Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vladimir William | -- | 3021 | 2024-01-02 16:24:11 | | | |
| 2 | Lindsay Dong | -2 word(s) | 3019 | 2024-01-03 02:21:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
William, V.U.; Magpantay, H.D. The Interactions between Microorganisms and Arsenic. Encyclopedia. Available online: https://encyclopedia.pub/entry/53329 (accessed on 13 January 2026).
William VU, Magpantay HD. The Interactions between Microorganisms and Arsenic. Encyclopedia. Available at: https://encyclopedia.pub/entry/53329. Accessed January 13, 2026.
William, Vladimir U., Hilbert D. Magpantay. "The Interactions between Microorganisms and Arsenic" Encyclopedia, https://encyclopedia.pub/entry/53329 (accessed January 13, 2026).
William, V.U., & Magpantay, H.D. (2024, January 02). The Interactions between Microorganisms and Arsenic. In Encyclopedia. https://encyclopedia.pub/entry/53329
William, Vladimir U. and Hilbert D. Magpantay. "The Interactions between Microorganisms and Arsenic." Encyclopedia. Web. 02 January, 2024.
Copy Citation
While arsenic is a natural and inevitable part of the biogeochemical cycle, the rise in anthropogenic activities has led to its continued increase in arsenic concentrations in various environmental matrices. High arsenic concentration is considered a threat due to its recalcitrant nature as well as its capacity for highly toxic effects in plants, animals, and humans. Among all domains of life, microorganisms have been dealing with arsenic since life arose and are the most resilient to its lethal effects. Strides in elucidating the biochemical pathways of their ability to detoxify arsenic has allowed us to utilize their potential in bioremediation processes.
arsenic
heavy metal
microbial bioremediation
tolerance genes
1. Introduction
Arsenic (chemical symbol: As; atomic number: 33; atomic mass: 74.921 amu) is a steel-grey metalloid that is naturally present in the environment but is most commonly found in rocks and soils, particularly in sulfide minerals [1][2]. While arsenic can exist in four oxidation states (As(III), As(V), As(0), As−3), the most common are the trivalent arsenite, As(III), and the pentavalent arsenate, As(V) [3]. Ecological niches differ in the form of arsenic present in them—As(III) prefers anoxic and reducing conditions, while aerobic environments have a preponderance of As(V) [4][5]. However, between the two oxyanions, As(III) is considered more toxic due to its high mobility in both aqueous and solid phases [6].
Currently, the World Health Organization estimates that at least 140 million people worldwide are exposed to arsenic at levels above provisional guideline values [7]. Considering the classification of arsenic as a Group 1 carcinogen and its declaration as the most pervasive hazardous substance found in the environment, its continuous, widespread impact has renewed recent interest in it as a pollutant of concern and has pushed the United States Centers for Disease Control and Prevention to put it at the top of the ATSDR’s Substance Priority List [8][9]. The impact of arsenic contamination is particularly seen in vulnerable regions highly reliant on groundwater as a primary source of potable water [10][11][12]. Global notoriety of arsenic was reached when an estimated 125 million people in Bangladesh were exposed to inorganic arsenic from contaminated tube wells, constituting what the World Health Organization calls “the largest mass poisoning of a population in history” [13]. In addition, the incessant use of soil amendments [14], as well as the constant conversion of land for agricultural and urban use [15], has led to a substantial increase in arsenic in areas with normally low concentrations.
2. The Genetic Basis of Microbial Arsenic Detoxification
Due to the ubiquitous nature of arsenic in the environment, it is generally assumed that evolution has conferred all living things the necessary molecular machinery to detoxify arsenic [16][17]. As mentioned, the two most naturally abundant forms of arsenic are As(III) and As(V). Between the two, however, As(III) is more biologically important due to its ability to disrupt protein function by strongly binding to sulfhydryl groups, reducing cysteines, and effectively preventing proper protein folding. While As(V) also exerts toxic cell function by abrogating phosphate anion transporters, the danger lies in its susceptibility to be reduced to the more toxic As(III) [18]. Selective pressure from constant environmental arsenic stress have allowed microorganisms to evolve niche-specific gene expressions to facilitate arsenic uptake [19]. This includes the genes which confer the ability to utilize As(III) as an electron donor and As(V) as an electron acceptor [20].
2.1. The aio Gene Systems
Presumed habitat vestiges of primordial life, such as thermal vents, were found to contain arsenic in the form of As(III), leading researchers to conclude that primitive microorganisms may have had bioenergetic use for these ions [21][22][23]. Since survival is reliant on the immediate detoxification of the more toxic As(III), it has been surmised that the mechanisms of As(III) oxidation is the most ancient As detoxification system, which recent phylogenetic analyses have confirmed [24].
