oct), a cylindrical-shaped BFO (BFO-Cyl), and their heterostructures as Au/BFO-Ns, Au/BFO-Oct, and Au/BFO-Cyl. (e) Effect of internal electrical field of the BFO on charge separation in CdS. (f) UV–vis absorption spectra of WO3, BVO, BFO, WO3/BVO, and WO3/BVO/BFO photoanodes.
Zhang et al. fabricated BiFeO3/Bi2Fe4O9 heterojunction nanofibers through a facile wet chemical process followed by an electro-spinning technique. The inclusion of Bi2Fe4O9 within the BFO matrix resulted in a red-shift of its absorption edge, thereby enabling the enhanced absorption of visible light and improved efficiency in the separation of photogenerated carriers. Furthermore, the synthesized BiFeO3/Bi2Fe4O9 nanofibers exhibited heightened photocatalytic activity in the generation of H2 from water under visible-light irradiation conditions. Notably, the BiFeO3/Bi2Fe4O9 (BB02) sample demonstrated H2 evolution rates (~800 μmol·g−1) approximately 2.7 times and 2.0 times higher than those observed for pure BiFeO3 and pure Bi2Fe4O9 samples, respectively (Figure 4b). It was observed that the photocurrent density of the BB02 sample reached 1.8 μA⋅cm−2, far exceeding those achieved through pure BiFeO3 (0.6 μA⋅cm−2) and pure Bi2Fe4O9 (0.9 μA⋅cm−2) samples, respectively. Notably, the order of variation in photocurrent density was identified as follows: BB02 > BB03 > BB01 > BB04 > Bi2Fe4O9 > BFO [97]. More recently, high-quality Bi2O3, BFO, Bi2O3/BFO films on indium tin oxide (ITO) were produced using pulse laser deposition (PLD). It was observed that the BFO film exhibited both cathodic and anodic photocurrents in the potential range of −0.7–0.2 V compared with the Ag/AgCl reference electrode. Notably, the photocathodic current was significantly higher, indicating pronounced p-type photocathodic behavior suitable for potential photoelectrochemical (PEC) applications. In particular, the BFO film exhibited an onset potential of around −0.10 V versus the Ag/AgCl reference electrode, with a photocurrent density of −40.1 μA⋅cm−2 obtained at −0.68 V versus the Ag/AgCl reference electrode. Remarkably, the cathodic photocurrent density showed a significant increase in the Bi2O3/BFO heterojunction film compared with the BFO film, reaching a value as high as −84.07 μA⋅cm−2 at −0.68 V compared with the Ag/AgCl reference electrode. This clear improvement represents a doubling of the corresponding value obtained for the BFO film at the same potential, with an onset potential of 0.14 V relative to the Ag/AgCl reference electrode. In addition, the effect of Bi2O3 overlayer thickness was explored, showing that the maximum photocurrent is achieved for 4 nm Bi2O3/BFO. Moreover, electrochemical impedance spectroscopy (EIS) results illustrated interfacial charge transfer processes on the photoelectrodes. The equivalent circuit utilized in the analysis comprises an electrolyte resistor (R1), a ground resistor (R2), a charge transfer resistor (R3), and two constant-phase elements (CPE1 and CPE2). As shown in Figure 4c, the findings indicate a significant reduction in the low-frequency arc when transitioning from Bi2O3 to BFO and Bi2O3/BFO heterojunction films. The charge transfer resistance, denoted as R3, in the Bi2O3/BFO film measures approximately 10 kΩ, a value lower than the approximate 18 kΩ observed in the BFO film. Thus, a more rapid charge separation process can be achieved by adding a Bi2O3 overlayer onto BFO film [98].
References
- Barbir, F.; Veziro, T.N.; Plass, H.J. Environmental damage due to fossil fuels use. Int. J. Hydrogen Energy 1990, 15, 739–749.
- Hassan, A.; Ilyas, S.Z.; Jalil, A.; Ullah, Z. Monetization of the environmental damage caused by fossil fuels. Environ. Sci. Pollut. Res. 2021, 28, 21204–21211.
- Geerken, T.G.; Timmermans, V.T.; Lassaux, S.L. Hydrogen and its Applications: Review of Life Cycle Assessment Studies and Well-to-Wheel Studies. Hysociety 2005, 1–11. Available online: http://www2.ulg.ac.be/cior-fsa/publicat/erscp_h2.pdf (accessed on 1 December 2023).
