Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rachid Touzani | -- | 1451 | 2024-01-03 10:51:15 | | | |
| 2 | Rita Xu | Meta information modification | 1451 | 2024-01-03 10:55:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bouammali, H.; Zraibi, L.; Ziani, I.; Merzouki, M.; Bourassi, L.; Fraj, E.; Challioui, A.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; et al. Rosemary for Natural Antioxidants and Anticancer Agents. Encyclopedia. Available online: https://encyclopedia.pub/entry/53363 (accessed on 06 March 2026).
Bouammali H, Zraibi L, Ziani I, Merzouki M, Bourassi L, Fraj E, et al. Rosemary for Natural Antioxidants and Anticancer Agents. Encyclopedia. Available at: https://encyclopedia.pub/entry/53363. Accessed March 06, 2026.
Bouammali, Haytham, Linda Zraibi, Imane Ziani, Mohammed Merzouki, Lamiae Bourassi, Elmehdi Fraj, Allal Challioui, Khalil Azzaoui, Rachid Sabbahi, Belkheir Hammouti, et al. "Rosemary for Natural Antioxidants and Anticancer Agents" Encyclopedia, https://encyclopedia.pub/entry/53363 (accessed March 06, 2026).
Bouammali, H., Zraibi, L., Ziani, I., Merzouki, M., Bourassi, L., Fraj, E., Challioui, A., Azzaoui, K., Sabbahi, R., Hammouti, B., Jodeh, S., Hassiba, M., & Touzani, R. (2024, January 03). Rosemary for Natural Antioxidants and Anticancer Agents. In Encyclopedia. https://encyclopedia.pub/entry/53363
Bouammali, Haytham, et al. "Rosemary for Natural Antioxidants and Anticancer Agents." Encyclopedia. Web. 03 January, 2024.
Copy Citation
Rosmarinus officinalis L. compounds, especially its main polyphenolic compounds, carnosic acid (CA) and rosmarinic acid (RA), influence various facets of cancer biology, making them valuable assets in the ongoing fight against cancer. These two secondary metabolites exhibit formidable antioxidant properties that are a pivotal contributor against the development of cancer.
carnosic acid
cell signaling
rosmarinic acid
Rosmarinus officinalis L.
1. Introduction
Aromatic plants have emerged as promising contenders in the realm of cancer treatment. They offer bioactive compounds with potent medicinal properties that can stop tumors from growing and induce cancer cell death. Notable studies have revealed that these secondary metabolites exhibit formidable antioxidant properties which are a pivotal contributor against the development of cancer [1]. Additionally, compounds derived from this natural resource have demonstrated their ability to thwart the activity of cancer stem cells, which are pivotal actors in tumor progression and metastasis. This compelling body of evidence underscores the significant promise held by aromatic plants as a wellspring of natural compounds for advancing cancer treatment [2].
In the vast realm of bioactive compounds, polyphenols are a prominent class of secondary plant metabolites that demand attention due to their multifaceted bioactive properties [3]. Despite their structural diversity, polyphenols play pivotal roles in various biological processes within plants, substantially contribute to the sensory and nutritional attributes of plant-based foods, and have potential applications in a wide array of practical contexts (formulation of traditional medicine, pharmaceutical industry, food processing and preservation) [4][5].
Rosemary, scientifically known as Rosmarinus officinalis L., as a cornerstone in both economic and social realms, plays a vital role in the countries where it thrives. Its significance is multifaceted, contributing substantially to local economies and societal practices [3]. With an estimated annual production ranging from 150 to 200 tons, rosemary has emerged as a key player in agriculture and industry. Notably, Tunisia leads production with 80 tons, followed by Morocco with 40 tons, Spain with 28 tons, and France contributing 5 to 10 tons of rosemary oil [4][6]. The cultivation of rosemary not only supports farmers but also fuels the agribusiness sector, providing employment opportunities in rural areas [7]. The extraction of its essential oil, widely utilized in the fragrance and flavoring industries, contributes to international trade, fostering economic growth and generating export revenue. Beyond its economic impact, rosemary is deeply interwoven into the social fabric of cultures [8]. Its aromatic and flavorful properties make it a staple in culinary traditions, enhancing the taste of diverse dishes and contributing to a sense of cultural identity. Additionally, rosemary holds a rich history in traditional medicine, where its potential health benefits, including hepatoprotective, antifungal, insecticidal, antioxidant and antibacterial effects [9], contribute to traditional healing practices. The cultural significance of rosemary extends to rituals, ceremonies, and festivals, adding to the cultural richness of communities [10]. Apart from the wide spread’s use of its essential oils in various applications, numerous scientific studies have explored the possible benefits of rosemary polyphenols [8] and their individual components, particularly those with antioxidant effects, for treating inflammation, liver damage, and cancer. Nowadays, in the European Union, rosemary extracts are added to food products and beverages at levels of up to 400 mg/kg, assigning them the label E392 [11].
