Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Donna Oldbury-Thomas | -- | 3639 | 2024-01-02 13:22:58 | | | |
| 2 | Lindsay Dong | -3 word(s) | 3636 | 2024-01-03 02:16:45 | | | | |
| 3 | Lindsay Dong | -9 word(s) | 3627 | 2024-01-12 09:10:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Davys, D.; Rayns, F.; Charlesworth, S.; Lillywhite, R. Different Biochar Characteristics on Soil Nitrogen Transformation Processes. Encyclopedia. Available online: https://encyclopedia.pub/entry/53322 (accessed on 16 January 2026).
Davys D, Rayns F, Charlesworth S, Lillywhite R. Different Biochar Characteristics on Soil Nitrogen Transformation Processes. Encyclopedia. Available at: https://encyclopedia.pub/entry/53322. Accessed January 16, 2026.
Davys, Donna, Francis Rayns, Susanne Charlesworth, Robert Lillywhite. "Different Biochar Characteristics on Soil Nitrogen Transformation Processes" Encyclopedia, https://encyclopedia.pub/entry/53322 (accessed January 16, 2026).
Davys, D., Rayns, F., Charlesworth, S., & Lillywhite, R. (2024, January 02). Different Biochar Characteristics on Soil Nitrogen Transformation Processes. In Encyclopedia. https://encyclopedia.pub/entry/53322
Davys, Donna, et al. "Different Biochar Characteristics on Soil Nitrogen Transformation Processes." Encyclopedia. Web. 02 January, 2024.
Copy Citation
Biochar, a carbonaceous product, is formed from organic feedstock pyrolysised in the absence of air and, therefore, is a potential means of recycling organic waste. However, different feedstock and pyrolysis conditions result in a biochar with a range of altered characteristics. These characteristics influence nitrogen transformation processes in soil and result in the metabolism of different substrates and the formation of different products, which have different effects on agricultural yield.
biochar
nitrogen fixation
mineralisation
denitrification
soil amendment
1. Introduction
Every year, 2 billion tonnes of solid waste are generated globally [1], 30 billion tonnes of carbon dioxide (CO2) [2] are emitted and 12 million hectares of agricultural land are lost to soil degradation, leading to a potential loss of 20 million tonnes of grain per annum [3]. This, as well as an ever-growing food and energy requirement as tastes shift towards a more technologically driven lifestyle, means that further stress is placed on Earth’s already depleted and increasingly polluted resources [4][5]. Hence, there is now a focus on technologies that can reuse waste, reduce emissions, mitigate climate change, generate energy and support food production. One such technology is pyrolysis and the production of biochar. Pyrolysis generally refers to a reaction in an inert environment [6].
Since 1993, interest in biochar has focused on whether it can be used to store carbon in soil whilst improving soil properties for improved crop production [7][8]. Carbon storage in soils is seen as a means to mitigate greenhouse gas (GHG) emissions through the sequestration of CO2. Woolf et al. [9] analysed what level of carbon sequestration, through the production of dedicated biomass for biochar, might be practical to mitigate climate change, without compromising food security, habitat or soil conservation. They estimated that a maximum of 1.8 Pg CO2-C equivalent (CO2-Ce) per year (or 12% of current anthropogenic emissions) might be achievable, although estimates range from 0.7 to 1.8 Gt CO2 eq y−1 [10].
Biochar improves the fertility of some soils through the direct provision of nutrients such as nitrogen, phosphorus and potassium. Biochar can also have a liming effect, influencing pH such that nutrients become more available [11][12]. Most biochars have a high pH (when compared to soil), which results from their ash content and base cations, but also due to intrinsic alkaline organic functional groups [13]. However, these values are governed by both feedstock and pyrolysis conditions. For instance, one study found that the pH of herbaceous biochars was two units higher (9.4) than woody biochars (7.4) due to higher concentrations of ash [14]. pH was also found to be higher in biochars from leguminous feedstock (9.02 to 10.35) than in non-leguminous feedstock (8.00 to 9.24) [15].
Hence, these differences in biochar qualities result in different effects on crop yield. For instance, corncob biochar on infertile acidic soil reduced maize yield, which may have resulted from the biochar’s high volatile matter content and bioavailable carbon. This labile carbon fuelled an increase in micro-organism growth, drawing nutrients from the soil and resulting in nitrogen immobilisation [16]. The availability of nitrogen for crop growth is a key agronomic parameter. Nitrogen is an essential plant nutrient and a key element in the development of organic molecules including amino acids, amino sugars, proteins, deoxynucleic acid (DNA), chlorophyll, etc. Nitrogen is very reactive and therefore has a very complex cycle through the soil system, sometimes transforming quickly between inorganic forms as gasses such as NH3, N2, N2O, and NO, in ionic form in soil, NH4+, NO2− and NO3−, and organic forms. Soils vary considerably in their total nitrogen concentrations from around 0.06% to 0.30%, and more than 90% of this is in organic form [17].