- Burdack, A.; Duarte-Herrera, L.; López-Jiménez, G.; Polklas, T.; Vasco-Echeverri, O. Techno-economic calculation of green hydrogen production and export from Colombia. Int. J. Hydrogen Energy 2022, 48, 1685–1700.
- Shahin, M.S.; Orhan, M.F.; Saka, K.; Hamada, A.T.; Uygul, F. Energy assessment of an integrated hydrogen production system. Int. J. Thermofluids 2023, 17, 100262.
- Younas, M.; Shafique, S.; Faisal, A.; Hafeez, A.; Javed, F.; Mustafa, M.; Rehman, F. Hydrogen Production through Water Vapors using Optimized Corona-DBD Hybrid Plasma Micro-Reactor. Fuel 2023, 331, 125838.
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113.
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964.
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317.
- Ardo, F.M.; Lim, J.W.; Ramli, A.; Lam, M.K.; Kiatkittipong, W.; Abdelfattah, E.A.; Shahid, M.K.; Usman, A.; Wongsakulphasatch, S.; Sahrin, N.T. A review in redressing challenges to produce sustainable hydrogen from microalgae for aviation industry. Fuel 2022, 330, 125646.
- Aydin, M.I.; Dincer, I. An assessment study on various clean hydrogen production methods. Energy 2022, 245, 123090.
- Mehanovic, D.; Peloquin, J.F.; Dufault, J.F.; Fréchette, L.; Picard, M. Comparative techno-economic study of typically combustion-less hydrogen production alternatives. Int. J. Hydrogen Energy 2022, 48, 7945–7958.
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412.
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111.
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264.
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567.
- Tahir, M.B.; Riaz, K.N. Fundamentals of Photocatalysis for Energy Conversion. In Nanomaterials and Photocatalysis in Chemistry; Springer: Singapore, 2021; pp. 5–17.
- Zeeshan, H.M.; Sharma, S.; Panahi, M.; Voloshina, E.; Dedkov, Y. Semiconducting eutectic materials for photocatalysis and photoelectrochemistry applications: A perspective. Phys. Chem. Chem. Phys. 2022, 24, 25720–25734.
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12.
- Sun, W.; Zhu, J.; Zhang, M.; Meng, X.; Chen, M.; Feng, Y.; Chen, X.; Ding, Y. Recent advances and perspectives in cobalt-based heterogeneous catalysts for photocatalytic water splitting, CO2 reduction, and N2 fixation. Chin. J. Catal. 2022, 43, 2273–2300.
- Isaacs, M.; Garcia-Navarro, J.; Ong, W.J.; Jiménez-Calvo, P. Is Photocatalysis the Next Technology to Produce Green Hydrogen to Enable the Net Zero Emissions Goal? Glob. Chall. 2022, 7, 2200165.
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577.
- Wang, G.; Chang, J.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002.
- Wang, Z.; Wang, L. Progress in designing effective photoelectrodes for solar water splitting. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 369–378.
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660.
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817.
- Minggu, L.J.; Daud, W.R.W.; Kassim, M.B. An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2010, 35, 5233–5244.
- Bhatt, M.D.; Lee, J.S. Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells. J. Mater. Chem. A Mater. 2015, 3, 10632–10659.
- Jeong, S.Y.; Song, J.; Lee, S. Photoelectrochemical Device Designs toward Practical Solar Water Splitting: A Review on the Recent Progress of BiVO4 and BiFeO3 Photoanodes. Appl. Sci. 2018, 8, 1388.
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, e1904717.
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A Mater. 2016, 4, 11446–11452.
- Sharma, P.; Jang, J.W.; Lee, J.S. Key Strategies to Advance the Photoelectrochemical Water Splitting Performance of α-Fe2O3 Photoanode. ChemCatChem 2019, 11, 157–179.
- Seabold, J.A.; Neale, N.R. All first row transition metal oxide photoanode for water splitting based on Cu3V2O8. Chem. Mater. 2015, 27, 1005–1013.
- Guo, L.J.; Luo, J.W.; He, T.; Wei, S.H.; Li, S.S. Photocorrosion-Limited Maximum Efficiency of Solar Photoelectrochemical Water Splitting. Phys. Rev. Appl. 2018, 10, 064059.
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994.