2. Potential Antioxidant and Anticancer Effects of Res
2.1. Polyphenols: Diverse Roles in Plants and Humans
Polyphenols are secondary metabolites that are widely found in different plants and play a pivotal role in both plant biology and human health [12]. These compounds exhibit a wide range of structural diversity and encompasses phenolic acids, phenolic alcohols, and other molecules with multiple hydroxyl groups on aromatic rings [13]. In our diet, polyphenols are commonly found in foods like fruits, vegetables, cereals, legumes, tea, coffee, and chocolate [14]. However, the quantity and quality of polyphenols in these foods depend on factors such as plant genetics, growing conditions, soil composition, harvest maturity, and post-harvest handling [15]. Polyphenols have antioxidant effects and can scavenge free radicals due to their chemical structure. However, the antioxidant activity depends on some parameters such as the type of compound, the level of methoxylation, and the number of hydroxyl groups [16]. Indeed, this property is responsible for the protection against several diseases (cancer, Alzheimer’s disease, cardiovascular disorders) Richheimer et al. [17]. Recent research by Birtić et al. [18] sheds light on the potential of natural products, including polyphenols, to interact with microtubule affinity regulatory kinase (MARK 4) and promising candidate for cancer treatment. Interestingly, polyphenolic antioxidants can exhibit a dual role as prooxidants. This can trigger apoptosis in cancer cells and damage biological macromolecules, mainly in systems that contain redox-active metals such as copper [19] (Figure 1).
