Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrzej Pawlak | -- | 4462 | 2023-12-29 08:29:39 | | | |
| 2 | Catherine Yang | Meta information modification | 4462 | 2023-12-29 08:31:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pawlak, A.; Krajenta, J. Disentangled Polymers and Composites. Encyclopedia. Available online: https://encyclopedia.pub/entry/53261 (accessed on 07 February 2026).
Pawlak A, Krajenta J. Disentangled Polymers and Composites. Encyclopedia. Available at: https://encyclopedia.pub/entry/53261. Accessed February 07, 2026.
Pawlak, Andrzej, Justyna Krajenta. "Disentangled Polymers and Composites" Encyclopedia, https://encyclopedia.pub/entry/53261 (accessed February 07, 2026).
Pawlak, A., & Krajenta, J. (2023, December 29). Disentangled Polymers and Composites. In Encyclopedia. https://encyclopedia.pub/entry/53261
Pawlak, Andrzej and Justyna Krajenta. "Disentangled Polymers and Composites." Encyclopedia. Web. 29 December, 2023.
Copy Citation
Macromolecule entanglements are common in polymers. The chains of macromolecules with carbon skeletons are flexible. The isolated chain easily takes the shape of a coil. When a macromolecule is surrounded by other macromolecules, its coils interpenetrate, and entanglements arise between these macromolecules. The condition of their occurrence is that their molecular weight exceeds a certain limit. The entanglements may be topological (these are common) or cohesive. Entanglement with another macromolecule limits the movement of the macromolecule's chain, so it is an obstacle to this movement.
entanglements
crystallization
mechanical behavior
polyethylene
polymer composites
1. Disentangling of Polymers
It is difficult to find a way to increase equilibrium entanglement of macromolecules, but it is possible to reduce entanglement. Three approaches are proposed for disentangling macromolecules: during polymerization, via dissolving, and via shear in the melt. The effectiveness of each of these methods depends on its characteristic parameters, such as temperature, the molecular mass of the polymer, or the type of solvent. Roles are described for each method. Typically, when the literature mentions “disentangling”, the authors mean only a partial, not complete, reduction in entanglements. A standard confirmation of reduced contacts between macromolecules is a change in the rheological modulus and viscosity.
Entanglements can be reduced during polymerization [1][2]. For this purpose, the freshly polymerized macromolecules should immediately form crystals, thus limiting the amorphous phase content and the possibility of entanglement. In traditional Ziegler–Natta (Z-N) polymerization, the polymerization rate is usually faster than the crystallization rate; the active sites are close to each other, so adjacent chains easily form entanglements. In homogeneous synthesis, the metallocene catalyst and co-catalyst are dispersed in the polymerization medium, and it may be possible to control the polymerization and crystallization rates and the separation of polymerization sites. As a result, a disentangled polymer can be obtained [3][4][5]. The reactor powder is usually called nascent. Polymers that disentangle during the polymerization process include PE, PP, and poly(tetrafluoroethylene)(PTFE). The disadvantages of this method are low output and a limitation on synthesizable polymers [6].
The most common disentangling method used In laboratories is dissolving the polymer, followed by freezing and drying [7][8][9][10][11][12][13][14]. This is called the freeze-drying method. In a concentrated solution, the chains of macromolecules are still entangled, but as the concentration decreases, the so-called critical concentration, c*, is reached, below which, the macromolecular coils are separated. The critical overlap concentration is usually less than a few percent. If the dissolved polymer is quickly frozen using liquid nitrogen, and the solvent is then removed, for example, via sublimation, a solid polymer with limited entanglements can be obtained. Instead of freezing, disentanglement stabilization can be achieved via crystallization in solution. A long list of polymers disentangled using the solvent method can be found in Pawlak’s review [15]. Unfortunately, freeze-drying diluted polymer solutions is not suitable for large-scale production given the small amount of material obtained in a single process [16] and the harmfulness of the solvent to the environment.
Recently, much attention has been paid to the development of a method of disentangling polymer via shearing. It has been known for many years that the application of shear to the polymer melt causes the partial orientation of macromolecules, which is responsible, among other things, for the faster crystallization of the polymer. However, the nature of the process is often not recognized or discussed in terms of the disentangling necessary to achieve the high orientation of macromolecules. During processing, the orientation and disentangling of the chains lead to the rheological phenomenon of shear-thinning, i.e., a reduction in steady-state viscosity. This happens when the shear rate exceeds the reciprocal of the linear relaxation time [16].
The shearing of molten polymer can be performed using laboratory equipment. Examples are shearing stages or rheometers, in which the polymer is placed between parallel plates, one of which rotates or oscillates in a controlled manner. Shearing changes the rheological properties of the polymer, which allows the effectiveness of the method to be assessed. One variant of shear application is large amplitude oscillatory shear (LAOS) flow, which can be realized in a plate–plate configuration for the rheometer. Examples of test conditions used for PP are as follows: strain amplitude, 50–100%; frequency, 1 Hz; T = 180 °C; time, 200 s [6][17]. The disentanglement of polymer using LAOS can be controlled by adjusting the shear conditions. For example, a larger amplitude and a longer time promote disentangling. LAOS flow produces a less disentangled melt state with respect to steady-shear flow, which is another disentangling possibility. In a steady-shear experiment, the shear rate and time are constant. For example, Liu et al. [18] used a steady-state shear of 5 s−1 for 360 s to disentangle linear PP. LAOS, as an experimental method, simulates the large and rapid deformations experienced by polymers during industrial processing and subsequent use well [19].