The archetypal aio system—the aioBA operon—was first identified and completely sequenced from the β-proteobacteria Herminiimonas arsenicoxydans. It is a bicomponent, regulatory pair composed of aioB and aioA, which respectively encodes for the small, Rieske 2Fe-2S cluster and the large molybdopterin subunit of the enzyme, arsenite oxidase (Aio) [25]. Structural elucidation of the Aio expressed by Alcaligenes faecalis provided a more intimate look at not only the enzyme, but also what came to be known as “arsenic gene islands”. These gene islands are functionally related As(III)-resistance genes in mutually independent microorganisms [26][27].
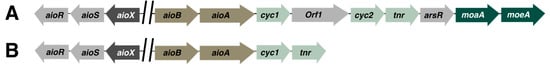
Explorations into the aio gene islands of various microorganisms revealed that differences in the organization of intergenic sequences between flanking genes, as well as homologous aioBA systems, are unique to each organism (see Figure 1). An in silico investigation of gene clusters of 55 phylogenetically distinct bacterial species confirmed this by concluding that although the aioBA domain is widespread across As(III) oxidizing species, multiplicity in the occurrence, orientation, and sequence of specific regulatory genes is only present in some species, as is seen in the case of the aioXSR operon, which can only be found in Proteobacteria [28].

Figure 1. Examples of genetic organization of aio operons in a few arsenic-resistant microorganisms. Arrows represent open reading frame and the direction of transcription. Orthologs are represented in the same color. (A) Thiomonas arsenitoxydans [29], (B) Acidovorax sp. NO-1 [30].
Another example of the divergence of As(III)-resistance genes can be found in the acidophilic Thiomonas arsenitoxydans, which was found to cotranscribe two unique genes, orf1 and cyc2, together with the aioBA cluster [29]. orf1, which encodes for a DNA binding metalloregulator, and cyc2, which encodes for a monohemic cytochrome c, suggests that these distinct genes regulate both the oxidation of As(III), as well as the activation of the operon in the bacterium.
2.2. The ars Gene Systems
Since the archaic Earth contained much higher concentrations of environmental arsenic, life would have had needed to evolve arsenic-resistance genes to thrive [16]. Additionally, the eventual oxygenation of the Earth’s atmosphere lead to the conversion of inorganic arsenic into As(V), giving microorganisms selective pressure to evolve genes required to reduce As(V) [31]. These environmental challenges have allowed microorganisms to diversify their genetic toolkit and gave rise to arsenic-resistance genes, encoded in the ars operons [17].
Serendipitously, the ars genes were isolated not from directly examining arsenic-resistant microorganisms but from the study of antibiotic-resistance genes in Staphylococcus aureus more than 50 years ago. The isolated plasmid, pI258, has been found to not only confer resistance to antibiotics but also to other metals, arsenic included. Using similar techniques, the R773 plasmid was isolated from E. coli, from which the main arsenic resistance phenotype is derived: the arsRDABC operon.
Each individual component of the arsRDABC gene cluster codes for a specific type of protein related to arsenic removal: The arsR encodes for a trans-acting transcriptional repressor member of the SmtB/ArsR family [32]; arsA codes for an ATPase [33], which together with the ArsB efflux pump can form their own ATP-dependent As(III) efflux pump [34]; arsD codes for a another trans-acting regulator metallochaperone, which has the ability to bind As(III) and to expel it via transfer to the ArsAB efflux pump [35][36]; and arsC, which codes for the canonical prokaryotic As(V) reductase, ArsC [37]. In fungi, the equivalent As(V) reductase is coded by the ACR2 gene [38].
2.3. The arr Gene Systems
In contrast with the other gene systems, the genes that code for dissimilatory As(V) reduction (also known as respiratory As(V) reduction) are a later evolutionary response to environmental arsenic stress. Known as the arr gene cluster, to date, only Bacteria and Archaea have been shown to express arr. Because of this, research on the arr genes and its resultant proteins is still in its nascent stages.
First described in Shewanella sp. ANA-3, the arr genes code for an As(V) reductase, similar to the ars cluster. The arr operon codes for the heterodimer respiratory As(V) reductase ArrAB, made up of a large and small subunit encoded by arrA and arrB, respectively. The large ArrA subunit is composed of a bismolybdopterin guanine dinucleotide cofactor and an [4Fe-4S], both of which act as the binding and catalytic site of As(V).
While continued searches for microorganisms which can express genes related to arrA are underway, phylogenetic analysis suggests that the eventual prevalence of As(V) in the environment coincided with the split of Bacteria and Archaea, explaining the specificity and preponderance of arr genes in only the two domains [39].