Biochar has been reported to reduce nitrogen leaching losses [18]. However, these ameliorative effects can vary depending on the carbon content of the amended soil, the biochar’s carbon content, soil textural differences, soil water-holding capacity and the soil micro-organism community amongst other parameters [19][20][21][22][23].
However, biochar’s influence on the native micro-organism community can exacerbate nitrogen losses from soil systems, depleting nitrogen from soil but also increasing pollutant release. For example, whilst several studies have reported that the incorporation of biochar into different soils reduced N2O emissions [24][25][26][27][28], other studies have reported that biochar incorporation results in an increase in N2O emissions [29][30]. However, the magnitude of change in N2O emission because of biochar incorporation is dependent upon experimental conditions, biochar type, application rate, soil properties, and chemical forms of added fertilizer [27].
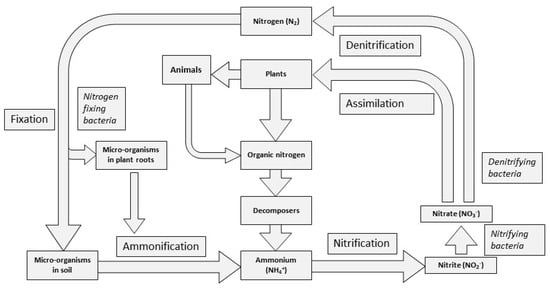
2. Phases of the Nitrogen Cycle
The soil system is open to the atmosphere and nitrogen cycles between them. The first input of nitrogen from the atmosphere is either through lightening, deposition or biological nitrogen fixation (BNF) (Figure 1). This is an important phase of the nitrogen cycle, capturing N2 and transforming it to NH4+. This process fixes a considerable level of nitrogen year on year. For instance, prior to human intervention, BNF is estimated to have provided 58 Tg N yr−1 worldwide, and it still plays a critical role in maintaining soil fertility [31]. It uses the soil’s natural resources (native micro-organisms) and is, therefore, a free and potentially ecologically sound means of crop fertilisation.

Figure 1. Simplified nitrogen cycle.
BNF is carried out by several groups of prokaryotes, including Azotobacter, Azospirillum, Rhizobium and Bradyrhizobium [32]. These are either free living in the soil or form symbiotic relationships with plants (around or on the root surface) and both utilise enzymatic processes to catalyse the conversion of N2 into NH3. This conversion to ammonia (or ammonification) is then followed by a process of nitrification to nitrites and nitrates. This process is undertaken by both heterotrophic and autotrophic micro-organisms with the bacterium, such as Nitrosomonas, converting NH3 into NO2−, and Nitrobacter converting NO2− to NO3− [32]. It is in these forms that plants and micro-organisms can assimilate NH3 and NO3− into their structures and the nitrogen then exists in organic form. Upon their death, or through excreta, these forms are then mineralised back again into inorganic form.
They are mineralised by micro-organisms that derive energy from the oxidation of organic nitrogen to NH4+. This is then available to be assimilated and incorporated into amino acids or used for other metabolic purposes. If micro-organisms produce NH4+ in excess of their own requirements, the surplus is excreted into, in this case, the soil, and is available for assimilation by plants, or as the substrate for nitrification [33].
Finally, denitrification releases the N2 originally acquired from the atmosphere by nitrogen-fixing bacteria. Again, it is a process facilitated by micro-organisms where NO3− is reduced through a series of nitrogen oxide products of decreasing oxidation states until molecular nitrogen (N2) is all that remains. The process is of particular interest to climate change researchers as some of the intermediate products, nitric oxide and N2O, are GHGs [34].
3. The Influence of Key Biochar Parameters on the Nitrogen Cycle
3.1. The Influence of Recalcitrant and Metabolisable Carbon
Critical to biochar’s ability to store carbon in soil are its aromatic rings, which are highly stable. Aromatic rings include six carbon structures such as benzene, which have strong chemical bonds very resistant to attack by micro-organisms [35]. It is this resistance that confers recalcitrance to biochar stored in soil and, hence, its role as a climate change mitigation tool. However, biochar contains other carbon compounds, such as aliphatic carbon chains. These structures have weaker chemical bonds easily broken by micro-organism enzymes and are thus used as an energy source for respiration and therefore result in the ultimate release of CO2. Hence, although biochar has a carbon content that can range from 50 to 95%, some of this is metabolically available and will therefore influence micro-organism growth in the short term [36][37]. The amount of metabolically available carbon depends on the biochar feedstock and pyrolysis condition.
In general, the effect of biochar on soil micro-organism mass (and therefore soil respiration) is not well understood [38]. For example, some studies have found a 30% degradation of carbon in the first 30 years and very little thereafter (over a 100-year timeframe) [39], whereas others estimated a loss of 47% C within a 50-year timeframe [40]. Confounding factors include whether the response is dictated by the metabolisable fraction of the biochar or whether the native micro-organisms have the necessary enzymes to mineralise that available carbon [41][42].