The steady-state shear has been compared with another variant of shear using rheometer, a ramping-up shear, which is a deformation with an increasing shear rate during an experiment. A comparison of the steady-state shear at a rate of 5 s−1 and a ramping-up shear (0–10 s−1), both within 360 s, for PP disentanglement was performed by [18]. The measured Me masses were 11,442 g/mol for “initial”, 18,974 g/mol for “steady”, and 67,181 g/mol for “ramping-up” PP. The reason for the ramp-up shear efficiency is that, with a slow rate of ramp-up, shear banding, which is typical of steady shear, was avoided; thus, the bulk sample was sheared efficiently, and the more tangled network could undergo structural breakdown because of the buildup of retractive force [20].
Attempts have been made to achieve the effective disentangling of polymers by shearing them in processing machines such as extruders, mixers, or injection molding machines. The difficulty in this case is that not only must the polymer be disentangled inside the machine but it must also be retained while forming the product outside the machine. One of the first to attempt the large-scale production of “in-pellet” materials from disentangled polymers was Ibar [21]. The concept was based on an additional, simultaneous simple shearing treatment for the polymer in the machine. As pointed out by Ibar, the orientation of an entangled polymer, which causes it to flow more easily, has often been incorrectly interpreted as “disentangling” the polymer.
In processing equipment, the shear process can be assisted by applying a vibration field or adding a pressure field. A vibrational force field added to polymer extrusion affects the molecular motion and rheological behavior of the polymer melt [22]. An example of a system with vibrations was proposed by An et al. [23]. The polymer was sheared by two rods vibrating in the opposite direction. Vibration support improved the rheological properties of molten PP. Both the tensile strength and elongation at the break of the PP increased after applying a shear vibration field, as did the melting temperature and crystallinity. A similar device was previously built by Isayev [24]. Often, these systems constitute a modified end part of the extruder. Another solution, involving the use of a vibration field in the entire extruder, was proposed by Chen [23].
Please note that the disentangled polymers obtained with the three methods above do not have exactly the same internal structure because, in the case of polymerization, the amount of the amorphous phase is smaller than usual; in the case of the freeze-drying methods; a solvent may still be present; and in the case of disentangling via shear, one can expect at least a local orientation of macromolecules.
2. Change in Properties Due to Polymer Disentangling
Studies on partially disentangled materials have provided further information on the impact of entanglements on polymer properties. Previous research up to 2016 was described in detail in Pawlak’s review [15]. The latest results, which clearly illustrate the current state of knowledge, are summarized below. This research has focused on crystallization, mechanical properties, and glass transition.
Crystallization from disentangled melt has been examined for isotactic PP [18][25][26][27][28], syndiotactic PP [12], PLA [8][29], PS [13], PEO [9], and UHMWPE [10][30]. Cold crystallization was studied for PC in [31] and PET in [32]. Generally, these studies confirmed conclusions from studies of equilibrium-entangled polymers, i.e., that both crystal nucleation and crystal growth may depend on reducing entanglement. Romano et al. [33] showed that the degree of polymer disentanglement can be evaluated based on differences in the melting kinetics of entangled and disentangled crystals. Wang et al. [34] crystallized PP after a LAOS process and concluded that the nucleation density and growth rate of spherulites increase as the entanglement density decreases. According to Fu et al. [16], less entanglement in sheared HDPE samples results in thicker lamellae in the non-isothermally crystallized polymer. Wang et al. [35] studied the crystallization kinetics of freeze-extracted PLA as a function of the concentration in solution below and above critical concentrations for a chain-overlapping c*, which was calculated to be 11 g/L. It was found that crystallization was faster and lamellae were thicker when the concentration of the precursor solution decreased, but only when it was lower than the c*. The crystallization of PLA was also studied by Hu et al. [29] in shear-disentangled samples. They concluded that, under isothermal and non-isothermal conditions, crystallization was faster and the degree of crystallinity was higher when the degree of entanglement was lower.
In another study, similar results were obtained from a comparison of fully entangled PET with another PET prepared from a trifluoroacetic acid solution, called reorganized PET (RPET) [36]. Observations of the transition of crystallization regimes I-II were interesting. The temperature was 7 °C lower for RPET. Using Hoffman–Weeks extrapolation, the calculated equilibrium melting temperature was found to be several degrees higher for RPET.
Many polymers, such as isotactic polybutene-1 (PB), can crystallize in different crystallographic forms. Ni et al. [37] showed the role of chain entanglement in the kinetics of polymorphic crystallization and the solid–solid phase transition. The temperature of crystallization, the temperature of melting, and the crystallinity of form II were found to decrease with the degree of disentanglement. This was accompanied by lower nucleation density and unusually lower spherulite growth rate. The reasons for this behavior were unclear, although the loose packing of the crystal lattice or highly mobile side ethyl groups were indicated as probable causes. Form II in PB is metastable and spontaneously transforms at room temperature into thermodynamically stable form I. Disentangling the chain significantly increases the phase transition rate and final degree of transition.
The crystallization of poly(ε-caprolactone) and its blends with poly(styrene-co-acrylonitrile) was studied by Liu et al. [38]. The authors prepared disentangled materials using strong shear or the “self-nucleation” process. The second term means that disentangled polymer was created by melting crystals and holding them just above the melting temperature. The ease of disordering and re-entangling such material was studied after applying weak shear immediately after disentangling. The weak shear decelerated the crystallization of the partially disentangled melt because of chain re-entanglement. However, when the weak shear rate was higher, additional disentanglement was attained, and crystallization occurred more quickly.
Zhang et al. [39] increased the mobility of poly(L-lactide) (PLLA) macromolecules using a complex shear method. As a result of disentangling, the crystallization kinetics of PLlA was accelerated. Moreover, PLLA macromolecular chains are usually aligned along the flow direction under strong shear fields, resulting in thicker shear layers and the formation of denser and more perfect shish–kebab structures.