3. Biomolecular Pathways Involved in the Microbial Detoxification of Arsenic Related to Bioremediation
3.1. Divergent Arsenic Detoxification Strategies Used by Microorganisms
In the biogeochemical cycling of arsenic, microorganisms play a central role in the transformation of arsenic from one form to another. Depending on the ecological niche, the predominant arsenic species may vary, which is why genes for arsenic resistance are encoded by all microorganisms. The process of biotransformation ultimately governs the speciation and mobilization of arsenic in all environmental matrices. Because of this, the most well-known mechanisms of arsenic detoxification in microorganisms involve the metabolic use of arsenic as an electron donor or acceptor in respiratory redox processes. The two processes are distinct in how they metabolically utilize environmental arsenic. When microorganisms are faced with As(III), cellular energy is gained from the oxidation of the As(III) by using it as an electron donor.
3.2. Microbial As(III) Oxidation
The oxidation of the more toxic As(III) is the prime arsenic detoxification mechanism used by microorganisms. Recent phylogenetic analyses have conclusively proven that the enzymes required for As(III) oxidation predate all other arsenic detoxification processes [40]. While this important process was first observed as early as 1918 [41], only recently with the development of new, more sophisticated structural elucidation techniques have researchers been given a clearer picture of the regulatory mechanisms and proteins involved in the process. Because of the rise in interest in exploiting microbial As(III) oxidation mechanisms for potentially novel bioremediation approaches, various papers have also reviewed the topic [42][43].
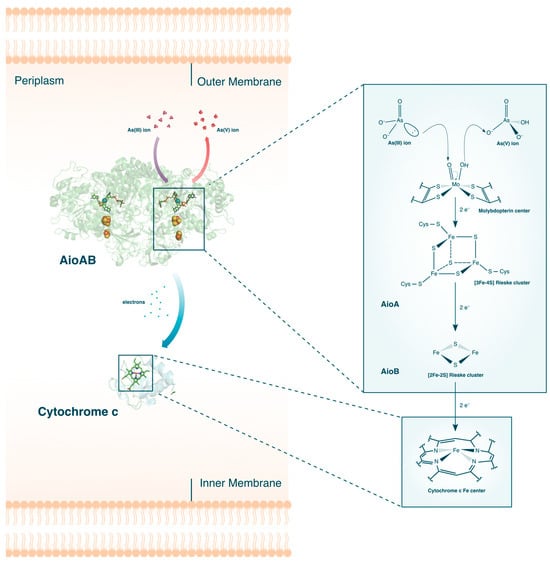
The aerobic oxidation of As(III) is primarily catalyzed by the As(III) oxidase, Aio (or AioBA) (see Figure 2). When challenged with As(III) stress, upregulation of aioBA genes occurs, which codes for the two subcomponents of Aio: the large α-subunit, AioA, and the small β-subunit, AioB. Crystal structures [26][44] of the protein complex revealed that the two subunits can be further subdivided into their individual redox active sites. The first active site is a molybdenum (Mo)-centered bis-pterin guanine dinucleotide surrounded by a network of protein residues and water molecules all connected by hydrogen bonds and salt bridges. Also found inside the AioA domain is a [3Fe-4S] Rieske cluster, while AioB contains a [2Fe-2S] Rieske cluster. Once expressed, these proteins are then translocated either to the periplasm or the cytoplasm and coupled with a c-type cytochrome [45].

Figure 2. The aerobic oxidation of As(III) in bacteria. The crystal structures for AioAB [44] and Cytochrome c [46] are shown. Description of the electron flow in the active sites are given in the text.
A combination of stopped-flow spectroscopy and isothermal titration calorimetry revealed that the Aio catalyzed oxidation of As(III) into As(V) occurs via four distinct electron transfer events [47]. When As(III) enter the vicinity of the funnel-liked opening of AioA, highly polar residues attached to the molybdopterin molecule binds the As(III). An electron pair from the bound As(III) performs a nucleophilic attack on the Mo(VI) center of the molybdopterin molecule, effectively reducing Mo(V) to Mo(IV). The reduction of Mo(V) and simultaneous oxidation of As(III) to As(V) happens almost instantaneously, with a reported rate of >4000 s−1. This is followed by sequential electron transfers from the molybdenum center to the two Rieske clusters, where the Fe and S atoms are also reduced rapidly. The last step, and coincidentally the rate-limiting step, is the electron transfer from the [2Fe-2S] Rieske cluster in AioB to the final electron acceptor, cytochrome c.
Interestingly, comparison of different As(III)-oxidizing microorganisms revealed that some species not only possess unique ligands surrounding the redox active sites but also differ in which cellular membranes Aio translocates to [48]. For instance, the Aio of Alcaligenes faecalis, whose structure was the first to be completely determined, contains a canonical disulfide bridge anchoring the AioB Rieske cluster [26].
3.3. Microbial As(V) Reduction
Microorganisms can reduce As(V) through two different mechanisms: the first is through the activation of cytoplasmic As(V) reductases via the ars operons and the second is through dissimilatory As(V) reduction encoded in the arr gene system.