There are potentially negative agronomic consequences resulting from the provision of metabolisable carbon. Although biochar may improve nutrient assimilation by micro-organisms, there may be a consequent decrease in assimilation by crop plants. For instance, biochars with a high aliphatic concentration are more easily degraded by soil organisms, causing an increase in their growth rates and consequent short-term assimilation and immobilisation of nitrogen, impeding supply to crop plants and reducing yield [16][43].
Conversely, biochar also stimulates the growth of micro-organisms able to mineralise recalcitrant nitrogen found in soil organic matter [44]. For instance, one N15 isotope tracing study using maize biochar found that nitrogen mineralised to NH4+ came from soil organic matter, which was otherwise generally resistant to micro-organism attack [25]. They hypothesised that this was due mainly to improved metabolisable carbon availability supplied by biochar pyrolysised at a low temperature. The subsequent elevated growth in micro-organism population could not be met with available nitrogen sources in the soil resulting in the mineralisation of recalcitrant soil organic matter [25][45][46].
A key phase within nitrogen transformations is denitrification, because, if incomplete, it can lead to the emission of N2O. In their work with wood and poultry manure biochars, Singh et al. [20] found that, generally, biochar-amended soils produced significantly lower N2O emissions than their respective controls but its efficacy in this regard was soil-dependent. They subjected two contrasting soils with different biochar treatment rates to wet–dry cycles. By the third cycle, they found that all biochar treatments consistently decreased N2O emissions, cumulatively by 14 to 73% from the Alfisol and by 23 to 52% from the Vertisol, relative to their controls. However, some biochars produce higher N2O emissions [20][27][47].
Biochar influences the cycles of carbon already in the soil, most notably organic matter and humus. Organic matter plays a vital role in the functioning of a healthy soil. Derived from the biomass of both plants and animals (for example, roots and manure), organic matter has a high CEC, helps retain water in the soil and provides soil structure. Organic matter decays over time, releasing its nutrients. When the level of decay slows significantly or stops, and the organic matter becomes stable, it is referred to as humus. Humic acids comprise a large group of chemicals, which perform a vital role in soil health contributing to soil moisture and nutrient retention, as well as the bulk density of soil [48]. There is now some evidence to suggest that biochar serves to protect humic acids from decomposition [49].
3.2. The Influence of Mineral Ions, Provision, Availability and Uptake
Biochar offers the opportunity to improve soil fertility through the direct provision of mineral ions. The quantity and type of minerals depend on pyrolysis conditions and feedstock. Hardwood biochars tend to have lower mineral contents (5–10%), whereas chicken litter waste can have an ash content of up to 64% [50]. The presence of mineral ions can have a significant effect on fixation rates. For instance, Rondon et al. [28] found that biochar significantly enhanced biological fixation by Phaseolus vulgaris and accounted for the increase in fixation rate through the increased availability of molybdenum and boron provided by biochar. They noted that the improvement in BNF, as well as the increase in biomass, of Phaseolus vulgaris (5–39%) was above those normally provided by recommended commercial fertiliser applications. Similarly, in Glycine max L., the enhancement of nodulation and BNF response to carbonized organic materials was due to an increase in available sulphur [51].
Even low-nutrient biochar has the potential to elevate nutrient availability. In their greenhouse experiment with seven different biochars, [52] stripped the biochars of mineral or volatile matter, or both, and left some untreated. The untreated biochar soil treatment planted with Phaseolus vulgaris resulted in an increase of 2126% in nitrogen fixation over the control average (as well as a 262% increase in shoot biomass, a 164% increase in root biomass and a 3575% increase in nodule biomass). The stripped biochar revealed that simple mineral nutrients provided by the biochar were only slightly responsible for these increases. For instance, although the amount of nitrogen fixed was significantly correlated with plant phosphorus uptake, it was not correlated with biochar phosphorus addition but rather improved phosphorus nutrition resulting from 360% greater mycorrhizal colonization with biochar additions.
Mycorrhizal root colonisation and hyphal responses to different biochars can vary substantially [53]. This may be due to different responses to a range of volatile matter compounds from contrasting biochars with mycorrhizal response being governed by carbohydrate availability or due to biochar providing physical protection from fungal grazers or the facilitation of root and hyphal exploration, facilitating improved access to nutrients for crop plants [52][53][54].
The provision of nutrients by biochar influences several different assimilation mechanisms. For instance, where biochar provides an increased cation concentration, plant water uptake increases due to the net increase in accumulated osmotically active ions such as potassium. This is key because it improves drought tolerance [55]. In addition, biochar appears to increase tap-root growth (and potentially fine root mass), which would increase water uptake from biochar pores [56]. Evidence for this improved water status with biochar has been demonstrated through lower proline (an amino acid associated with cell osmotic adjustment in leaves) concentrations and higher osmotic values in leaves, which may reflect an increased tolerance to drought conditions [57].