- Xu, X.; Zhou, G.; Dong, X.; Hu, J. Interface Band Engineering Charge Transfer for 3D MoS2 Photoanode to Boost Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2017, 5, 3829–3836.
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band Structure Tuning of TiO2 for Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. C 2014, 118, 7451–7457.
- Lv, R.; Wang, T.; Su, F.; Zhang, P.; Li, C.; Gong, J. Facile synthesis of ZnO nanopencil arrays for photoelectrochemical water splitting. Nano Energy 2014, 7, 143–150.
- Rahman, G.; Joo, O.S. Photoelectrochemical water splitting at nanostructured α-Fe2O3 electrodes. Int. J. Hydrogen Energy 2012, 37, 13989–13997.
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076.
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, e1903378.
- Muzakkar, M.Z.; Umar, A.A.; Ilham, I.; Saputra, Z.; Zulfikar, L.; Maulidiyah, M.; Wibowo, D.; Ruslan, R.; Nurdin, M. Chalcogenide material as high photoelectrochemical performance Se doped TiO2/Ti electrode: Its application for Rhodamine B degradation. In Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2019.
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron-hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957.
- Wang, Z.; Huang, H.; Li, G.; Yan, X.; Yu, Z.; Wang, K.; Wu, Y. Advances in engineering perovskite oxides for photochemical and photoelectrochemical water splitting. In Applied Physics Reviews; American Institute of Physics Inc.: New York, NY, USA, 2021; Volume 8.
- Guerrero, A.; Bisquert, J. Perovskite semiconductors for photoelectrochemical water splitting applications. Curr. Opin. Electrochem. 2017, 2, 144–147.
- Grinberg, I.; West, D.V.; Torres, M.; Gou, G.; Stein, D.M.; Wu, L.; Chen, G.; Gallo, E.M.; Akbashev, A.R.; Davies, P.K.; et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 2013, 503, 509–512.
- Young, S.M.; Rappe, A.M. First principles calculation of the shift current photovoltaic effect in ferroelectrics. Phys. Rev. Lett. 2012, 109, 116601.
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42.
- Jung, H.S.; Park, N.G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25.
- Sophocleous, M. Global and regional water availability and demand: Prospects for the future. Nat. Resour. Res. 2004, 13, 61–75.
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water Air Soil Pollut. 2010, 215, 3–29.
- Pandey, A.; Kumar, R.R.; Kalidasan, B.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300.
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2022, 77, 677–701.
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022.
- Li, H.; Zhu, J.; Wu, Q.; Zhuang, J.; Guo, H.; Ma, Z.; Ye, Y. Enhanced photovoltaic properties of PbTiO3-based ferroelectric thin films prepared by a sol-gel process. Ceram. Int. 2017, 43, 13063–13068.
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64.
- Castillo, M.E.; Shvartsman, V.V.; Gobeljic, D.; Gao, Y.; Landers, J.; Wende, H.; Lupascu, D.C. Effect of particle size on ferroelectric and magnetic properties of BiFeO3 nanopowders. Nanotechnology 2013, 24, 355701.
- Qiao, X.; Geng, W.; Sun, Y.; Zheng, D.; Yang, Y.; Meng, J.; He, J.; Bi, K.; Cui, M.; Chou, X. Robust in-plane polarization switching in epitaxial BiFeO3 films. J. Alloys Compd. 2021, 852, 156988.
- Deng, J.; Banerjee, S.; Mohapatra, S.K.; Smith, Y.R.; Misra, M. Bismuth Iron Oxide Nanoparticles as Photocatalyst for Solar Hydrogen Generation from Water. J. Fundam. Renew. Energy Appl. 2011, 1, 1–10.
- Gao, T.; Chen, Z.; Zhu, Y.; Niu, F.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Synthesis of BiFeo3 nanoparticles for the visible-light induced photocatalytic property. Mater. Res. Bull. 2014, 59, 6–12.
- Wang, N.; Luo, X.; Han, L.; Zhang, Z.; Zhang, R.; Olin, H.; Yang, Y. Structure, Performance, and Application of BiFeO3 Nanomaterials. Nano-Micro Lett. 2020, 12, 81.
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C Mater. 2018, 6, 1239–1247.
- Shah, J.H.; Malik, A.S.; Idris, A.M.; Rasheed, S.; Han, H.; Li, C. Intrinsic photocatalytic water oxidation activity of Mn-doped ferroelectric BiFeO3. Chin. J. Catal. 2021, 42, 945–952.