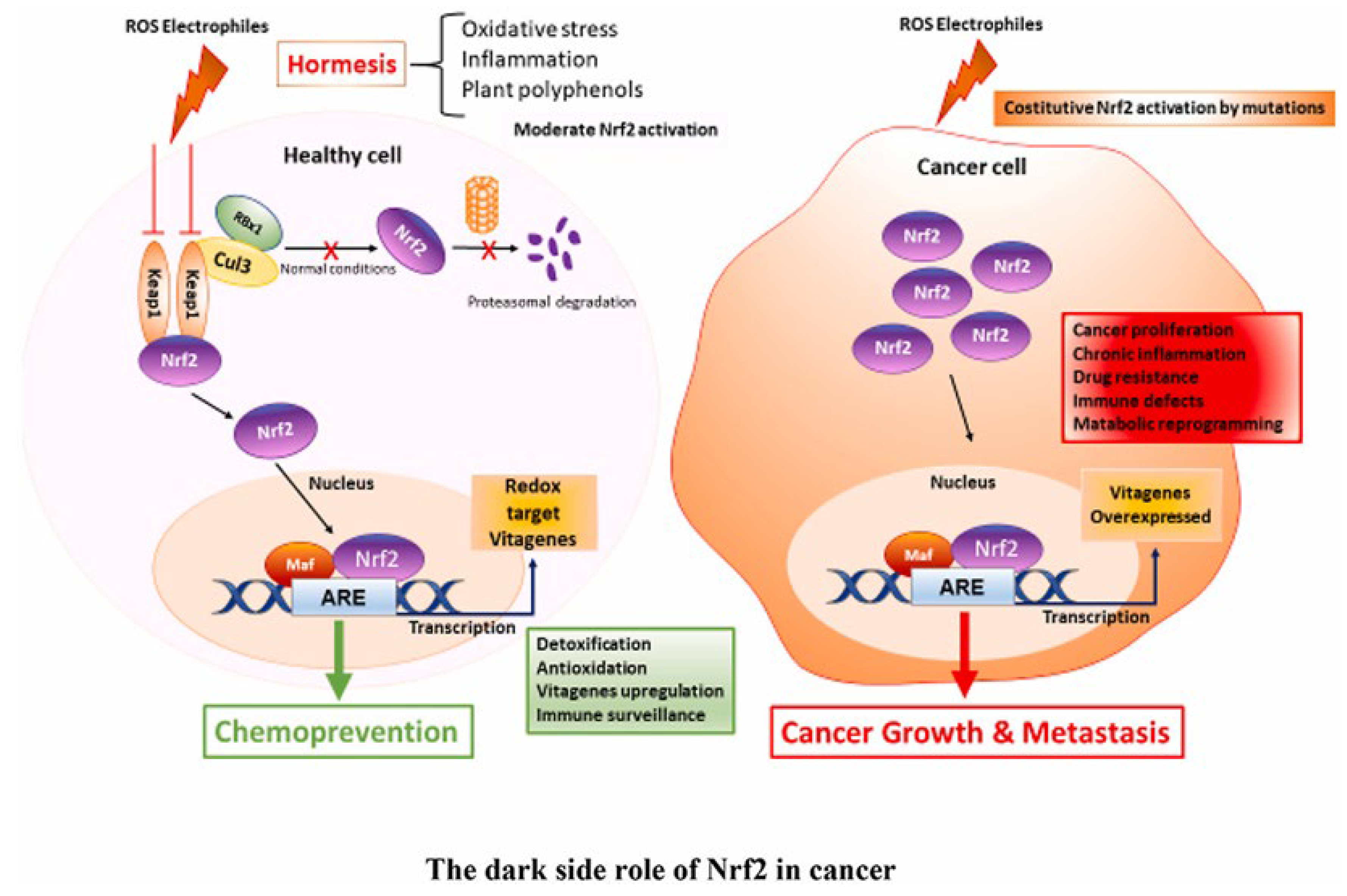
Figure 1. Mechanism of chemopreventive action of plant polyphenols.
In line with the exploration of polyphenols, Fadili et al. [20] conducted a comprehensive analysis to quantify the polyphenol content in two distinct species of rosemary. Their investigation unveiled the presence of a rich array of secondary metabolites within both plant species, with a particular focus on the abundance of polyphenols. These polyphenols exhibited variable concentrations, ranging from 0.02 to 0.185 g, signifying the remarkable diversity in secondary metabolite content. Furthermore, the researchers endeavored to assess the antioxidant potential of these compounds using the DPPH method. The results were short and impressive, particularly for the ethyl acetate fractions, which displayed robust DPPH radical-scavenging abilities. In fact, the inhibitory concentrations responsible for 50% of the antiradical activity (IC50) were measured at an astonishing 103.86 ± 3.5 μg/mL for Rosmarinus officinalis L. To determine the precise polyphenol content within the plant extracts, UV/visible spectrophotometry was employed. The outcomes of this meticulous analysis are visually presented in Figure 2, showcasing the rich polyphenolic landscape of these rosemary species.