3.3. The Influence of Porosity on Soil Structure, Water and Gas Dynamics
Due to its porous nature, biochar can significantly increase gas transport in soil as well as a soil’s water-holding capacity (WHC) [58][59][60][61]. This is because pyrolysis results in an interconnected network of micropores, mesopores and macropores [41]. The distribution of pore sizes is governed by feedstock, with wood feedstock developing larger pores [59], and by pyrolysis conditions, with those biochars produced at high temperatures via a slow process more likely to produce more macropores (that is, greater than 50 μm in diameter) [59][62].
Biochar has been reported to improve crop yield through its effect on soil structure [63]. In some cases, biochar addition increased aggregate stability and reduced the detachment of colloidal material, improving soil structure [64]. However, in coarser soils, there was no such enhancement [65]. Where improvements were found, this may have been due to mechanisms such as carboxylic and phenolic functional groups on aged biochar surfaces, which form attachments with soil mineral surfaces. Also, a high CEC allows for cation bridge formation contributing to structural stabilisation [43].
Differences in biochar porosity resulting from different feedstocks have a direct influence on micro-organism population. This is because the adhesion of bacteria to biochar may be influenced by pore size [66]. Bacillus mucilaginosus and Acinetobacter sp. need a pore size of 2–4 μm if they are to enter [67]. In pores, they are better protected from dehydration and grazers and competitors. Surface tension holds water in the biochar, but it does so preferentially, with smaller pores exerting a greater holding capacity than larger ones.
3.4. The Sorption of Mineral Ions, Signalling Compounds, Heavy Metals and Organic Pollutants
Some biochars can be effective in adsorbing NH4+ and NO3− from the soil [68]. This apparent disadvantage, however, may lead to an increase in the ability of the soil ecosystem to feed plants as this reduced nitrogen availability to plant roots stimulates increased nodulation in legumes [49][69]. Root nodulation can influence the rate of biological fixation, and both the nodulation rate and development, as well as nitrogenase activity, can be affected by the presence of biochar.
Even though biochar can influence the structure of a micro-organism community to promote one nitrogen process or another, its sorption powers can often confound the result. In an experiment comparing biochar alone and biochar that had been shaken with dairy effluent for 24 h, both biochar treatments reduced net ammonification by 220% compared with soil alone. This suggested that the rate of nitrification was higher than the rate of ammonification. However, it appears that these rates were not changed in response to an increase in nitrifiers because CO2 emissions did not rise. Hence, it was postulated that the reduction in NH4+ was more likely due to its adsorption to biochar rather than immobilisation [70].
Again, biochar may influence the denitrification process by limiting micro-organism access to substrates. For instance, in one study, an acidic biochar absorbed NH4+, not only reducing the NH4+ leaching rate but also decreasing the NH3 volatilisation rate due to a reduction in substrate for the denitrification process [71]. However, the decrease in volatilisation rate was driven mainly by the acid–base reaction. Findings from another study concurred, concluding not only that the effectiveness of biochar to reduce N2O emission was, in part, dependent on the sorption of NH4+, decreasing the overall availability of nitrogen to denitrifiers, but that this ability changed over time [20]. With ageing, the effectiveness of biochar to reduce N2O emission (and NH4+ leaching) increased, as biochar surfaces become increasingly oxidised due to biotic and abiotic processes, potentially leading to an increase in cation exchange capacity [23], which may explain the reduction in available nitrogen [20].
The mechanism of root nodule formation and BNF in leguminous plants requires infection by nodule-forming bacteria. This process is governed by chemotaxis involving signalling pathways, which are initiated by polyphenolic signalling compounds (for example, flavonoids) released by the host plant [72][73][74][75][76][77]. Biochar is highly effective at adsorbing signalling compounds so any incorporation of biochar into soil, certainly at higher rates, may interfere with these signalling pathways, potentially interrupting nodule development and therefore nitrogen fixation [72][74][75][76][77].
3.5. The Influence of pH, Cation Exchange Capacity and Electron Shuttle Services
The pH of soil is critical because it affects plant nutrient availability by controlling the chemical forms of various nutrients and influencing the chemical reactions they undergo. For instance, phosphorus, molybdenum and calcium become increasingly unavailable with decreasing pH, with a corresponding decrease in crop productivity [78]. Biochar influences pH because it is generally alkaline due to its ash content and release of base cations, but also due to intrinsic alkaline organic functional groups [12]. However, the pH of a biochar is governed by both feedstock and pyrolysis conditions. Streubel et al. [13] found that the pH of herbaceous biochars was two units higher (9.4) than woody biochars (7.4) due to higher concentrations of ash in their study on contrasting biochar types (all pyrolysed at 350 °C).