The crystallization of the sterocomplex of polylactide enantiomers is usually restricted to high-molecular-weight components. Sun et al. [40] demonstrated the crucial role of chain entanglement in regulating this kind of crystallization. PLLA/PDLA blends with various degrees of entanglement were prepared by freeze-drying a dioxane solution. The disentangling not only increased the crystallization rate but also the crystallinity under both non-isothermal and isothermal conditions. The less-entangled samples crystallized exclusively as high-crystallinity complexes, in contrast to the predominant homo-crystallization that occurred in the entangled samples.
The crystallization of PP with its entanglements halved was the subject of several works by Pawlak et al. [26][41]. It was found that, similar to other polymers, the crystallization temperature and degree of crystallinity are higher in the disentangled polymer. The thickness of the lamellae also increases. It was shown for the first time that, in polymers under the influence of disentangling, the transition temperature between the II and III crystallization regimes decreases by 4 °C [26]. Krajenta et al. [42] studied the crystallization of PLA after limiting the density of entanglements to 20% of the initial density. The crystal growth was found to be 10% faster, and it was shown that the boundary temperatures of the crystallization regimes shift under the influence of disentangling.
Another polymer in which the course of isothermal and non-isothermal crystallization was studied was poly(ethylene oxide) (PEO) [43]. It turned out that with a decrease in entanglement density spherulitic nucleation increases, and the resulting spherulites grow faster. With fewer macromolecular entanglements, the temperature of transition between crystallization regimes I and II shifted in the direction of lower temperatures. Equilibrium melting temperature measurements using the Hoffman-Weeks method showed little difference between entangled and disentangled PEO. X-ray observations of the size of the formed lamellae have shown that slightly thicker and more perfect crystals grew from a partially disentangled melt. The macromolecules in the samples annealed in the melt gradually re-entangled, but this process was slow.
The experimental crystallization studies have been supported by simulations. For example, Zhai et al. [44] performed coarse-grained molecular dynamics simulations of the isothermal crystallization of bimodal and unimodal polymers with equivalent average Mw. They analyzed the evolution of entanglement during crystallization. With the onset of crystal growth, the entanglement concentration decreased rapidly, but at the end of crystallization, it saturated at a level lower than the initial one. The actual crystallinity and lamellae thickness were linearly proportional to the degree of disentanglement. The authors built a scenario out of the entire process of chain disentangling and lamellar thickening based on the chain-sliding diffusion mechanism. Compared with the unimodal system, the increase in the crystallinity of the bimodal system was faster than disentanglement. The reason was that the chains of the component with longer chains slid more slowly.
Peng et al. [45] performed molecular dynamics simulations to account for the role of entanglement in the nucleation of polymer melts. In highly entangled polymers like PC, nucleation is completely suppressed. The inhibition of nucleation may result from an entanglement-enhanced nucleation barrier or from constrained chain dynamics. Which of these is important is not clear. The determined nucleation-free energy barriers and critical nucleus size decreased at lower entanglement densities.
Still, the effect of entanglement on the glass transition temperature (Tg) is not entirely clear, even as new results are available for disentangled polymers. A decrease in Tg has been observed for disentangled polymers, mainly PS but also PC, PLLA, and PET [34][36][46][47][48][49][50][51][52][53]. The decrease in Tg is interpreted in the literature as the enhanced degree of freedom of the bond rotation due to a reduction in entanglements, affecting the chain relaxation process [54]. Another possible explanation for the decrease in the Tg is that, after freeze-drying, the proliferation of free-volume holes facilitates the movement of molecular chains [55]. Since a decrease in Tg has also been observed in non-entangled, low-Mw PS prepared from solution, Simon and co-workers [49][56] suggested that the reason for the glass transition shift is not disentanglement but the presence of residual solvent.
Research has also been carried out to better understand the relationship between the entanglements of macromolecules and the mechanical properties of polymers. Three of these experiments focused on impact behavior. Yue et al. [57] examined the Izod impact strength of two sintered UHMWPE, including one with reduced entanglements. Interestingly, the polymer in the weakly entangled state was characterized by significantly better impact strength (80 vs. 70 kJ/m2). The reason may be better sintering of fewer entangled chains.
More traditional were the studies on the tensile properties of PC conducted by Wang et al. [34]. The novelty of their approach was the use of a modified extruder, in which the disentangling of PC was achieved by applying different shear procedures (vibration, rotation, or together). Surprisingly, no significant changes were observed between the tensile properties of disentangled and raw polymers. While examining the tensile properties of PP, Pawlak et al. [58] noticed that the differences between the entangled and non-entangled polymers become visible when, instead of engineering parameters, the values of true stress and true strain were calculated. It was found that properties at yield were not changed by the reduction in entanglements. However, in the strain-hardening phase, the increase in stress was slower when the polypropylene was less entangled. Increased cavitation was observed in partially disentangled polypropylene.
The process of pore formation in amorphous polyethylene during uniaxial stretching was modeled using the molecular dynamics method by Logunova and Orekhova [59]. The results of the statistical analysis of pore sizes indicate that intermolecular entanglements slow down the processes of their growth and aggregation in the bulk of the polymer.
In recent years, publications have appeared concerning other studies of materials containing disentangled chains, e.g., those that are not electrically neutral [60][61]. For example, the idea of studying relaxation processes using broadband dielectric spectroscopy has been presented. Dielectric spectroscopy allows for the identification of polarization and conductivity phenomena, the measurement of charge transport, and other things. In the case of non-polar polymers, it may be necessary to introduce a substance with a constant dipole moment. In Drakopoulos et al.’s [60] research on disentangled UHMWPE, Al2O3 catalytic ash played such a role. Five relaxation processes were identified in the polymer, two of which were attributed to the disentangled and entangled amorphous phase. Based on the results of dielectric spectroscopy, the formation of entanglements was also analyzed, estimating the minimum temperature at which the re-entanglement begins (58 °C).