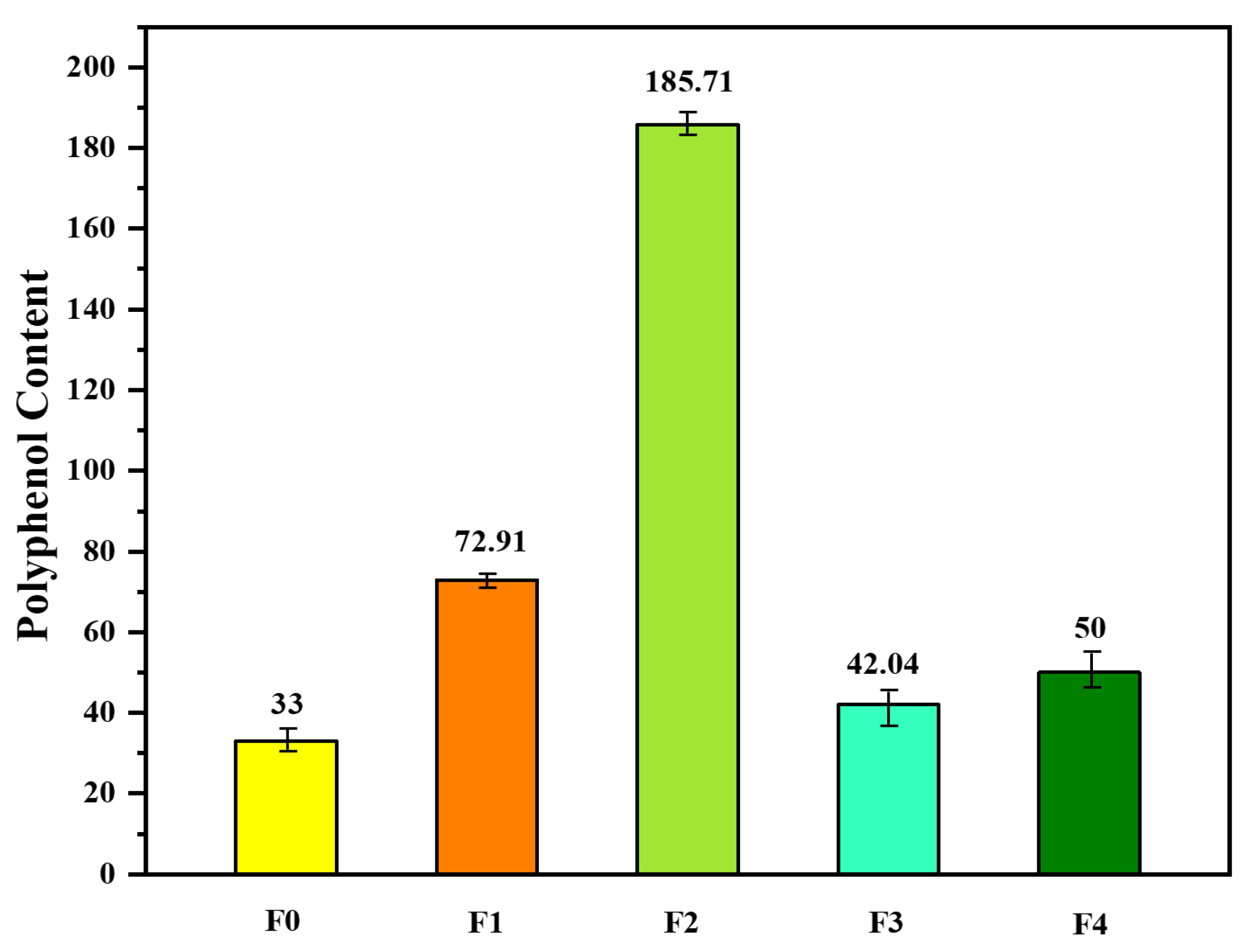
Figure 2. Polyphenol content expressed as gallic acid equivalents (mg GAE) per gram of Rosmarinus officinalis L. (F0 = methanol/water (80/20) extract; F1–3 are obtained from F0 by extraction using successively chloroform (F1), ethyl acetate (F2), and n-butanol (F1); F4 is the remaining final aqueous phase).
2.2. Phenolic Powerhouses in Rosemary: Carnosic and Rosmarinic Acids
A great number of compounds were identified in a solid liquid extract from rosemary, using, most often, HPLC–MS and GC-MS as methods of analysis. These secondary metabolites belong mainly to the classes of phenolic diterpenes (carnosic acid, carnosol, rosmadial, rosmanol), phenolic acids (rosmarinic acid, caffeic acid), flavonoids (genkwanin, homoplantaginin, scutellarein and cirsimaritin), and triterpenes (ursolic acid, oleanolic acid) (Figure 3) [21]. Their levels vary significantly based on the parts of the plant (leaves, stems, sepals, petals, seeds, roots), seasonal variation, and the extraction method [22].