As pH influences the chemical form and availability of substrates, it can affect change in both ammonia-oxidising archaea (AOA) and ammonia-oxidising bacteria (AOB) communities, thereby affecting mineralisation rates [79][80]. Accordingly, the addition of biochar has been shown to increase fixation rates, albeit to a lesser extent, and this capability appears to diminish over time [28][52].
pH, as amended by biochar, may have a greater influence on mineralisation rates, but the results are highly inconsistent and will depend on the existing pH levels of the amended soil. For instance, autotrophic nitrification generally occurs in neutral and alkaline soils because a critical enzyme, ammonia monooxygenase, uses NH3 as a substrate rather than NH4+ with the balance affected by pH, with a higher pH favouring NH3 [81]. Hence, although in an already alkaline soil, the addition of biochar resulted in a decrease in the number of nitrifiers [82]. However, an amended-acid soil resulted in a significant increase in the abundance of AOB correlating with an increased pH resulting from wheat biochar application [38].
3.6. The Influence of Inhibitory Substances
After pyrolysis of biomass, compounds toxic to micro-organisms may be present including polyaromatic hydrocarbons (PAHs) [83][84][85]. The presence of inhibitory substances due to biochar addition is of particular concern as once added to soil, it is almost impossible to remove, therefore any negative environmental consequences may be long-lasting. For instance, Anderson et al. [45] found that applying biochar to a silt-loam soil decreased the abundance of Nitrosovibrio—an AOB. As this is a rate-limiting step for nitrification, rates fell, which may be due to the introduction of inhibitory substances [30]. Wang et al. also found that phenolic compounds, which are retained by biochars, especially at low-pyrolysis temperatures, may inhibit microbial activity [86].

4. Summary of the Influence of Biochar on the Nitrogen Cycle
To conclude the above discussion, as the nitrogen cycle is governed by the activities of micro-organisms and biochar influences both micro-organism action and community composition, as well as other physical and chemical processes in the soil, it has a profound influence on fixation, assimilation, mineralisation and denitrification.
The main influences on fixation are through the promotion of mycorrhizal root colonisation due to the provision of carbohydrates that form part of the metabolisable fraction of carbon supplied by biochar or by providing physical protection from fungal grazers [52][53][54]. Biochar sorption capabilities may be hugely influential as they sorb nitrogen, reducing its availability in soil, thereby promoting nodulation [87]. It sorbs pollutants detrimental to micro-organism growth including fixers [72][88], but can also interfere with signal pathways, potentially interrupting nodule development [74][76]. Biochar can alter soil pH such that soil fixers benefit, although this process may diminish over time [89]. The provision of a metabolisable form of carbon, as well as recalcitrant forms, has been found to increase nitrogen-fixing organisms [28][52]. Figure 2 gives a summary of the substrates and products of fixation and how biochar influences the chemical and biological processes that lead to the products.

Figure 2. Potential mechanisms of biochar influence on fixation of atmospheric N2 and production of inorganic nitrogen (arrows denote change in product formation rate).
Biochar influences the assimilation of nitrogen into micro-organisms and plants via several mechanisms. Firstly, it can reduce leaching, keeping nitrogen in the rhizosphere and available for uptake. It can do this through the adsorption of NH4− or organic nitrogen onto biochar, intercalation or cation or anion exchange reactions [90]. Secondly, it improves soil water-holding capacity in some soils, which aids root and hyphal elongation and nutrient capture [53][89] even under conditions of high water evaporation stress [61].