The role of entanglement on the rheological and mechanical properties of polymerized ionic liquids, a class of polyelectrolytes, was investigated by Liu et al. [61]. Samples with a wide range of molecular weights were tested. The high density of electrical charges in the polymer backbone of this material provides unique rheological properties, such as resistance to entanglement. The molecular weight of the entanglement of the tested polyelectrolyte was 1.6 × 105 g/mol, which was much higher than that of conventional polymers. Disentangling the polymerized ionic liquid under shear in the LAOS process was also observed, with re-entangling occurring within 6 min after the high shear had ceased.
3. Composites Created Using Disentangled Polymers
Over the last ten years, interest in polymer nanocomposites has been steadily increasing. Classic composites contain a micrometer-sized dispersed phase (fillers, particles, plates) with a relatively high amount of reinforcing agent (10–50 wt.%). The progress in composites involves the use of smaller dispersed phase elements, nanometer-sized, which allow the content to be reduced to 1–5 wt.% while also improving the properties of the matrix. An additional aspect of introducing very small dispersed phase elements into the composite is that some new effects or behaviors may be revealed. An example is unexpected rheological behavior. It is known that in micro-composites the dispersed phase increases the viscosity of the melt, but when nanoparticles are used in a polymer composite, the behavior may be the opposite, i.e., a reduction in viscosity, as long as the size of the nanoparticles is smaller than the mesh size of the matrix [62][63][64][65][66].
For example, such an effect was observed for poly(methyl methacrylate) (PMMA) with 2 wt.% dispersed 11 nm grafted silicon nanoparticles [65]. A decrease in the storage modulus with grafted polyoxometalate particle content was observed by Chai et al. [62]. A decrease in the GNo modulus of a commercial UHMWPE composite with POSS compared with UHMWPE was observed by Zhang et al. [53]. The effect was best visible at a filler content of 0.5% by weight and less at higher contents. Sui et al. [67] formulated a UHMWPE composite with 0.1–0.5 wt.% TiO2 and observed that the viscosity of the solution in the presence of nanoparticles decreased by 91% compared with the pure UHMWPE solution. Also, the critical overlap concentration, i.e., the concentration in the solution at which the separation of the macromolecular coils occurs, increased from 1 wt.% in UHMWPE up to 1.4 wt.% in the composite.
The reason for the observed rheological behavior is that sufficiently small nanoparticles can increase the free volume in the melt, thereby reducing chain entanglement and accelerating the relaxation process. As a result of disentanglement, a lower melt viscosity (or modulus) can be observed compared with the polymer used as the matrix [62]. Table 1 shows the nanocomposites prepared in which the matrix polymer was disentangled.
Developed nanocomposites with partial disentanglement have been used to study their properties. In one study, in PMMA/SiO2 composites, the glass transition did not change in relation to the PMMA-to-SiO2 content below 10 wt.% but increased with higher amounts [65]. For PS/polyoxymetalate, a regular shift in Tg toward lower temperatures was observed with an increase in nanofiller content in [62]. Flash DSC studies of UHMWPE with 0.8 nm POSS particles showed that the composite had lower melting activation energy than pure UHWMPE, confirming the lower entanglement level, and the chain segment activity was improved with the addition of POSS.
The subject of studies from Luo’s group was the properties of a PP micro-composite with a high amount (35 wt.%) of boron nitride [68]. The PP in the composite was disentangled via a steady-state shear, and the re-entangling was examined. The viscosities of the non-sheared composites were higher compared with PP, were stable during the experiment, and depended on the size of microparticles. After shearing, the order changed, and PP had a higher viscosity than the composites. This confirmed the disentanglement. After the cessation of shear, the viscosity of each materias increased rapidly during the first 30 s of the time sweep experiment and then remained stable (at least 300 s); however, it was far from the equilibrium value of PP. The authors interpreted this as maintaining a state of disentanglement in the case of the composites. In many experiments, reaching a viscosity plateau is interpreted as full entanglement. According to Luo, the attachment of disentangled molecular chains to the surface of particles and, thus, the limitation on the mobility of the macromolecular chains are the cause of the reduced plateau viscosity of composites. The crystallization of PP/boron nitride composites was slightly faster when the PP matrix was partially disentangled.
The entanglement dynamic of the oriented UHMWPE/1 wt.% Au nanocomposite was the subject of publication by Drakopoulos et al. [69]. Dielectric spectroscopy was used to study the transition of a composite into a molten state. A decrease in DC conduction was observed with the formation of entanglements.
Barangizi and Pawlak [70] investigated the crystallization of solution-disentangled PP in a composite with 1 wt.% Al2O3 nanopowder. For comparison, entangled nanocomposite, solution-disentangled PP, and entangled PP were examined. It was observed that isothermal crystallization occurred faster and non-isothermal crystallization occurred at a higher temperature if the composite had fewer entanglements. However, it was also observed that crystallization in the nanocomposite was slower compared with the homopolymer at the same entangling level. The dispersed alumina nanoparticles hindered the movement of macromolecules into the growing crystals. The re-entanglement of macromolecules as a result of annealing at 200 °C was also investigated. The time to equilibrium for the less-entangled composite and similarly entangled PP was around 80 min. More entangled samples reached equilibrium after 40–50 min.
Recently, Barangizi et al. [71] published the results of studies on the mechanical and thermal properties of PLA composites containing 0.1–1.0 wt.% multi-wall carbon nanotubes (MWCNT). The authors compared fully and partially entangled composites and PLA. The partial disentangling of macromolecules improved filler dispersion during composite fabrication via extrusion. The increased mobility of the less-entangled PLA macro-molecules accelerated crystallization in the nanocomposites, which had occurred already during the cooling of the melt and not only as a cold crystallization. Isothermal crystallization studies showed faster crystallization because of a favorable combination of matrix disentanglement and increased nucleation in the nanotubes. The reduction in entanglements influenced the mechanical properties of the composites (Figure 1). The initiation of plastic deformation was easier, i.e., at lower yield stress, and the increase in stress in the strain-hardening phase was slower for the disentangled homopolymers. In the composites, the strain-hardening was stronger with a higher concentration of nanotubes, but it also depended on their dispersion, which was better in the less-entangled polylactide matrix, resulting in the fastest increase in stress.