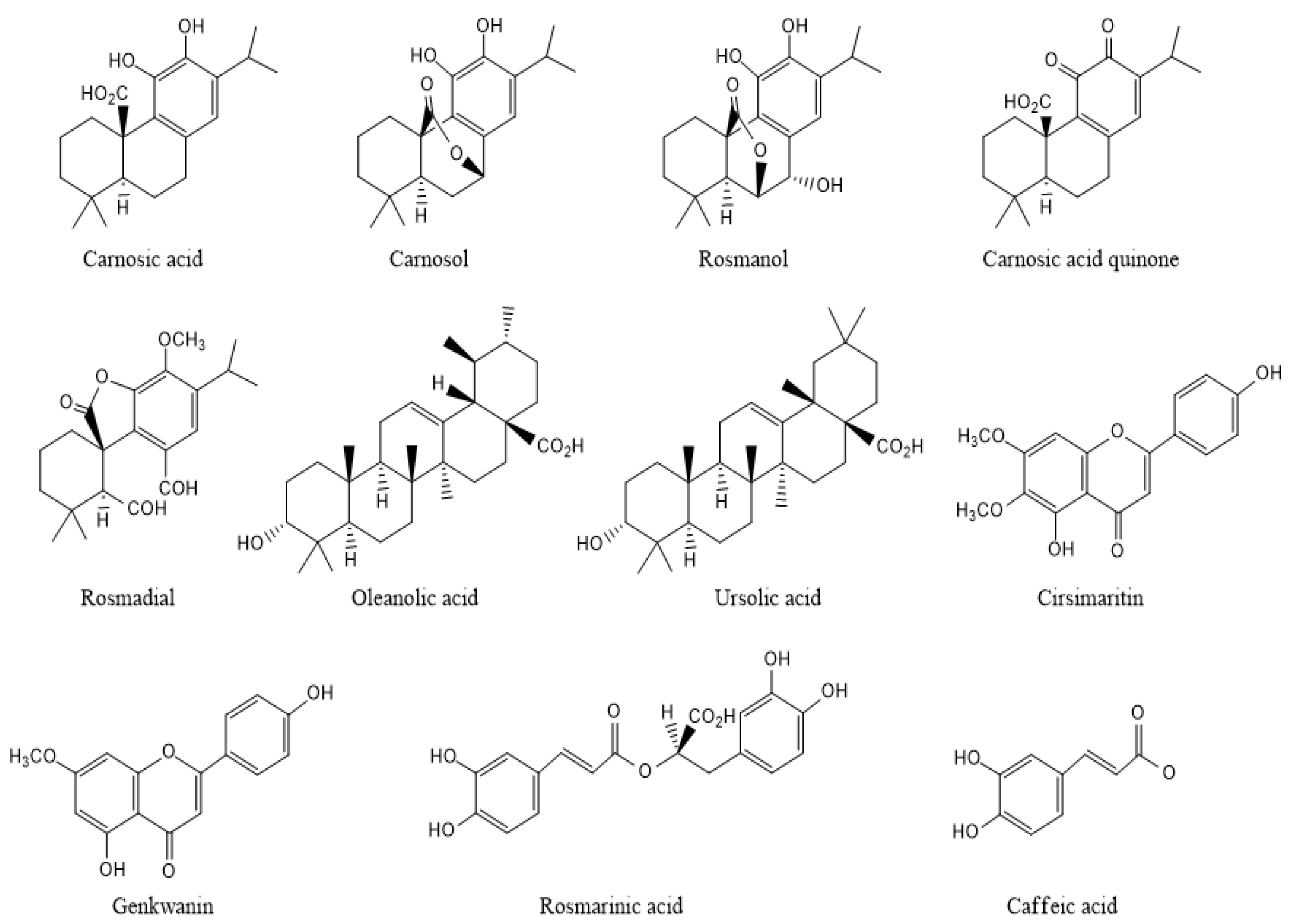
Figure 3. Chemical structures of varied bioactive compounds of rosemary.