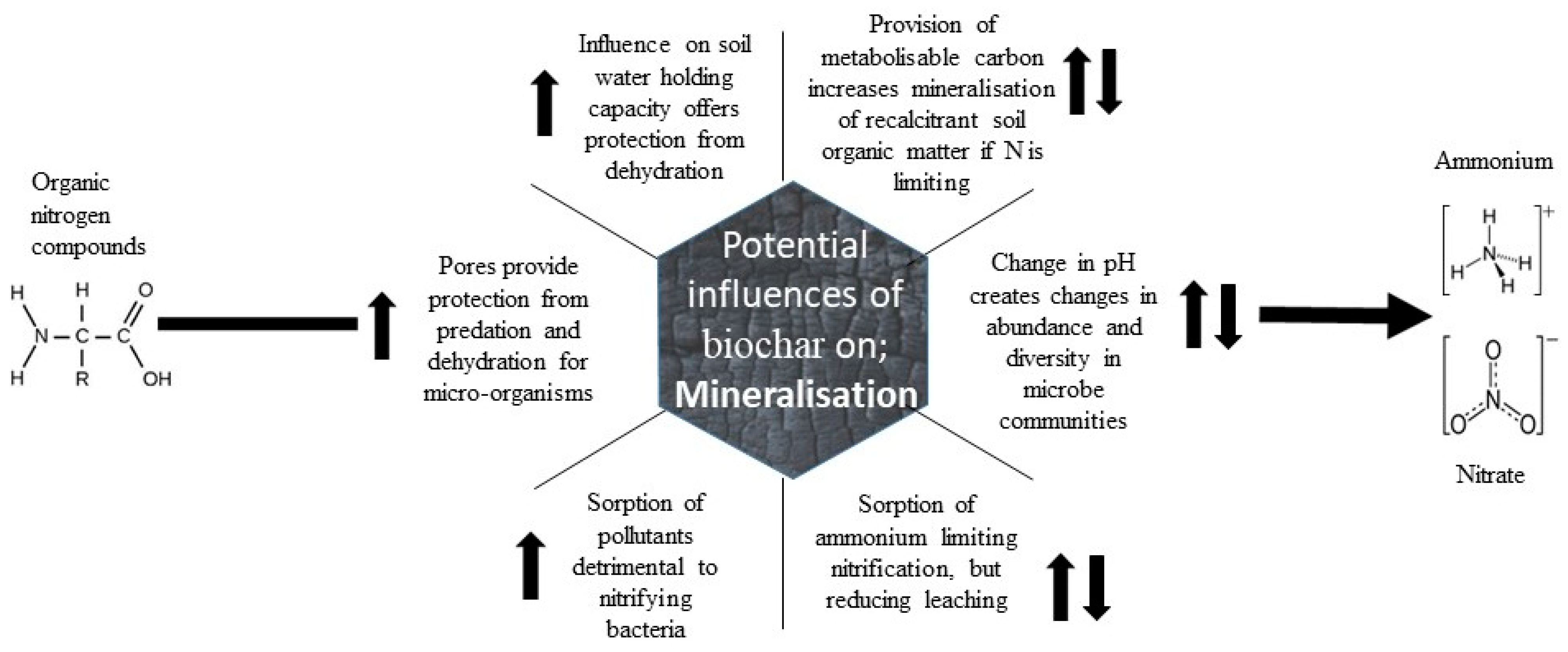
Much research has been conducted on the influence of biochar on mineralisation rates and responses vary with soil type. However, the main mechanisms of influence include the provision of a metabolisable carbon, which influences micro-organism growth (Figure 3).

Figure 3. Potential mechanisms of the influence of biochar on mineralisation of organic nitrogen to non-organic forms (arrows denote change in product formation rate).
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development Series; World Bank: Washington, DC, USA, 2018.
- IEA. Global Energy Review 2020; IEA: Paris, France, 2020.
- Rickson, R.J.; Deeks, L.K.; Graves, A.; Harris, J.A.H.; Kibblewhite, M.G.; Sakrabani, R. Input constraints to food production: The impact of soil degradation. Food Secur. 2015, 7, 351–364.
- Dekker, S.C.; Kraneveld, A.D.; van Dijk, J.; Kalfagianni, A.; Knulst, A.C.; Lelieveldt, H.; Moors, E.H.M.; Müller, E.; Pieters, R.H.H.; Pieterse, C.M.J.; et al. Towards Healthy Planet Diets—A Transdisciplinary Approach to Food Sustainability Challenges. Challenges 2020, 11, 21.
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of the global food system. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2769–2777.
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology (the Gold Book), 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997.
- Seifritz, W. Should we store carbon in charcoal? Int. J. Hydrogen Energy 1993, 18, 405–407.
- Sombroek, W.G.; Nachtergaele, F.O.; Hebel, A. Amounts, dynamics and sequestering of carbon in tropical and subtropical soils. Ambio 1993, 22, 417–426.
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56.
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55.
- Tan, S.; Zhou, G.; Yang, Q.; Ge, S.; Liu, J.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Kong, S.H.; Chang, J.-S.; et al. Utilization of current pyrolysis technology to convert biomass and manure waste into biochar for soil remediation: A review. Sci. Total Environ. 2023, 864, 160990.
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of Soil pH Increase by Biochar on NO, N2O and N2 Production during Denitrification in Acid Soils. PLoS ONE 2015, 10, e0138781.
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413.
- Yuan, J.; Xu, R. Effects of biochars generated from crop residues on chemical properties of acid soils from tropical and subtropical China. Soil Res. 2012, 50, 570–578.
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357.
- Deenik, J.L.; Cooney, M.J. The Potential Benefits and Limitations of Corn Cob and Sewage Sludge Biochars in an Infertile Oxisol. Sustainability 2016, 8, 131.
- Kelley, K.R.; Stevenson, F.J. ;Characterization and extract ability of immobilized 15N from the soil microbial biomass. Soil Biol. Biochem. 1985, 17, 517–523.
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94.
- Bateman, E.; Baggs, E.M. Contributions of nitrification and denitrifcation to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils Coop. J. Int. Soc. Soil Sci. 2005, 41, 379–388.