Table 1. Nano- and micro-composites prepared for studies of the matrix disentangling effect.
| Polymer Matrix | Filler | Contents of Filler (wt.%) | Method of Dispersion | Disentangling of Matrix | Reference |
|---|---|---|---|---|---|
| PMMA | Grafted SiO2, 11 nm | 2 | Solution | During mixing | [65] |
| PS | Grafted polyoxometalate, 0.5–6 nm | 1–5 | Solution | During mixing | [62] |
| UHMWPE | POSS | 0.2–3 | During polymerization | Previously disentangled | [10] |
| UHMWPE | POSS, 0.8 nm | 0.1–1 | Melt mixing | During mixing | [53] |
| UHMWPE | TiO2 | 0.1–0.5 | Solution | During mixing | [67] |
| UHMWPE | Gold, nano | 1 | Solution | Previously disentangled | [72] |
| PP | Graphene | 0.1–4 | Shear in melt | During mixing | [73] |
| PP | Boron nitride, 1, 5, 27 µm | 35 | Steady-state shear | During shear | [68] |
| PP | Al2O3, 78 nm | 1 | Melt mixing | Previously disentangled | [70] |
| Waterborne acrylic coatings | TiO2 | 1–3 | Solution | During mixing | [74] |
| PLA | MWCNT | 0.1–1 | Melt mixing | Previously disentangled | [71] |
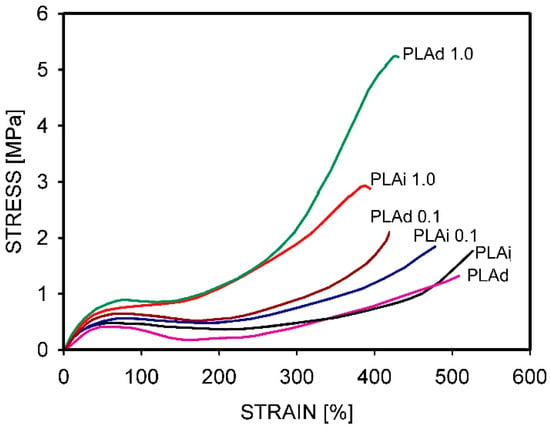
Figure 1. Strain–stress curves showing the mechanical properties of PLA and PLA/MWCNT composite samples in a tensile test at 70 °C. PLAi—entangled polymer; PLAd—disentangled polymer; PLAi 0.1 and PLAi 1.0—composites of entangled polymers with 0.1 and 1.0 wt.% MWCNT, respectively; PLAd 0.1 and PLAd 1.0—composites of entangled polymers with 0.1 and 1.0 wt.% MWCNT, respectively [71].
A specific approach to formulating composites is the polymerization of UHMWPE in the presence of POSS nanoparticles, such as in [10]. In that study, extending the polymerization time resulted in a decrease in the POSS content in the composite, which was 0.74–2.31%. Rheological tests showed that the highest G′ modulus value (higher than UHMWPE) was obtained for the polymer composite with the highest molecular weight and the lowest POSS content, which resulted from the longest polymerization process. During the time sweep test, the composites gradually reached the equilibrium G′ values, which, however, were not the same as for the homopolymer. The times to reach equilibrium were 103–104 min and were the longest for UHMWPE. The crystallinity and lamella thickness of the resulting UHMWPE increased with increasing POSS loading. This suggested that the POSS particles were nucleating agents.
Disentangled polymers can also be used to produce special types of composites called all-polymer composites. These are composites in which not only the matrix but also the reinforcement is polymer. Smith and Lemstra [75] showed that very strong fibers can be obtained by spinning from solution. At that time, it was not known that this was possible by limiting entanglements. A similar concept was later used by Galeski [28][76]. It is known that the plastic deformation of a semi-crystalline polymer is limited by the network of macromolecules in the amorphous phase. It was assumed that if the network was less dense, it would be possible to obtain much greater deformations and transform the lamellar structure of the polymer into a fibrous one. Such a transformation can be achieved by blending a solid, partially disentangled polymer powder with a second molten polymer. The concept was experimentally verified via extrusion, and all-polymer composites were created from HDPE/PP and PS/PP pairs [28]. Microscopic observations confirmed that long, flexible micro- and nanofibers were obtained, well dispersed in the second polymer matrix. Tests of mechanical properties showed effective strengthening in the polymer matrix.
References
- Huang, Y.F.; Xu, J.Z.; Zhang, Z.C.; Xu, L.; Li, L.B.; Li, J.F.; Li, Z.M. Melt processing and structural manipulation of highly linear disentangled ultrahigh molecular weight polyethylene. Chem. Eng. J. 2017, 315, 132–141.
- Pandey, A.; Champouret, Y.; Rastogi, S. Heterogeneity in the Distribution of Entanglement Density during Polymerization in Disentangled Ultrahigh Molecular Weight Polyethylene. Macromolecules 2011, 44, 4952–4960.
- Rastogi, S.; Kurelec, L.; Cuijpers, J.; Lippits, D.; Wimmer, M.; Lemstra, P.J. Disentangled state in polymer melts; a route to ultimate physical and mechanical properties. Macromol. Mater. Eng. 2003, 288, 964–970.
- Westfahl, H., Jr.; Cardoso, M.B. Accessing the hidden lamellar nanostructure of semi-crystalline nascent polymers by small-angle X-ray scattering contrast variation. J. Appl. Crystall. 2011, 44, 1123–1126.