The principal bioactive components of the rosemary extracts are carnosic acid (CA), rosmarinic acid (RA), and carnosol (CAR) [5]. CA and RA, prominent phenolic compounds in rosemary leaves, boast unique attributes that contribute to their significant roles in health and well-being. CA, characterized by its abietane-like structure fused with a catechol group and O-phenolic hydroxyl groups at positions C11 and C12 [23], distinguishes itself by accumulating preferentially in rosemary’s glandular trichomes. Its lipophilic nature empowers CA to excel the antioxidant activity, effectively countering singlet oxygen, hydroxyl radicals, and lipid peroxyl radicals. Beyond its antioxidative prowess, CA exhibits a diverse repertoire of benefits, including anti-inflammatory, antiproliferative, antitumorigenic, and neuroprotective properties [24]. Recent research underscores CA’s substantial contribution, along with its primary oxidation product, carnosol (CAR), comprising around 90% of rosemary extract’s antioxidant capabilities [25]. This remarkable antioxidant response owes much to CA’s abundant presence relative to other phenolic compounds. Intriguingly, CA’s superior protection against lipid peroxidation, as evidenced in accelerated aging experiments, surpasses that of α-tocopherol, while its rapid consumption leads to the absence of accumulated conjugated hydroperoxides of polyunsaturated fatty acids [26].
Rosemarinic acid (RA) has a chemical structure termed 3,4-dihydroxyphenyllactic acid and displays versatility through a spectrum of health-enhancing qualities. Widely distributed across plant families, including Boraginaceae and Lamiaceae [27], RA shines with its antiviral, antibacterial, anti-inflammatory, and antioxidant effects [28]. What is intriguing is RA’s potential for synthesis in undifferentiated plant cell cultures, often yielding higher levels than the original plant source. Its documented ability to alleviate inflammation by reducing Cyclooxygenase 2 enzyme (COX 2) expression and prostaglandin levels further highlights its significance. Nevertheless, when assessed for antioxidant potency through DPPH tests, CA, with its two phenolic hydroxyl groups, surpasses RA, which boasts four such groups (Table 1) [29]. Together, CA and RA, as major polyphenolic constituents in rosemary extract, exhibit formidable antioxidant properties. Research has explored how these substances protect against oxidative stress and glycation in diabetic rats, revealing a decrease in oxidative stress markers and advanced glycation end products. This signifies their ability to combat oxidative damage and glycation, offering potential benefits in mitigating oxidative stress in diabetes [30].
Table 1. Antioxidant and antitumor properties of carnosic acid and rosmarinic acid derived from Rosmarinus officinalis L.
| Tested Compounds in IC50 (μΜ) | References | |||
|---|---|---|---|---|
| Rosemary Extract | Carnosic Acid | Rosmarinic Acid | ||
| Radical-scavenging activity DPPH | 54.0 ± 1.4 | 33.1 ± 1.7 | 72.3 ± 3.3 | [31] |
| Oral cancer | 27.4–68.2 μM | 7.5–20 μM | 50–104.2 μM | [32][33][34] |
| Kidney cancer | - | 20 μΜ | 240.2 µM | [35][36] |
| Pancreatic Cancer | 20–120 µM | 6.02–54.15 µM | 100 μΜ | [35][37] |
| Colon cancer | 20–40 µg | 8.29–27.43 mg/mL | 240.2 μM | [38][39][40] |
| Prostate cancer | 19.72 μg/mL | 41.1 μM | 14 μM | [41][42][43] |
References
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline Containing Natural Products as Anticancer Agents: A Review. Eur. J. Med. Chem. 2014, 77, 121–133.
- Chaitanya, M.V.N.L.; Ramanunny, A.K.; Babu, M.R.; Gulati, M.; Vishwas, S.; Singh, T.G.; Chellappan, D.K.; Adams, J.; Dua, K.; Singh, S.K. Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives. Pharmaceutics 2022, 14, 2401.
- Ziani, I.; Bouakline, H.; Yahyaoui, M.I.; Belbachir, Y.; Fauconnier, M.L.; Asehraou, A.; Tahani, A.; Talhaoui, A.; El Bachiri, A. The Effect of Ethanol/Water Concentration on Phenolic Composition, Antioxidant, and Antimicrobial Activities of Rosmarinus Tournefortii de Noé Hydrodistillation Solid Residues. J. Food Meas. Charact. 2022, 17, 1602–1615.
