Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuji Isegawa | -- | 3865 | 2023-12-28 16:01:38 | | | |
| 2 | Jason Zhu | Meta information modification | 3865 | 2023-12-29 02:23:15 | | | | |
| 3 | Jason Zhu | Meta information modification | 3865 | 2023-12-29 03:32:55 | | | | |
| 4 | Jason Zhu | -57 word(s) | 3808 | 2024-03-01 11:45:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Isegawa, Y. Immune and Antiviral Effects of Euglena Extracts. Encyclopedia. Available online: https://encyclopedia.pub/entry/53230 (accessed on 07 February 2026).
Isegawa Y. Immune and Antiviral Effects of Euglena Extracts. Encyclopedia. Available at: https://encyclopedia.pub/entry/53230. Accessed February 07, 2026.
Isegawa, Yuji. "Immune and Antiviral Effects of Euglena Extracts" Encyclopedia, https://encyclopedia.pub/entry/53230 (accessed February 07, 2026).
Isegawa, Y. (2023, December 28). Immune and Antiviral Effects of Euglena Extracts. In Encyclopedia. https://encyclopedia.pub/entry/53230
Isegawa, Yuji. "Immune and Antiviral Effects of Euglena Extracts." Encyclopedia. Web. 28 December, 2023.
Copy Citation
Influenza is an acute respiratory illness caused by influenza virus infection, which is managed using vaccines and antiviral drugs. Recently, the antiviral effects of plants and foods have gained attention. Euglena is a motile unicellular alga and eukaryotic photosynthetic microorganism. It has secondary chloroplasts and is a mixotroph able to feed by photosynthesis or phagocytosis.
Euglena
immunostimulation
antiviral activity
β-1,3-glucan
paramylon
1. Effects of Euglena Intake on Allergic Diseases
Allergic rhinitis (AR) is the most common allergic disease and represents a health problem worldwide. In Japan, the number of patients with AR has increased. Recently, the number of patients with pollinosis, particularly Japanese cedar pollinosis (JCP), has markedly increased beyond that associated with house dust mites (HDM) or pollinosis other than JCP. An epidemiological study revealed a marked increase (approximately 10%) in the prevalence of AR between 1998 and 2008 [1]. JCP is an immediate-type (type I) allergic disease that causes allergic symptoms due to a specific reaction with IgE antibodies. Cry j1 and Cry j2 in the pollen have been identified as the major antigens (allergens) causing JCP [2][3]. Although immunotherapy and drug therapy are available as treatments for JCP, there is still no effective cure. Recently, there have been reports that functional foods suppress allergic symptoms [4][5][6], raising the expectations for functional foods.
Helper T cells (Th), which are classified into Th1 and Th2 according to their cytokine production patterns, are involved in the pathogenesis of allergy [7]. Th1 participates in cellular immunity and secretes cytokines such as IL-2 and IFN-γ. Th2 is involved in humoral immunity and secretes cytokines such as IL-4 and IL-5. When Th1 and Th2 are unbalanced, allergies are thought to be triggered. For example, Th1 predominates in delayed (type IV) allergic development and Th2 in type I allergic development. Therefore, pollinosis is thought to develop when Th2 predominates.
Euglena is attracting attention as a new functional food, and the paramylon (β-1,3-D-glucan) contained in Euglena has immunostimulatory effects involving cytokines [8], hepatoprotective effects on acute liver injury [9] and anti-human immunodeficiency virus (HIV) [10], and antibacterial effects [11]. Furthermore, Sugiyama et al. [12] indicated that paramylon treatment could provide an effective alternative therapy for the management of atopic dermatitis (AD). Oral administration of paramylon was suggested to suppress the development of atopic dermatitis in NC/Nga mice, which spontaneously develop an eczematous AD-like skin lesion when kept under conventional care but not under specific pathogen-free (SPF) conditions [13][14], by inhibiting Th1 and Th2 responses [12]. The effects of Euglena and paramylon were observed in the early stages of A4gnt KO mice, which are mutant models that spontaneously develop gastric cancer through hyperplasia–dysplasia–adenocarcinoma mechanisms in the antrum of the stomach. The results suggest that the administration of Euglena and paramylon may ameliorate the early involvement of A4gnt mice through inflammatory modulation in the gastric mucosa [15]. Amorphous paramylon had a greater effect on intestinal immunity than paramylon, inhibiting colon cancer [16].