- Yamazaki, S.; Gu, F.; Watanabe, K.; Okada, K.; Toda, A.; Hikosaka, M. Two-step formation of entanglement from disentangled polymer melt detected by using nucleation rate. Polymer 2006, 47, 6422–6428.
- Wang, B.; Cavallo, D.; Zhang, X.; Zhang, B.; Chen, J. Evolution of chain entanglements under large amplitude oscillatory shear flow and its effect on crystallization of isotactic polypropylene. Polymer 2020, 186, 121899.
- Bu, H.S.; Gu, F.M.; Bao, L.; Chen, M. Influence of entanglements on crystallization of macromolecules. Macromolecules 1998, 31, 7108–7110.
- Hao, H.; Liu, R.J.; Zhao, Y.L. Concentration Dependence of Crystalline Poly(L-lactide) Prepared by Freeze-drying Solutions. Polym. Polym. Compos. 2009, 17, 31–35.
- Gu, F.M.; Bu, H.S.; Zhang, Z. A unique morphology of freeze-dried poly(ethylene oxide) and its transformation. Polymer 2000, 41, 7605–7609.
- Li, W.; Guan, C.; Xu, J.; Mu, J.; Gong, D.; Chen, Z.R.; Zhou, Q. Disentangled UHMWPE/POSS nanocomposites prepared by ethylene in situ polymerization. Polymer 2014, 55, 1792–1798.
- Huang, B.; Ito, M.; Kanamoto, T. Deformation mechanism of amorphous poly(ethylene terephthalate) as function of molecular weight and entanglements. Polymer 1994, 35, 1210–1216.
- Sun, Q.; Fu, Q.; Xue, G.; Chen, W. Crystallization Behavior of Syndiotactic Poly(propylene) Freeze-Dried from Toluene at very Dilute Concentration. Macromol. Rapid Comm. 2001, 22, 1182–1185.
- Xue, G.; Wang, Y.; Liu, S.; Liao, Y.T. FT-IR Study of Concentration Dependence for Crystallization of Isotactic Polystyrene Arising from Freeze-Drying Dilute Solutions. Macromolecules 1995, 28, 4344–4346.
- Ikeda, Y.; Ohta, T. The influence of chain entanglement density on ultra-drawing behavior of ultra-high-molecular-weight polypropylene in the gel-casting method. Polymer 2008, 49, 621–627.
- Pawlak, A. The Entanglements of Macromolecules and Their Influence on the Properties of Polymers. Macromol. Chem. Phys. 2019, 220, 1900043.
- Fu, J.; Wang, Y.; Shen, K.; Fu, Q.; Zhang, J. Insight into Shear-Induced Modification for Improving Processability of Polymers: Effect of Shear Rate on the Evolution of Entanglement State. J. Polym. Sci. B Polym. Phys. 2019, 57, 598–606.
- Wang, B.; Cavallo, D.; Chen, J. Delay of re-entanglement kinetics by shear-induced nucleation precursors in isotactic polypropylene melt. Polymer 2020, 210, 123000.
- Liu, M.; Chen, J.; Luo, J.; Min, J.; Fu, Q.; Zhang, J. Investigating the disentanglement of long chain branched polypropylene under different shear fields. J. Appl. Polym. Sci. 2022, 139, 51642.
- Kamkar, M.; Salehiyan, R.; Goudoulas, T.B.; Abbasi, M.; Saengow, C.; Erfanian, E.; Sadeghi, S.; Natale, G.; Rogers, S.A.; Giacomini, A.J.; et al. Large amplitude oscillatory shear flow: Microstructural assessment of polymeric systems. Prog. Polym. Sci. 2022, 132, 101580.
- Wang, S.-Q.; Ravindranath, S.; Wang, Y.; Boukany, P. New theoretical considerations in polymer rheology: Elastic breakdown of chain entanglement network. J. Chem. Phys. 2007, 127, 064903.
- Ibar, J.P. Processing Polymer Melts under Rheo-Fluidification Flow Conditions, Part 1: Boosting Shear-Thinning by Adding Low Frequency Nonlinear Vibration to Induce Strain Softening. J. Macromol. Sci. B Phys. 2013, 52, 407–441.
- Chen, K.-Y.; Zhou, N.-Q.; Liu, B.; Jin, G. Improved Mechanical Propertes and Structure of Polypropylene Pipe Prepared Under Vibration Force Field. J. Appl. Polym. Sci. 2009, 114, 3612–3620.
- An, F.Z.; Gao, X.Q.; Lei, J.; Deng, C.; Li, Z.M.; Shen, K.Z. Vibration assisted extrusion of polypropylene. Chin. J. Polym. Sci. 2015, 33, 688–696.
- Isayev, A.I.; Wong, C.M.; Zeng, X. Effect of oscillations during extrusion on rheology and mechanical properties of polymers. Adv. Polym. Technol. 1990, 10, 31–45.
- Wang, X.H.; Liu, R.; Wu, M.; Wang, Z.; Huang, Y. Effect of chain disentanglement on melt crystallization behavior of isotactic polypropylene. Polymer 2009, 50, 5824–5827.
- Pawlak, A.; Krajenta, J.; Galeski, A. The crystallization of polypropylene with reduced density of entanglements. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 748–756.
- Xiao, Z.G.; Sun, Q.; Xue, G.; Yuan, Z.; Dai, Q.; Hu, Y.L. Thermal behavior of isotactic polypropylene freeze-extracted from solutions with varying concentrations. Europ. Polym. J. 2003, 39, 927–931.
- Krajenta, J.; Pawlak, A.; Galeski, A. Deformation of disentangled polypropylene crystalline grains into nanofibers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1983–1994.