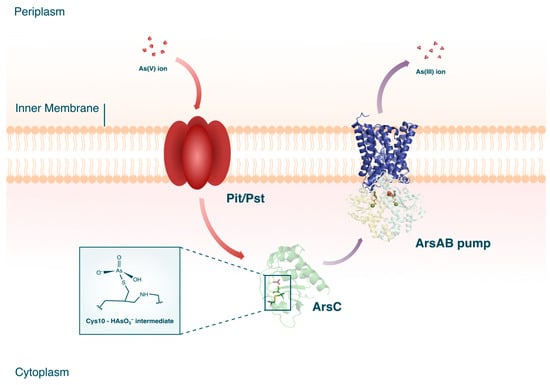
Cytoplasmic As(V) reductases are believed to be ubiquitous among microorganisms. Current knowledge on the ars genes separates the ArsC proteins into three different clades which, while evolutionarily divergent, all effect the same molecular function [49]. The biochemical cascade of cytoplasmic As(V) reduction begins with the intracellular uptake of As(V) through phosphate membrane systems (see Figure 3). Due to As(V) being a chemical analog of phosphorus, As(V) ions can enter the bacterial cell through the periplasmic binding proteins, phosphate inorganic transport (Pit), or phosphate-specific transport (Pst).

Figure 3. Cytoplasmic As(V) reduction in microorganisms. The crystal structures for ArsC [50], ArsA [51], and ArsB (PDB accession code AF_AFP45946F1) are shown. The crystal structures for ArsA and ArsB are combined in the figure to form the ArsAB efflux pump.
3.4. Microbial Arsenic Biomethylation
Biomethylation, also sometimes known as biovolatilization, is defined as the biological conversion of metals and metalloids to both volataile and nonvolatile methylated metabolites [52]. First observed in fungi, biomethylation plays an important role in microbial arsenic detoxification as well as in the biogeocycling of arsenic. The ability of microorganisms to both biomethylate and resist arsenic is conferred through the activation of the arsM gene [53]. Comparing orthologs of the arsM gene in different microorganisms revealed its prokaryotic origin, explaining its similarity to the ars operon genes used by bacteria [54].
4. Recently Isolated Arsenic Tolerant Microorganisms and Approaches Used in the Microbial Bioremediation of Arsenic
4.1. Bacteria
Numerous bacterial species are known to not only tolerate high concentrations of arsenic but also transform it from one ionic species to another. Since the ionic species of arsenic decides its toxicity as well as its mobility in the environment, bioremediation approaches usually rely on the biotransformation capability of microorganisms.
The oxidation of As(III) is of particular interest to bioremediation studies due to its potential application in large scale pre-treatment of arsenic-contaminated groundwater. In typical water treatment processes, As(V) can be easily removed through conventional physico-chemical techniques such as adsorption or ion exchange [55]. However, treating As(III) is considered more challenging due to its solubility and its low affinity for absorbents [56]. To remedy this, most operations rely on converting As(III) to As(V) through chemical pre-oxidation, which, by itself, is inherently inefficient due to the slow reaction rate and its tendency to form potentially toxic oxidation byproducts. Recent technologies are now shifting towards utilizing the inherent ability of some microorganisms to oxidize As(III) and coupling it with other arsenic removal strategies.
Some of the most effective microorganisms used in arsenic bioremediation, both in batch reactor experiments and in situ applications, are sulfate-reducing bacteria (SRB) [57]. Investigating the effect of inoculating Desulfovibrio vulgaris in waters to simulate arsenic-contaminated aquifers, researchers were able to observe the successful reduction of As(V) with and without sulfate amendment [58]. To probe the biotransformation of arsenic compounds, researchers screened for native arsenic-tolerant microorganisms in dam tailings from a gold mine from Iran [59].
Bacterial species belonging to the genus Bacillus are among the most robust microbes used in the treatment of arsenic in soils. In a study which screened for potential arsenic hyper-tolerant microorganisms from a gold mine in Brazil, a strain of Bacillus cereus was isolated and found to be resistant to up to 3000 ppm of As(III) in lab conditions [60]. Taking advantage of the arsenic resistance of the bacterial strain, its ability to bioaccumulate and oxidize As(III) in vitro was also assessed.
Perhaps one of the most remarkably arsenic-tolerant bacterial strains isolated so far is a strain of Bacillus firmus isolated from soil near the Lonar lake in India [61]. The strain, characterized as Bacillus firmus L-148, showed exceptional tolerance to exceedingly high concentrations of As, capable of thriving in concentrations of up to 247,241 ppm of As(III) and 299,686 ppm of As(V). The hyper-tolerant strain was also able to oxidize As(III) in the presence of other heavy metals and in alkaline conditions. While the latter is expected due to the fact that the lake where it is indigenous is naturally basic, the bacteria were capable of oxidizing As(III) even in buffered conditions.