- Yun, Q.; Xing, W.; Chen, J.; Gao, W.; Bai, Y.; Zhao, S. Effect of Ho and Mn co-doping on structural, ferroelectric and ferromagnetic properties of BiFeO3 thin films. Thin Solid Films 2015, 584, 103–107.
- Preethi, A.J.; Ragam, M. Effect of doping in multiferroic BFO: A review. J. Adv. Dielectr. 2021, 11, 2130001.
- Xian, T.; Yang, H.; Dai, J.F.; Wei, Z.Q.; Ma, J.Y.; Feng, W.J. Photocatalytic properties of BiFeO3 nanoparticles with different sizes. Mater. Lett. 2011, 65, 1573–1575.
- Dhawan, A.; Sudhaik, A.; Raizada, P.; Thakur, S.; Ahamad, T.; Thakur, P.; Singh, P.; Hussain, C.M. BiFeO3-based Z scheme photocatalytic systems: Advances, mechanism, and applications. J. Ind. Eng. Chem. 2023, 117, 1–20.
- Li, S.; Lin, Y.H.; Zhang, B.P.; Wang, Y.; Nan, C.W. Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors. J. Phys. Chem. C 2010, 114, 2903–2908.
- Ponraj, C.; Vinitha, G.; Daniel, J. Visible light photocatalytic activity of Mn-doped BiFeO3 nanoparticles. Int. J. Green Energy 2020, 17, 71–83.
- Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y. A review: Preparation of bismuth ferrite nanoparticles and its applications in visible-light induced photocatalyses. Rev. Adv. Mater. Sci. 2015, 40, 97–109.
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396.
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Structural, magnetic and photocatalytic properties of La and alkaline co-doped BiFeO3 nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 796–802.
- Sakar, M.; Balakumar, S.; Saravanan, P.; Bharathkumar, S. Compliments of confinements: Substitution and dimension induced magnetic origin and band-bending mediated photocatalytic enhancements in Bi1−xDyxFeO3 particulate and fiber nanostructures. Nanoscale 2015, 7, 10667–10679.
- Zhou, J.; Jiang, L.; Chen, D.; Liang, J.; Qin, L.; Bai, L.; Sun, X.; Huang, Y. Facile synthesis of Er-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic degradation of tetracycline hydrochloride. J. Sol-Gel Sci. Technol. 2019, 90, 535–546.
- Chen, Z.; Wu, Y.; Wang, X.; Jin, W.; Zhu, C. Ferromagnetism and enhanced photocatalytic activity in Nd doped BiFeO3 nanopowders. J. Mater. Sci. Mater. Electron. 2015, 26, 9929–9940.
- Sharmin, F.; Basith, M.A. Highly efficient photocatalytic degradation of hazardous industrial and pharmaceutical pollutants using gadolinium doped BiFeO3 nanoparticles. J. Alloys Compd. 2022, 901, 163604.
- Hu, Z.; Chen, D.; Wang, S.; Zhang, N.; Qin, L.; Huang, Y. Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater. Sci. Eng. B 2017, 220, 1–12.
- Soltani, T.; Lee, B.K. Comparison of benzene and toluene photodegradation under visible light irradiation by Ba-doped BiFeO3 magnetic nanoparticles with fast sonochemical synthesis. Photochem. Photobiol. Sci. 2017, 16, 86–95.
- Ponraj, C.; Kumar, P.S.; Sarkar, S.; Krishnamoorthi, C.; Manikandan, N.; Vinitha, G.; Daniel, J. Enhanced visible light photocatalytic activity of magnetic cobalt doped BiFeO3. Surf. Interfaces 2022, 31, 102050.
- Jaffari, Z.H.; Lam, S.M.; Sin, J.C.; Zeng, H.; Mohamed, A.R. Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 2020, 236, 116195.
- Umar, M.; Mahmood, N.; Awan, S.U.; Fatima, S.; Mahmood, A.; Rizwan, S. Rationally designed La and Se co-doped bismuth ferrites with controlled bandgap for visible light photocatalysis. RSC Adv. 2019, 9, 17148–17156.
- Kebede, M.T.; Devi, S.; Tripathi, B.; Chauhan, S.; Dillu, V. Structural transition and enhanced magnetic, optical and photocatalytic properties of novel Ce–Ni co-doped BiFeO3 nanoparticles. Mater. Sci. Semicond. Process. 2022, 152, 107086.