- Hu, H.; Chen, J.; Yang, T.; Wang, P.; Min, J.; Fu, Q.; Zhang, J. Regulation of Entanglement Networks under Different Shear Fields and Its Effect on the Properties of Poly(L-lactide). Ind. Eng. Chem. Res. 2023, 62, 7434–7446.
- Pandey, A.; Toda, A.; Rastogi, S. Influence of Amorphous Component on Melting of Semicrystalline Polymers. Macromolecules 2011, 44, 8042–8055.
- Ji, G.; Xue, G.; Ma, J.; Dong, C.; Gu, X. Concentration dependence of crystallinity of polycarbonate by shock-cooling and subsequent freeze-drying of its various solutions. Polymer 1996, 37, 3255–3258.
- Xie, Z.P.; Liu, D.; Zhu, P.P.; Yang, H.Y. Crystallization behavior of chain-disentangled poly(ethylene terephthalate). Acta Polym. Sinica 2010, 5, 522–529.
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of Polymerization Conditions on Melting Kinetics of Low Entangled UHMWPE and Its Implications on Mechanical Properties. Macromolecules 2014, 47, 4750–4760.
- Wang, Y.; Liu, M.; Chen, J.; Luo, J.; Min, J.; Fu, Q.; Zhang, J. Efficient disentanglement of polycarbonate melts under complex shear field. Polymer 2020, 201, 122610.
- Wang, Y.; Fu, J.; Liu, M.; Fu, Q.; Zhang, J. Understanding the effect of chain entanglement state on melt crystallization of the polymer freeze-extracted from solution: The role of critical overlap concentration. Polymer 2019, 178, 121588.
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472.
- Ni, L.; Xu, S.; Sun, C.; Qin, Y.; Zheng, Y.; Zhou, J.; Yu, C.; Pan, P. Retarded Crystallization and Promoted Phase Transition of Freeze-Dried Polybutene-1: Direct Evidence for the Critical Role of Chain Entanglement. ACS Macro Lett. 2022, 11, 257–263.
- Liu, X.; Yu, W. Weak Shear-Induced Slowdown in Crystallization of Less-Entangled Poly(ε-caprolactone). Macromolecules 2021, 54, 3347–3357.
- Zhang, Y.; Chen, J.; Fu, Q.; Zhang, J. Novel Strategy to Improve the Performance of Poly(L-lactide): The Synergistic Effect of Disentanglement and Strong Shear Field. ACS Sustain. Chem. Eng. 2023, 11, 9630–9642.
- Sun, C.; Zheng, Y.; Xu, S.; Ni, L.; Li, X.; Shan, G.; Bao, Y.; Pan, P. Role of Chain Entanglements in the Stereocomplex Crystallization between Poly(lactic acid) Enantiomers. ACS Macro. Lett. 2021, 10, 1023–1028.
- Krajenta, J.; Safandowska, M.; Pawlak, A. The re-entangling of macromolecules in polypropylene. Polymer 2019, 175, 215–226.
- Krajenta, J.; Safandowska, M.; Pawlak, A.; Galeski, A. All-polymer composites—A new approach with the use of disentangled semi-crystalline polymers. Part 1. Disentangling and properties of disentangled polylactide. Polimery 2020, 65, 167–173.
- Krajenta, J.; Polinska, M.; Lapienis, G.; Pawlak, A. The crystallization of poly(ethylene oxide) with limited density of macromolecular entanglements. Polymer 2020, 197, 122500.
- Zhai, Z.; Fusco, C.; Morthomas, J.; Perez, M.; Lame, O. Disentangling and Lamellar Thickening of Linear Polymers during Crystallization: Simulation of Bimodal and Unimodal Molecular Weight Distribution Systems. ACS Nano. 2019, 13, 11310–11319.
- Peng, F.; Nie, C.; Xu, T.Y.; Sheng, J.F.; Chen, W.; Yu, W.C.; Li, L.B. Entanglement on nucleation barrier of polymer crystal. Chin. J. Polym. Sci. 2022, 40, 1640–1650.
- Dai, Q.; Lu, Y.; Xue, G.; Liao, Y.T. Glass transition of atactic polystyrene with less chain entanglements. Polym. Bull. 1995, 35, 209–214.
- Sasaki, T.; Yamauchi, N.; Irie, S.; Sakurai, K. Differential scanning calorimetry study on thermal behaviors of freeze-dried poly(L-lactide) from dilute solutions. J. Polym. Sci. B Polym. Phys. 2005, 43, 115–124.
- Huang, D.H.; Yang, Y.; Zhuang, G.; Li, B. Influence of Entanglements on the Glass Transition and Structural Relaxation Behaviors of Macromolecules. 1. Polycarbonate. Macromolecules 1999, 32, 6675–6678.
- Bernazzani, P.; Simon, S.L.; Plazek, D.J.; Ngai, K.L. Effects of entanglement concentration on Tg and local segmental motions. Europ. Phys. J. E 2002, 8, 201–207.
- Rong, W.; Fan, Z.; Yu, Y.; Bu, H.; Wang, M. Influence of entanglements on glass transition of atactic polystyrene. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2243–2251.
- Huang, D.H.; Yang, Y.; Zhuang, G.; Li, B. Influence of Intermolecular Entanglements on the Glass Transition and Structural Relaxation Behaviors of Macromolecules. 2. Polystyrene and Phenolphthalein Poly(ether sulfone). Macromolecules 2000, 33, 461–464.
- Singh, M.K.; Hu, M.; Cang, Y.; Hsu, H.P.; Therien-Aubin, H.; Koynov, K.; Fytas, G.; Landfester, K.; Kremer, K. Glass Transition of Disentangled and Entangled Polymer Melts: Single-Chain-Nanoparticles Approach. Macromolecules 2020, 53, 7312–7321.
- Zhang, X.; Zhao, S.; Xin, Z. The chain dis-entanglement effect of polyhedral oligomeric silsesquioxanes (POSS) on ultra-high molecular weight polyethylene (UHMWPE). Polymer 2020, 202, 122631.