4.2. Fungi
Compared to bacteria, fungi have the advantage of being deemed as the dominant living biomass present in soils [62]. The intimate association between fungi and soil is due to the low degree of shear strain experienced by soils, allowing the development of the fungal hyphal network [63]. Despite this, the potential of fungi in bioremediating arsenic in soils remains restricted. Nevertheless, recent studies have gradually started exploiting the various metabolic capabilities of these organisms against arsenic contamination.
Explorations into filamentous fungi such as Penicillum [64][65], Fusarium [66], Trichoderma [67], Humicola [68], and Aspergillus [65][66] showed notable resistance to high arsenic concentrations. Screening of As(V)-contaminated agricultural soil in India revealed that a strain of Penicillium coffeae can tolerate As(V) concentrations of up to 37,461 ppm in vitro [64]. The fungus was also able to tolerate the same concentration of As(V) under basic conditions, whether living, dead, or as treated biomass. However, the exact mechanism the fungus utilizes in tolerating As(V) remains unknown.
Among the numerous filamentous fungi used for arsenic bioremediation, the most used and studied is Aspergillus. Past research [69][70][71] has already attested to the efficacy of using indigenous Aspergillus sp. present in soils to mitigate arsenic contamination.
4.3. Microbial Consortium
A relatively unexplored approach to the bioremediation of arsenic is the identification and application of arsenic-resistant mixed microbial consortia. When using pure cultures, bioremediation efficiency may be limited by the difficulty of maintaining pure cultures, especially in in situ applications. Another important consideration is the ability of pure cultures to bioremediate complex contamination scenarios. Efficient bioremediation using mixed microbial cultures has consistently been observed for a myriad of pollutants, which has been attributed to the symbiotic and co-metabolic action between the different species in a specific consortium [72].
References
- Flora, S.J.S. Arsenic: Chemistry, Occurrence, and Exposure; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124199552.
- Reis, V.; Duarte, A.C. Occurrence, Distribution, and Significance of Arsenic Speciation, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 85, ISBN 9780444642646.
- Oremland, R.S.; Stolz, J.F. Arsenic, Microbes and Contaminated Aquifers. Trends Microbiol. 2005, 13, 40–49.
- RoyChowdhury, A.; Datta, R.; Sarkar, D. Heavy Metal Pollution and Remediation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128095492.
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944.
- Cordos, E.A.; Frentiu, T.; Ponta, M.; Marginean, I.; Abraham, B.; Roman, C. Distribution Study of Inorganic Arsenic (III) and (V) Species in Soil and Their Mobility in the Area of Baia-Mare, Romania. Chem. Speciat. Bioavailab. 2006, 18, 11–25.
- World Health Organization Preventing Disease through Healthy Environment. Exposure to Arsenic: A Major Public Health Concern; World Health Organization Preventing Disease through Healthy Environment: Geneva, Switzerland, 2019; pp. 1–5.
- International Agency for Research on Cancer. Arsenic and Arsenic Compounds; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100C.
- United States Environmental Protection Agency ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 8 April 2020).
- Polya, D.A. Arsenic in Groundwaters of South-East Asia: With Emphasis on Cambodia and Vietnam. Appl. Geochem. 2008, 23, 2968–2976.
- Kim, K.W.; Chanpiwat, P.; Hanh, H.T.; Phan, K.; Sthiannopkao, S. Arsenic Geochemistry of Groundwater in Southeast Asia. Front. Med. China 2011, 5, 420–433.
- McCarty, K.M.; Hanh, H.T.; Kim, K.W. Arsenic Geochemistry and Human Health in South East Asia. Rev. Environ. Health 2011, 26, 71–78.
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of Drinking Water by Arsenic in Bangladesh: A Public Health Emergency. Bulletin of the World Health Organization 78: Contamination of Drinking-Water by Arsenic in Bangladesh: A Public Health Emergency. World Health Organ. Bull. World Health Organ. 2000, 78, 1093–1103.
- Suda, A.; Baba, K.; Yamaguchi, N.; Akahane, I.; Suda, A.; Baba, K.; Yamaguchi, N.; Akahane, I. The Effects of Soil Amendments on Arsenic Concentrations in Soil Solutions after Long-Term Flooded Incubation. Soil Sci. Plant Nutr. 2015, 61, 592–602.
- Li, Y.; Bi, Y.; Mi, W.; Xie, S.; Ji, L. Land-Use Change Caused by Anthropogenic Activities Increase Fluoride and Arsenic Pollution in Groundwater and Human Health Risk. J. Hazard. Mater. 2021, 406, 124337.
- Yang, H.C.; Rosen, B.P. New Mechanisms of Bacterial Arsenic Resistance. Biomed. J. 2016, 39, 5–13.