The production of allergen-specific IgE was significantly suppressed, and the production of IL-12 and IFN-γ increased when low-molecular-weight β-glucan was administered to mice [17]. It was also found that low-molecular-weight lentinan suppressed allergic symptoms, such as seasonal and perennial rhinorrhea, sneezing, nasal obstruction, itching, tearing in humans, and allergen-specific IgE and total IgE levels [18], indicating that the β-1,3-1,6-D-glucan found in mushrooms and yeast has immunomodulatory and allergy-suppressing effects. Although a decrease in serum IgE concentration was reported in atopic dermatitis-induced mice after the oral administration of paramylon, no decrease in IgE was observed in a pollinosis model mouse created by inoculation with Cry j1, because the hypersensitivity response to externally introduced specific antigens is biased toward Th2 dominance [19]. Furthermore, Euglena intake may directly reduce pollinosis symptoms, suggesting that components other than paramylon also relieve pollinosis [19].
2. Effects of Euglena’s Intake on the Intestinal Microbiota and Defecation
The intestinal tract hosts the gut microbiota, a complex bacterial community. The gut microbiota interacts with the host and strongly influences homeostasis and immunity in the host. Therefore, the gut microbiota is essential for maintaining the health of the host [20][21][22]. There is growing interest in optimizing the composition of the gut microbiota through dietary therapy using functional foods containing probiotics [23] or prebiotics [24].
For example, β-glucans in cereals are fermented by microorganisms living in the large intestine, and they are converted into short-chain fatty acids (SCFAs), such as acetic, propionic, and butyric acids [25]. SCFAs produced in the colon exert various effects, including immunomodulation [26], mediating apoptosis of colon cancer cells [27], and preventing obesity [28]. For example, the prevention of obesity is achieved by regulating energy metabolism via the SCFA receptor GPR43 and preventing the accumulation of excess lipids in adipose tissue [28]. In addition, SCFAs inhibit the growth of harmful bacteria such as Clostridium spp. and pathogenic Escherichia coli, thereby maintaining a healthy intestinal microflora [29].
The modulatory effects of diet on the gut microbiota are often investigated through in vivo studies in humans [30]. Information on the composition of the colon microbiota comes primarily from the analysis of fecal samples in human dietary intervention studies. However, this method has the experimental limitation that the production of certain metabolites, such as short-chain fatty acids (SCFAs), cannot be measured in situ (in the intestinal tract). A model culture system was developed to rigorously reproduce the microbial components of human fecal collections in vitro [31]. This in vitro human colon microbiota model was used to detect the decreased butyrate production in patients with ulcerative colitis [31]. Thus, combining in vitro human colon microbiota models with in vivo studies can help interpret changes in the human gut microbiota.
Since the effects of Euglena’s ingestion on the human gut microbiota are not yet clear, this section evaluates the effects of Euglena on the colon microbiota of healthy human subjects. Furthermore, by analyzing the effects of adding Euglena or paramylon to an in vitro human colon microbiota model, verifying the effects of Euglena on the gut microbiota is feasible.