- Lu, X.L.; Xue, G.; Mi, Y.L. Understanding the effect of chain entanglement on the glass transition of a hydrophilic polymer. J. Appl. Polym. Sci. 2011, 119, 2310–2317.
- Li, N.; Yang, Q.; Huang, Y.; Zhang, Q.; Zhao, W. Entropy reduction phenomenon in the non-equilibrium state of freeze-dried polymethyl methacrylate samples. J. Polym. Res. 2014, 21, 392.
- Zheng, W.; Simon, S.L. Polystyrene freeze-dried from dilute solution: Tg depression and residual solvent effects. Polymer 2006, 47, 3520–3527.
- Yue, Z.; Wang, N.; Cao, Y.; Li, W.; Dong, C.-D. Reduced Entanglement Density of Ultrahigh-Molecular-Weight Polyethylene Favored by the Isolated Immobilization on the MgCl2 (110) Plane. Ind. Eng. Chem. Res. 2020, 59, 3351–3358.
- Pawlak, A.; Krajenta, J.; Galeski, A. Cavitation phenomenon and mechanical properties of partially disentangled polypropylene. Polymer 2018, 151, 15–26.
- Logunova, M.A.; Orekhov, N.D. The Role of Intermolecular Entanglements in the Formation of Nanosized Pores during Deformation of Polyethylene: Atomistic Modeling. Polym. Sci. Series A 2021, 63, 591–599.
- Drakopoulos, S.X.; Psarras, G.C.; Forte, G.; Martin-Fabiani, I.; Ronca, S. Entanglement dynamics in ultra-high molecular weight polyethylene as revealed by dielectric spectroscopy. Polymer 2018, 50, 35–43.
- Liu, G.; Larson, R.G.; Li, L.; Luo, H.; He, X.; Niu, Y.; Li, G. Influence of Chain Entanglement on Rheological and Mechanical Behaviors of Polymerized Ionic Liquids. Macromolecules 2023, 56, 2719–2728.
- Chai, S.-C.; Xu, T.-Y.; Cao, X.; Wang, G.; Chen, Q.; Li, H.-L. Ultrasmall Nanoparticles Diluted Chain Entanglement in Polymer Nanocomposites. Chin. J. Polym. Sci. 2019, 37, 797–805.
- Mackay, M.E.; Dao, T.T.; Tuteja, A.; Ho, D.L.; Horn, B.V.; Kim, H.C.; Hawker, C.J. Nanoscale effects leading to non-Einstein-like decrease in viscosity. Nat. Mater. 2003, 2, 762–766.
- Nusser, K.; Schneider, G.J.; Pyckhout-Hintzen, W.; Richter, D. Viscosity decrease and reinforcement in polymer-silsesquioxane composites. Macromolecules 2011, 44, 7820–7830.
- Mangal, R.; Srivastava, S.; Archer, L.A. Phase stability and dynamics of entangled polymer-nanoparticle composites. Nat. Commun. 2015, 6, 7198.
- Senses, E.; Ansar, S.M.; Kitchens, C.L.; Mao, Y.; Narayanan, S.; Natarajan, B.; Faraone, A. Small Particle Driven Chain Disentanglements in Polymer Nanocomposites. Phys. Rev. Lett. 2017, 118, 147801.
- Sui, Y.; Yui, Y.; Wei, P.; Cong, C.; Meng, X.; Ye, H.-M.; Zhou, Q. Nanoscale effects of TiO2 nanoparticles on the rheological behaviors of ultra-high molecular weight polyethylene (UHMWPE). Soft Matter 2023, 19, 5459–5467.
- Luo, J.; Chen, J.; Liu, M.; Min, J.; Fu, Q.; Zhang, J. Investigating the Influence of Incorporation of Boron Nitride on the Kinetics of Isotactic Polypropylene Entanglement Recovery. Ind. Eng. Chem. Res. 2021, 60, 12901–12910.
- Drakopoulos, S.X.; Psarras, G.C.; Ronca, S. Oriented ultra-high molecular weight polyethylene/gold nanocomposites: Electrical conductivity and chain entanglement dynamics. Express Polym. Lett. 2021, 15, 492–502.
- Barangizi, H.; Pawlak, A. Crystallization of partially disentangled polypropylene in nanocomposites with aluminum oxide. Polymer 2022, 254, 125049.
- Barangizi, H.; Krajenta, J.; Pawlak, A. The influence of entanglements of macromolecules on the mechanical and thermal properties of polylactide composites with carbon nanotubes. Express Polym. Lett. 2023, 17, 738–758.
- Drakopoulos, S.X.; Tarallo, O.; Guan, L.; Martin-Fabiani, I.; Ronca, S. Nanocomposites of Au/Disentangled UHMWPE: A Combined Optical and Structural Study. Molecules 2020, 25, 3225.
- Luo, J.; Liu, M.; Chen, J.; Min, J.; Fu, Q.; Zhang, J. Effectively maintaining the disentangled state of isotactic polypropylene in the presence of graphene nanoplatelet. Polymer 2021, 226, 23806.
- Romo-Uribe, A. Dispersion at Single Unit TiO2 Nanoparticles Reduced Tg, Induced Chain Disentanglement and Reduced Tensile Modulus in Waterborne Acrylic Coatings. Macromol. Mater. Eng. 2021, 306, 200059.
- Smith, P.; Lemstra, P.J. Ultra-drawing of high molecular weight polyethylene cast from solution. Coll. Polym. Sci. 1980, 258, 891–894.
- Jurczuk, K.; Galeski, A.; Piorkowska, E. All-polymer nanocomposites with nanofibrillar inclusions generated in situ during compounding. Polymer 2013, 54, 4617.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