- Fekih, I.B.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473.
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113, 7769–7792.
- Mateos, L.M.; Villadangos, A.F.; de la Rubia, A.G.; Mourenza, A.; Marcos-Pascual, L.; Letek, M.; Pedre, B.; Messens, J.; Gil, J.A. The Arsenic Detoxification System in Corynebacteria: Basis and Application for Bioremediation and Redox Control. Adv. Appl. Microbiol. 2017, 99, 103–137.
- Lièvremont, D.; Bertin, P.N.; Lett, M.C. Arsenic in Contaminated Waters: Biogeochemical Cycle, Microbial Metabolism and Biotreatment Processes. Biochimie 2009, 91, 1229–1237.
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.T.; Silver, S. Microbial Arsenic: From Geocycles to Genes and Enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325.
- Sforna, M.C.; Philippot, P.; Somogyi, A.; Van Zuilen, M.A.; Medjoubi, K.; Schoepp-Cothenet, B.; Nitschke, W.; Visscher, P.T. Evidence for Arsenic Metabolism and Cycling by Microorganisms 2.7 Billion Years Ago. Nat. Geosci. 2014, 7, 811–815.
- Van Lis, R.; Nitschke, W.; Duval, S.; Schoepp-Cothenet, B. Arsenics as Bioenergetic Substrates. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 176–188.
- Lebrun, E.; Brugna, M.; Baymann, F.; Muller, D.; Lièvremont, D.; Lett, M.C.; Nitschke, W. Arsenite Oxidase, an Ancient Bioenergetic Enzyme. Mol. Biol. Evol. 2003, 20, 686–693.
- Muller, D.; Lievremont, D.; Simeonova, D.D.; Hubert, J.-C.; Lett, M.-C. Arsenite Oxidase Aox Genes from a Metal-Resistant_B-Proteobacterium. J. Bacteriol. 2002, 185, 135–141.
- Ellis, P.J.; Conrads, T.; Hille, R.; Kuhn, P. Crystal Structure of the 100 KDa Arsenite Oxidase from Alcaligenes Faecalis in Two Crystal Forms at 1.64 Å and 2.03 Å. Structure 2001, 9, 125–132.
- Silver, S.; Phung, L.T. Genes and Enzymes Involved in Bacterial Oxidation and Reduction of Inorganic Arsenic. Appl. Environ. Microbiol. 2005, 71, 599–608.
- Li, H.; Li, M.; Huang, Y.; Rensing, C.; Wang, G. In Silico Analysis of Bacterial Arsenic Islands Reveals Remarkable Synteny and Functional Relatedness between Arsenate and Phosphate. Front. Microbiol. 2013, 4, 347.
- Slyemi, D.; Moinier, D.; Talla, E.; Bonnefoy, V. Organization and Regulation of the Arsenite Oxidase Operon of the Moderately Acidophilic and Facultative Chemoautotrophic Thiomonas Arsenitoxydans. Extremophiles 2013, 17, 911–920.
- Huang, Y.; Li, H.; Rensing, C.; Zhao, K.; Johnstone, L.; Wang, G. Genome Sequence of the Facultative Anaerobic Arsenite-Oxidizing and Nitrate-Reducing Bacterium Acidovorax sp. Strain NO1. J. Bacteriol. 2012, 194, 1635–1636.
- Chen, S.C.; Sun, G.X.; Yan, Y.; Konstantinidis, K.T.; Zhang, S.Y.; Deng, Y.; Li, X.M.; Cui, H.L.; Musat, F.; Popp, D.; et al. The Great Oxidation Event Expanded the Genetic Repertoire of Arsenic Metabolism and Cycling. Proc. Natl. Acad. Sci. USA 2020, 117, 10414–10421.
- Busenlehner, L.S.; Pennella, M.A.; Giedroc, D.P. The SmtB/ArsR Family of Metalloregulatory Transcriptional Repressors: Structural Insights into Prokaryotic Metal Resistance. FEMS Microbiol. Rev. 2003, 27, 131–143.
- Rosen, B.P.; Bhattacharjee, H.; Zhou, T.; Walmsley, A.R. Mechanism of the ArsA ATPase. Biochim. Biophys. Acta-Biomembr. 1999, 1461, 207–215.
- Meng, Y.L.; Liu, Z.; Rosen, B.P. As(III) and Sb(III) Uptake by GlpF and Efflux by ArsB in Escherichia Coli. J. Biol. Chem. 2004, 279, 18334–18341.
- Wu, J.; Rosen, B.P. The ArsD Gene Encodes a Second Trans-Acting Regulatory Protein of the Plasmid-Encoded Arsenical Resistance Operon. Mol. Microbiol. 1993, 8, 615–623.