- Santana-Méridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Polyphenol Composition, Antioxidant and Bioplaguicide Activities of the Solid Residue from Hydrodistillation of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 125–134.
- Lesellier, E.; Lefebvre, T.; Destandau, E. Recent developments for analysis and the extraction of bioactive compounds from Rosmarinus officinalis and medicinal plants of the Lamiaceae family. Trends Anal. Chem. 2021, 135, 11658.
- Chen, H.; Gu, Z.; Yang, L.; Yang, R.; Ji, Y.; Zeng, Q.; Xiao, F.; Huang, P. Optimization Extraction of Rosemary Essential Oils Using Hydrodistillation with Extraction Kinetics Analysis. Food Sci. Nutr. 2021, 9, 6069–6077.
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction Techniques, Analytical Methods and Health-Promoting Biological Effects. Phytochem. Rev. 2021, 20, 1273–1328.
- Bouakline, H.; Elkabous, M.; Ziani, I.; Karzazi, Y.; Tahani, A.; El Bachiri, A. Antioxidative Activity of Pistacia Lentiscus Leaf Extract Main Components: Experimental and Theoretical Study. Mater. Today Proc. 2023, 72, 3275–3279.
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528.
- Vella, F.M.; Laratta, B. Rosmary Essential Oil Extraction and Residue Valorization by Means of Plyphenol Recovery. Biol. Life Sci. Forum 2023, 26, 8.
- Macheix, J.J.; Fleuriet, A.; Jay-Allemand, C. Phenolic Compounds Plants: An Example of Secondary Metabolites of Economic Importance; Polytechnic and University Romandes Presses: Lausanne, Italie, 2005; p. 192.
- Anwar, S.; Shahwan, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Microtubule-Affinity Regulating Kinase 4: A Potential Drug Target for Cancer Therapy. Cell. Signal. 2022, 99, 110434.
- Yar Khan, H.; Zubair, H.; Fahad Ullah, M.; Ahmad, A.; Mumtaz Hadi, S. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Curr. Drug Targets 2012, 13, 1738–1749.
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox Modulation by Plant Polyphenols Targeting Vitagenes for Chemoprevention and Therapy: Relevance to Novel Anti-Cancer Interventions and Mini-Brain Organoid Technology. Free Radic. Biol. Med. 2022, 179, 59–75.
- Yeddes, W.; Majdi, H.; Gadhoumi, H.; Affes, T.G.; Mohamed, S.N.; Aidi Wannes, W.; Saidani-Tounsi, M. Optimizing Ethanol Extraction of Rosemary Leaves and Their Biological Evaluations. J. Explor. Res. Pharmacol. 2022, 7, 85–94.
- Richheimer, S.L.; Bernart, M.W.; King, G.A.; Kent, M.C.; Beiley, D.T. Antioxidant Activity of Lipid-Soluble Phenolic Diterpenes from Rosemary. J. Am. Oil Chem. Soc. 1996, 73, 507–514.
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic Acid. Phytochemistry 2015, 115, 9–19.
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage. Food Chem. 2010, 118, 656–662.
- Fadili, K.; Amalich, S.; N’dedianhoua, S.K.; Bouachrine, M.; Mahjoubi, M.; El Hilali, F.; Zair, T. Polyphenols Content and Antioxidant Activity of Two Species from Moroccan High Atlas: Rosmarinus officinalis and Thymus satureioides. Int. J. Innov. Sci. Res. 2015, 4, 24–33.
- Borras Linares, I.; Arraez-Rman, D.; Herrero, M.; Ibanez, E.; Segura-Carretero, A.; Fernandez-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactve phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to elecrospray time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7682–7690.
- Almela, L.; Sanchez-Munoz, B.; Fernndez-Lopez, J.A.; Roca, M.J.; Rabe, V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from defferent raw material. J. Chromatogh. A 2006, 1120, 221–229.
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective Effect of Rosmarinic Acid and Carnosic Acid against Streptozotocin-Induced Oxidation, Glycation, Inflammation and Microbiota Imbalance in Diabetic Rats. Food Funct. 2018, 9, 851–860.