- Singh, B.; Singh, B.P.; Cowie, A. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 2010, 48, 516.
- Kimetu, J.; Lehmann, J. Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Soil Res. 2010, 48, 577–585.
- Yeboah, E.; Ofori, P.; Quansah, G.W.; Dugan, E.; Sohi, S.P. Improving soil productivity through biochar amendments to soils. Afr. J. Environ. Sci. Technol. 2009, 3, 34.
- Cheng, C.; Lehmann, J. Ageing of black carbon along a temperature gradient. Chemosphere 2009, 75, 1021–1027.
- Ameloot, N.; Maenhout, P.; De Neve, S.; Sleutel, S. Biochar-induced N2O emission reductions afer field incorporation in a loam soil. Geoderma 2016, 267, 10–16.
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27.
- Rittl, T.F.; Oliveira, D.M.S.; Canisares, L.P.; Sagrilo, E.; Butterbach-Bahl, K.; Dannenmann, M.; Cerri, C.E.P. High Application Rates of Biochar to Mitigate N2O Emissions from a N-Fertilized Tropical Soil under Warming Conditions. Front. Environ. Sci. 2021, 8, 1.
- Lee, S.-I.; Park, H.-J.; Jeong, Y.-J.; Seo, B.-S.; Kwak, J.-H.; Yang, H.I.; Xu, X.; Tang, S.; Cheng, W.; Lim, S.-S.; et al. Biochar-induced reduction of N2O emission from East Asian soils under aerobic conditions: Review and data analysis. Environ. Pollut. 2021, 291, 118154.
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699.
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235.
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle. J. Environ. Qual. 2010, 39, 1218–1223.
- Vitousek, P.M.; Menge, D.N.; Reed, S.C.; Cleveland, C.C. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 1621.
- Canali, S.; Di Bartolomeo, E.; Tittarelli, F.; Montemurro, F.; Verrastro, V.; Ferri, D. Comparison of different laboratory incubation procedures to evaluate nitrogen mineralization in soils amended with aerobic and anaerobic stabilized organic materials. J. Food Agric. Environ. 2011, 9, 540–546.
- Strock, J.S. Ammonification. In Encyclopedia of Ecology; Elsevier B.V.: Amsterdam, The Netherlands, 2008; pp. 162–165.
- Crutzen, P.J. The influence of nitrogen oxides on the atmospheric ozone content. QJR Meteorol. Soc. 1970, 96, 320–325.
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial biochar systems for atmospheric carbon removal: A review. Environ. Chem. Lett. 2021, 19, 3023.
- Thies, J.; Rillig, M. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: Oxford, UK, 2009; p. 85.
- Shackley, S.; Sohi, S.; Brownsort, P.; Carter, S.; Cook, J.; Cunningham, C.; Gaunt, J.; Hammond, J.; Ibarrola, R.; Mašek, O.; et al. An Assessment of the Benefits and Issues Associated with the Application of Biochar to Soil; Department for Environment, Food and Rural Affairs: London, UK, 2010.
- Zhang, Q.-Z.; Dijkstra, F.A.; Liu, X.-R.; Wang, Y.-D.; Huang, J.; Lu, N. Effects of Biochar on Soil Microbial Biomass after Four Years of Consecutive Application in the North China Plain. PLoS ONE 2014, 9, e102062.
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2009, 92, 163–176.
- Bird, M.I.; Moyo, C.; Veenendaal, E.M.; Lloyd, J.; Frost, P. Stability of elemental carbon in a savanna soil. Glob. Biogeochem. Cycles 1999, 13, 923–932.
- Shackley, S.; Ruysschaert, G.; Zwart, K.; Glaser, B. (Eds.) Biochar in European Soils and Agriculture: Science and Practice; Earthscan from Routledge: London, UK, 2016.
- Bruun, E.W.; Müller-Stöver, D.; Ambus, P.; Hauggaard-Nielsen, H. Application of biochar to soil and N2O emissions: Potential effects of blending fast-pyrolysis biochar with anaerobically digested slurry. Eur. J. Soil Sci. 2011, 62, 581–589.
- Lin, Y.; Munroe, P.; Joseph, S.; Kimber, S.; Van Zwieten, L. Nanoscale organo-mineral reactions of biochars in ferrosol: An investigation using microscopy. Plant Soil 2012, 357, 369–380.
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843.
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320.
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.; Kuzyakov, Y. Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur. J. Soil Sci. 2009, 60, 186–197.
- García-Sánchez, M.; Šípková, A.; Száková, J.; Kaplan, L.; Ochecová, P.; Tlustoš, P. Applications of organic and inorganic amendments induce changes in the mobility of mercury and macro- and micronutrients of soils. Sci. World J. 2014, 2014, 407049.
- Udall, D.; Rayns, F.; Mansfield, T. LIVING SOILS: A Call to Action; Soil Association: Bristol, UK.; Centre for Agroecology, Water and Resilience (CAWR) at Coventry University: Coventry, UK, 2014.