- Lin, Y.F.; Yang, J.; Rosen, B.P. ArsD Residues Cys12, Cys13, and Cys18 Form an As(III)-Binding Site Required for Arsenic Metallochaperone Activity. J. Biol. Chem. 2007, 282, 16783–16791.
- Kaur, S.; Kamli, M.R.; Ali, A. Diversity of Arsenate Reductase Genes (Arsc Genes) from Arsenic-Resistant Environmental Isolates of E. Coli. Curr. Microbiol. 2009, 59, 288–294.
- Mukhopadhyay, R.; Rosen, B.P. Saccharomyces Cerevisiae ACR2 Gene Encodes an Arsenate Reductase. FEMS Microbiol. Lett. 1998, 168, 127–136.
- Duval, S.; Ducluzeau, A.L.; Nitschke, W.; Schoepp-Cothenet, B. Enzyme Phylogenies as Markers for the Oxidation State of the Environment: The Case of Respiratory Arsenate Reductase and Related Enzymes. BMC Evol. Biol. 2008, 8, 206.
- Szyttenholm, J.; Chaspoul, F.; Bauzan, M.; Ducluzeau, A.L.; Chehade, M.H.; Pierrel, F.; Denis, Y.; Nitschke, W.; Schoepp-Cothenet, B. The Controversy on the Ancestral Arsenite Oxidizing Enzyme; Deducing Evolutionary Histories with Phylogeny and Thermodynamics. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148252.
- Green, H.H. Description of a Bacterium Which Oxidizes Arsenite to Arsenate, and of One Which Reduces Arsenate to Arsenite, Isolated from a Cattle-Dipping Tank. S. Afr. J. Sci. 1918, 14, 465–467.
- Shi, K.; Wang, Q.; Wang, G. Microbial Oxidation of Arsenite: Regulation, Chemotaxis, Phosphate Metabolism and Energy Generation. Front. Microbiol. 2020, 11, 569282.
- Yin, S.; Zhang, X.; Yin, H.; Zhang, X. Current Knowledge on Molecular Mechanisms of Microorganism-Mediated Bioremediation for Arsenic Contamination: A Review. Microbiol. Res. 2022, 258, 126990.
- Warelow, T.P.; Oke, M.; Schoepp-Cothenet, B.; Dahl, J.U.; Bruselat, N.; Sivalingam, G.N.; Leimkühler, S.; Thalassinos, K.; Kappler, U.; Naismith, J.H.; et al. The Respiratory Arsenite Oxidase: Structure and the Role of Residues Surrounding the Rieske Cluster. PLoS ONE 2013, 8, e72535.
- Kalimuthu, P.; Heath, M.D.; Santini, J.M.; Kappler, U.; Bernhardt, P.V. Electrochemically Driven Catalysis of Rhizobium sp. NT-26 Arsenite Oxidase with Its Native Electron Acceptor Cytochrome C552. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 112–120.
- Dobbs, A.J.; Anderson, B.F.; Faber, H.R.; Baker, E.N. Three-Dimensional Structure of Cytochrome c from Two Alcaligenes Species and the Implications for Four-Helix Bundle Structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 1996, 52, 356–368.
- Watson, C.; Niks, D.; Hille, R.; Vieira, M.; Schoepp-Cothenet, B.; Marques, A.T.; Romão, M.J.; Santos-Silva, T.; Santini, J.M. Electron Transfer through Arsenite Oxidase: Insights into Rieske Interaction with Cytochrome C. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 865–872.
- Van Lis, R.; Nitschke, W.; Warelow, T.P.; Capowiez, L.; Santini, J.M.; Schoepp-Cothenet, B. Heterologously Expressed Arsenite Oxidase: A System to Study Biogenesis and Structure/Function Relationships of the Enzyme Family. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1701–1708.
- Mukhopadhyay, R.; Rosen, B.P. Arsenate Reductases in Prokaryotes and Eukaryotes. Environ. Health Perspect. 2002, 110, 745–748.
- Zegers, I.; Martins, J.C.; Willem, R.; Wyns, L.; Messens, J. Arsenate Reductase from S. Aureus Plasmid PI258 Is a Phosphatase Drafted for Redox Duty. Nat. Struct. Biol. 2001, 8, 843–847.
- Zhou, T.; Radaev, S.; Rosen, B.P.; Gatti, D.L. Structure of the ArsA ATPase: The Catalytic Subunit of a Heavy Metal Resistance Pump. EMBO J. 2000, 19, 4838–4845.
- Bentley, R.; Chasteen, T.G. Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth. Microbiol. Mol. Biol. Rev. 2002, 66, 250–271.
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.G. Arsenic Biomethylation by Photosynthetic Organisms. Trends Plant Sci. 2012, 17, 155–162.