- Ben Jemia, M.; Tundis, R.; Maggio, A.; Rosselli, S.; Senatore, F.; Menichini, F.; Bruno, M.; Kchouk, M.E.; Loizzo, M.R. NMR-Based Quantification of Rosmarinic and Carnosic Acids, GC–MS Profile and Bioactivity Relevant to Neurodegenerative Disorders of Rosmarinus officinalis L. Extracts. J. Funct. Foods 2013, 5, 1873–1882.
- Lim, S.H.; Nam, K.H.; Kim, K.; Yi, S.A.; Lee, J.; Han, J.-W. Rosmarinic Acid Methyl Ester Regulates Ovarian Cancer Cell Migration and Reverses Cisplatin Resistance by Inhibiting the Expression of Forkhead Box M1. Pharmaceuticals 2020, 13, 302.
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic Acid Suppresses the Development of Oral Squamous Cell Carcinoma via Mitochondrial-Mediated Apoptosis. Front. Oncol. 2021, 11, 760861.
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic Acid and Its Derivatives: Current Insights on Anticancer Potential and Other Biomedical Applications. Biomed. Pharmacother. 2023, 162, 114687.
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731.
- Chen, X.; Su, H.Z.Z. Detailed Studies on the Anticancer Action of Rosmarinic Acid in Human Hep-G2 Liver Carcinoma Cells: Evaluating Its Effects on Cellular Apoptosis, Caspase Activation and Suppression of Cell Migration and Invasion. J. BUON. 2020, 25, 2011–2016.
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation Effect of Rosemary (Rosmarinus officinalis) on Human Ovarian Cancer Cells in Vitro. Phytomedicine 2012, 19, 436–443.
- González-Vallinas, M.; Reglero, G.; Ramirez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231.
- Khella, K.F.; El Maksoud, A.I.A.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102.
- Manimaran, A.; Manoharan, S.; Karthikeyan, S.; Nirmal, M. Anti-Cell Proliferative, Anti-Inflammatory and Anti-Angiogenic Potential of Lupeol in 7, 12-Dimethylbenz (a) Anthracene Induced Hamster Buccal Pouch Carcinogenesis. Br. J. Med. Med. Res. 2015, 6, 587–596.
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of Carnosic Acid to the Treatment of Several Health Disorders: Molecular Targets and Mechanisms. Biomed. Pharmacother. 2016, 84, 569–582.
- Petiwala, S.M.; Puthenveetil, A.G.; Johnson, J.J. Polyphenols from the Mediterranean Herb Rosemary (Rosmarinus officinalis) for Prostate Cancer. Front. Pharmacol. 2013, 4, 00029.
- Chou, S.T.; Ho, B.Y.; Tai, Y.T.; Huang, C.J.; Chao, W.W. Bidirect Effects from Cisplatin Combine with Rosmarinic Acid (RA) or Hot Water Extracts of Glechoma Hederacea (HWG) on Renal Cancer Cells. Chin. Med. 2020, 15, 77.
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739.
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) Extract Causes ROS-Induced Necrotic Cell Death and Inhibits Tumor Growth in Vivo. Sci. Rep. 2019, 9, 808.
- Faustino, C.; Neto, Í.; Fonte, P.; Macedo, A. Cytotoxicity and Chemotherapeutic Potential of Natural Rosin Abietane Diterpenoids and Their Synthetic Derivatives. Curr. Pharm. Des. 2018, 24, 4362–4375.
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-Warburg Effect of Rosmarinic Acid via MiR-155 in Gastric Cancer Cells. Drug Des. Devel. Ther. 2015, 9, 2695–2703.
- Jaglanian, A.; Termini, D.; Tsiani, E. Rosemary (Rosmarinus officinalis L.) Extract Inhibits Prostate Cancer Cell Proliferation and Survival by Targeting Akt and MTOR. Biomed. Pharmacother. 2020, 131, 110717.
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300.
- Min, K.; Jung, K.-J.; Kwon, T.K. Carnosic Acid Induces Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress Induction in Human Renal Carcinoma Caki Cells. J. Cancer Prev. 2014, 19, 170.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
03 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