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Effect of (Nd, Ni) co-doped on the multiferroic and photocatalytic properties of BiFeO3. Mater. Res. Bull. 2015, 72, 299–305.
- Subramanian, Y.; Ramasamy, V.; Karthikeyan, R.; Srinivasan, G.R.; Arulmozhi, D.; Gubendiran, R.K.; Sriramalu, M. Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3–GdFeO3 nanocomposite photocatalyst. Heliyon 2019, 5, e01831.
- Xu, J.; Qin, T.; Chen, W.; Lv, J.; Zeng, X.; Sun, J.; Li, Y.-Y.; Zhou, J. Synergizing piezoelectric and plasmonic modulation of Ag/BiFeO3 fibrous heterostructure toward boosted photoelectrochemical energy conversion. Nano Energy 2021, 89, 106317.
- Niu, F.; Chen, D.; Qin, L.; Zhang, N.; Wang, J.; Chen, Z.; Huang, Y. Facile Synthesis of Highly Efficient p–n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ChemCatChem 2015, 7, 3279–3289.
- Bargozideh, S.; Tasviri, M.; Kianifar, M. Construction of novel magnetic BiFeO3/MoS2 composite for enhanced visible-light photocatalytic performance towards purification of dye pollutants. Int. J. Environ. Anal. Chem. 2020, 102, 6390–6404.
- Soltani, T.; Tayyebi, A.; Lee, B.K. BiFeO3/BiVO4 p−n heterojunction for efficient and stable photocatalytic and photoelectrochemical water splitting under visible-light irradiation. Catal. Today 2020, 340, 188–196.
- Ghorbani, M.; Sheibani, S.; Abdizadeh, H.; Golobostanfard, M.R. Modified BiFeO3/rGO nanocomposite by controlled synthesis to enhance adsorption and visible-light photocatalytic activity. J. Mater. Res. Technol. 2023, 22, 1250–1267.
- Wang, X.; He, X.-S.; Li, C.-Y.; Liu, S.-L.; Lu, W.; Xiang, Z.; Wang, Y. Sonocatalytic removal of tetracycline in the presence of S-scheme Cu2O/BiFeO3 heterojunction: Operating parameters, mechanisms, degradation pathways and toxicological evaluation. J. Water Process. Eng. 2023, 51, 103345.
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268.
- Qi, J.; Liu, H.; Feng, M.; Xu, H.; Liu, H.; Wang, C.; Wang, A.; Lü, W. Enhanced hydrogen evolution reaction in Sr doped BiFeO3 by achieving the coexistence of ferroelectricity and ferromagnetism at room temperature. J. Energy Chem. 2020, 53, 93–98.
- Man, S.; Leng, X.; Bai, J.; Kan, S.; Cui, Y.; Wang, J.; Xu, L. Enhancement of photoelectrochemical performance of BiFeO3 by Sm3+ doping. Ceram. Int. 2023, 49, 10255–10264.
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.S.K.; Srivastava, O.N. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrogen Energy 2017, 42, 22677–22686.
- Khoomortezaei, S.; Abdizadeh, H.; Golobostanfard, M.R. Ferro-photocatalytic Enhancement of Photoelectrochemical Water Splitting Using the WO3/BiFeO3 Heterojunction. Energy Fuels 2021, 35, 9623–9634.
- Wu, X.; Li, H.; Wang, X.; Jiang, L.; Xi, J.; Du, G.; Ji, Z. Ferroelectric enhanced photoelectrochemical water splitting in BiFeO3/TiO2 composite photoanode. J. Alloys Compd. 2019, 783, 643–651.
- Zhang, T.; Shen, Y.; Qiu, Y.; Liu, Y.; Xiong, R.; Shi, J.; Wei, J. Facial Synthesis and Photoreaction Mechanism of BiFeO3/Bi2Fe4O9 Heterojunction Nanofibers. ACS Sustain. Chem. Eng. 2017, 5, 4630–4636.
- Yan, X.; Pu, R.; Xie, R.; Zhang, B.; Shi, Y.; Liu, W.; Ma, G.; Yang, N. Design and fabrication of Bi2O3/BiFeO3 heterojunction film with improved photoelectrochemical performance. Appl. Surf. Sci. 2021, 552, 149442.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
05 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