Studies on the effects of Euglena’s intake on gut microbiota and defecation showed that the occupancy of the genus Faecalibacterium was increased by Euglena in vitro and in vivo [32]. However, this effect may not be due to the paramylon contained in Euglena [32]. Faecalibacterium prausnitzii is one of the butyrate-producing bacteria with the highest occupancy in the intestinal tract [33]. Other butyrate-acid-producing bacteria, such as Roseburia, showed no differences. Thus, the changes in the gut microbiota induced by Euglena may be specific to Faecalibacterium. Gao et al. reported that butyrate improves insulin resistance [34] and Jia et al. showed that increasing the number of butyrate-producing bacteria may be useful to treat type 2 diabetes [35]. Such results of increased butyrate production induced by Euglena are discussed with the results of a study showing that Euglena consumption lowered blood glucose levels in a rat model of type 2 diabetes [36], indicating that butyrate production by the gut microbiota may be one of the mechanisms by which blood glucose levels are reduced. Euglena is also a source of vitamins, minerals, and unsaturated fatty acids [37][38]. These components promote the growth of Faecalibacterium. Okouchi et al. showed that ingestion of Euglena increased bifidobacteria in the intestinal microflora of mice [39]. However, in another experiment, there was no significant increase in the relative occupancy of bifidobacteria in vitro or in vivo. Therefore, Euglena consumption may promote acetic acid production by Bifidobacterium, which is then consumed by Faecalibacterium, promoting Bifidobacterium growth and butyrate production. In Bifidobacterium adolescentis and F. prausnitzii, a cross-feeding process has been reported using a carbon source of fructooligosaccharides [40].
Furthermore, stool volume was increased by the ingestion of Euglena [32], as shown by Asayama et al. [41]. These results are consistent with the findings of Kawano et al. that rats fed a diet containing cholesterol and Euglena had a shorter cholesterol retention time in the intestine than rats fed a diet containing only cholesterol [42]. Dietary fiber intake may increase stool frequency in patients with constipation [43]. The paramylon in Euglena is insoluble and is neither digested nor absorbed. Therefore, it is believed to exert the same effect as a dietary fiber. Moreover, the consumption of Euglena enhanced butyrate production by F. prausnitzii and is consistent with the results of a previous study showing that butyrate supplementation may alleviate defecation disorders [44]. Thus, ingesting Euglena may have beneficial effects on constipation, such as reducing pain during defecation. In addition, butyrate produced in the intestine increases the number of specific CD8+ T cells that eliminate influenza viruses and control the infection [45]. These findings indicate Euglena may contribute to immunomodulation and antiviral activity (Figure 1).
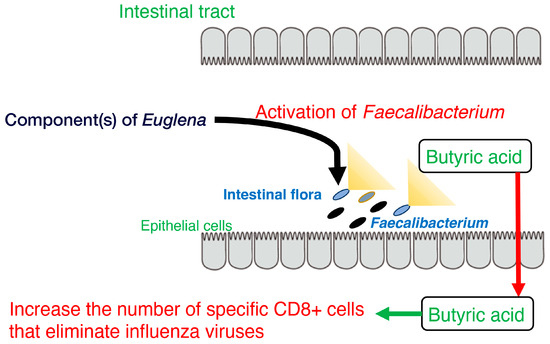
Figure 1. Model of the activation of Faecalibacterium, butyrate production, and CD8+ T cells by component(s) of Euglena.
Overall, these findings suggest that Euglena increases the occupancy of Faecalibacterium, which in turn promotes butyrate production (Figure 1). Future challenges for these studies include increasing the sample size for the in vivo analysis and identifying active components other than paramylon in Euglena. Thus, Euglena has great potential as a novel prebiotic.
3. Effect of Euglena Intake on Symptoms of Influenza Virus Infection
Influenza is an acute respiratory illness caused by the influenza virus [46][47]. Influenza increases in winter and is a serious social and economic problem in many countries. Influenza virus infection causes multiple systemic symptoms, including fever over 38 °C, headache, arthralgia, myalgia, and fatigue. Typically, healthy adults recover spontaneously without antiviral treatment through self-healing mechanisms involving the immune response. However, in the elderly, infants, and patients with an underlying respiratory disease, impaired immune function can exacerbate symptoms due to viral infection and, in the worst case, pneumonia or encephalitis, which can be fatal. Because influenza viruses are segmental-stranded RNA viruses, gene replication and reassortment are frequent. Therefore, vaccination does not provide adequate protection against influenza virus infection.