- Chen, S.C.; Sun, G.X.; Rosen, B.P.; Zhang, S.Y.; Deng, Y.; Zhu, B.K.; Rensing, C.; Zhu, Y.G. Recurrent Horizontal Transfer of Arsenite Methyltransferase Genes Facilitated Adaptation of Life to Arsenic. Sci. Rep. 2017, 7, 7741.
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406.
- ALSamman, M.T.; Sotelo, S.; Sánchez, J.; Rivas, B.L. Arsenic Oxidation and Its Subsequent Removal from Water: An Overview. Sep. Purif. Technol. 2023, 309, 123055.
- Alam, R.; McPhedran, K. Applications of Biological Sulfate Reduction for Remediation of Arsenic—A Review. Chemosphere 2019, 222, 932–944.
- Liu, E.; Yang, Y.; Xie, Z.; Wang, J.; Chen, M. Influence of Sulfate Reduction on Arsenic Migration and Transformation in Groundwater Environment. Water 2022, 14, 942.
- Parsania, S.; Mohammadi, P.; Soudi, M.R. Biotransformation and Removal of Arsenic Oxyanions by Alishewanella Agri PMS5 in Biofilm and Planktonic States. Chemosphere 2021, 284, 131336.
- Aguilar, N.C.; Faria, M.C.S.; Pedron, T.; Batista, B.L.; Mesquita, J.P.; Bomfeti, C.A.; Rodrigues, J.L. Isolation and Characterization of Bacteria from a Brazilian Gold Mining Area with a Capacity of Arsenic Bioaccumulation. Chemosphere 2020, 240, 124871.
- Bagade, A.; Nandre, V.; Paul, D.; Patil, Y.; Sharma, N.; Giri, A.; Kodam, K. Characterisation of Hyper Tolerant Bacillus Firmus L-148 for Arsenic Oxidation. Environ. Pollut. 2020, 261, 114124.
- Hoshino, Y.T.; Morimoto, S. Comparison of 18S RDNA Primers for Estimating Fungal Diversity in Agricultural Soils Using Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis. Soil Sci. Plant Nutr. 2008, 54, 701–710.
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped Potential: Exploiting Fungi in Bioremediation of Hazardous Chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192.
- Bhargavi, S.D.; Savitha, J. Arsenate Resistant Penicillium Coffeae: A Potential Fungus for Soil Bioremediation. Bull. Environ. Contam. Toxicol. 2014, 92, 369–373.
- Soares Guimarães, L.H.; Segura, F.R.; Tonani, L.; von-Zeska-Kress, M.R.; Rodrigues, J.L.; Calixto, L.A.; Silva, F.F.; Batista, B.L. Arsenic Volatilization by Aspergillus sp. and Penicillium sp. Isolated from Rice Rhizosphere as a Promising Eco-Safe Tool for Arsenic Mitigation. J. Environ. Manage. 2019, 237, 170–179.
- Singh, M.; Srivastava, P.K.; Verma, P.C.; Kharwar, R.N.; Singh, N.; Tripathi, R.D. Soil Fungi for Mycoremediation of Arsenic Pollution in Agriculture Soils. J. Appl. Microbiol. 2015, 119, 1278–1290.
- Govarthanan, M.; Mythili, R.; Kamala-Kannan, S.; Selvankumar, T.; Srinivasan, P.; Kim, H. In-Vitro Bio-Mineralization of Arsenic and Lead from Aqueous Solution and Soil by Wood Rot Fungus, Trichoderma sp. Ecotoxicol. Environ. Saf. 2019, 174, 699–705.
- Tripathi, P.; Khare, P.; Barnawal, D.; Shanker, K.; Srivastava, P.K.; Tripathi, R.D.; Kalra, A. Bioremediation of Arsenic by Soil Methylating Fungi: Role of Humicola sp. Strain 2WS1 in Amelioration of Arsenic Phytotoxicity in Bacopa monnieri L. Sci. Total Environ. 2020, 716, 136758.
- Mukherjee, A.; Das, D.; Kumar Mondal, S.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of Arsenate-Induced Stress in Aspergillus Niger, a Possible Candidate for Bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182.
- Čerňanský, S.; Kolenčík, M.; Ševc, J.; Urík, M.; Hiller, E. Fungal Volatilization of Trivalent and Pentavalent Arsenic under Laboratory Conditions. Bioresour. Technol. 2009, 100, 1037–1040.
- Choe, S.I.; Sheppard, D.C. Bioremediation of Arsenic Using an Aspergillus System; Elsevier, B.V.: Amsterdam, The Netherlands, 2016; ISBN 9780444635136.
- Li, X.; Feng, C.; Lei, M.; Luo, K.; Wang, L.; Liu, R.; Li, Y.; Hu, Y. Bioremediation of Organic/Heavy Metal Contaminants by Mixed Cultures of Microorganisms: A Review. Open Chem. 2022, 20, 793–807.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
894
Revisions:
2 times
(View History)
Update Date:
03 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