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 1st ed.; Earthscan: London, UK, 2009.
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.; Six, J.; Parikh, S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204.
- Scheifele, M.; Hobi, A.; Buegger, F.; Gattinger, A.; Schulin, R.; Boller, T.; Mäder, P. Impact of pyrochar and hydrochar on soybean (Glycine max L.) root nodulation and biological nitrogen fixation. J. Plant Nutr. Soil Sci. 2017, 180, 199–211.
- Güereña, D.T.; Lehmann, J.; Thies, J.E.; Enders, A.; Karanja, N.; Neufeldt, H. Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol. Fertil. Soils 2015, 51, 479–491.
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20.
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 2015, 395, 105.
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.-W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil-plant relations. Plant Soil 2011, 345, 195–210.
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379.
- González, J.A.; Gallardo, M.; Hilal, M.B.; Rosa, M.D.; Prado, F.E. Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and water logging stresses; dry matter partitioning. Bot. Stud. 2009, 50, 35–42.
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–A review. Soil Biol. Biochem. 2011, 43, 1812–1836.
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Routledge: Abingdon, UK, 2009; Chapter 2.
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230.
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58.
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796.
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2014, 123, 45–51.
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219–220, 162–167.
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.-L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72.
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215.
- Samonin, V.V.; Elikova, E.E. A study of the adsorption of bacterial cells on porous materials. Microbiology 2004, 73, 696–701.
- Rivka, F.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627.
- Gao, S.; Thomas, H.D.L. Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield. Adv. Plants Agric. Res. 2016, 4, 348–362.
- Sarkhot, D.V.; Berhe, A.A.; Ghezzehei, T.A. Impact of biochar enriched with dairy manure effluent on carbon and nitrogen dynamics. J. Environ. Qual. 2012, 41, 1107–1114.
- Esfandbod, M.; Phillips, I.R.; Miller, B.; Rashti, M.R.; Lan, Z.M.; Srivastava, P.; Singh, B.; Chen, C.R. Aged acidic biochar increases nitrogen retention and decreases ammonia volatilisation in alkaline bauxite residue sand. Ecol. Eng. 2017, 98, 157–165.
- Seneviratne, M.; Weerasundara, L.; Ok, Y.S.; Rinklebe, J.; Vithanage, M. Phytotoxicity attenuation in Vigna radiata under heavy metal stress at the presence of biochar and N fixing bacteria. J. Environ. Manag. 2017, 186, 293–300.
- Slattery, J.F.; Coventry, D.R.; Slattery, W.J. Rhizobial ecology as affected by the soil environment. Aust. J. Exp. Agric. 2001, 41, 289–298.
- Beare, M.H.; Gregorich, E.G.; St-Georges, P. Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biol. Biochem. 2009, 41, 611–621.
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2010; p. 419.
- Gundale, M.J.; Nilsson, M.C.; Pluchon, N.; Wardle, D.A. The Effect of Biochar Management on Soil and Plant Community Properties in a Boreal Forest. GCB Bioenergy 2016, 8, 777–789.
- Ni, J.; Pignatello, J.; Xing, B. Adsorption of Aromatic Carboxylate Ions to Black Carbon (Biochar) Is Accompanied by Proton Exchange with Water. Environ. Sci. Technol. 2011, 45, 9240–9248.
- Smith, K.S.; Balistrieri, L.S.; Smith, S.M.; Severson, R.C. Distribution and mobility of molybdenum in the terrestrial environment. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 23–46.
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25.
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978.
- Yao, H.; Gao, Y.; Nicol, G.W.; Campbell, C.D.; Prosser, J.I.; Zhang, L.; Han, W.; Singh, B.K. Links between Ammonia Oxidizer Community Structure, Abundance, and Nitrification Potential in Acidic Soils. Appl. Environ. Microbiol. 2011, 77, 4618–4625.
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzler, B.; Soja, G.; et al. Biochar Decelerates Soil Organic Nitrogen Cycling but Stimulates Soil Nitrification in a Temperate Arable Field Trial. PLoS ONE 2014, 9, e86388.
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67.
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728.
- Kim, E.J.; Oh, J.E.; Chang, Y.S. Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil. Sci. Total Environ. 2003, 311, 177–189.
- Wang, Z.; Zheng, H.; Luo, Y.; Deng, X.; Herbert, S.; Xing, B. Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ. Pollut. 2013, 174, 289–296.
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117.
- Spokas, K.; Koskinen, W.; Baker, J.; Reicosky, D. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581.
- Güereña, D.; Lehmann, J.; Hanley, K.; Enders, A.; Hyland, C.; Riha, S. Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil 2013, 365, 239–254.
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 2013, 42, 1545–1554.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
670
Revisions:
3 times
(View History)
Update Date:
12 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