In the previous section, it was shown that Euglena consumption contributes to the regulation of butyrate-producing bacteria occupancy in the gut and may contribute to antiviral activity by stimulating butyrate production; however, the effect of Euglena or paramylon on influenza virus infection is unknown.
This section reviews the alleviating effects of Euglena and paramylon on influenza virus infection symptoms in mice based on survival, lung virus titer, and cytokine production.
Euglena or paramylon administration prevented a decrease in survival after influenza virus infection. High lung virus titers and/or abnormal production of inflammatory cytokines frequently occur with the progression of influenza virus infection and are associated with the severity of the morbidity [48][49][50][51]. Therefore, lung homogenates from infected mice were used to measure lung viral titers and cytokine production. On day 1 post-infection, pulmonary viral titers were similar in the Euglena- and paramylon-fed groups. However, the paramylon-treated group showed lower viral titers on day 2 compared to those of the Euglena-treated group. The production of IL-1β, IFN-β, IFN-γ, and TNF-α was higher in the Euglena group than in the control group. Significant increases in IL-1β, IL-6, IL-12, IL-10, IFN-γ, and TNF-α were detected in the paramylon-treated group, and increased production of IFN-β was observed. These results suggest that paramylon is one of the functional substances in Euglena that alleviate influenza virus infection symptoms. The Euglena used in this experiment contained approximately 30% paramylon of a β-1,3-glucan.
Recent immunological studies have shown that influenza virus infection induces the production of type I interferons such as INF-β, leading to acute inflammation of the lungs, after which INF-β contributes to viral elimination via induction of NK and CD8+ T cells [52][53]. It has also been reported that β-1,3-glucan enhances NK cell activity in influenza virus-infected mice, increases IL-1β, TNF-α, and IFN-γ levels in the lungs [54][55], activates type I interferon, and induces CD8+ T cells via Dectin-1 in dendritic cells [56]. Furthermore, the proliferation, deviation, and activation of NK cells are induced by the IFN, TNF-α, and IL-1 secreted by dendritic cells and macrophages [57][58][59].
Generally, Dectin-1, a C-type lectin-like pattern recognition receptor on the surface of leukocytes, is the primary receptor for β-glucan [60]. The triple-helix structure formed by the backbone of β-1,3-glucan is specifically recognized by Dectin-1. As mentioned above, the crystal structure of paramylon consists of a triple helix [61][62], which is recognized by the β-glucan receptor Dectin-1, and it is presumed that paramylon activates Dectin-1. Moreover, the size of paramylon is typically 2–3 μm, which is comparable to the size of pathogenic bacteria. This suggests paramylons may pass through epithelial cells using the same mechanism as pathogenic bacteria. Indeed, consistent with the above data, paramylon has been shown to bind directly to the recombinant Dectin-1 [63] and upregulate inflammatory factors such as NO, TNF-α, IL-6, and COX-2 [64]. Studies have shown that the number and size of β-1,6 branches on the β-1,3 skeleton are crucial for the function of β-glucans [65][66]; however, despite its limited number of branches, paramylon shows functional diversity. In the results of the study by Nakashima et al. [67], mice were fed the same amount of Euglena and paramylon; however, the survival rate and viral titer in the lungs results tended to be better in the paramylon-fed than in the Euglena-fed group. However, the Euglena-fed group did not show the same results as those of the paramylon-fed group. In particular, the Euglena-fed group showed a different pattern of cytokine production, suggesting the involvement of components other than paramylon in Euglena treatment. These results indicate that Euglena may prevent influenza virus infection not only through the actions of components other than paramylon in the intestinal microflora, but also through systemic immune regulation, mainly through the contribution of paramylon in Euglena.
Oral intake of Euglena and paramylon significantly reduced pulmonary viral titers and increased survival. In addition, paramylon induced significant increases in cytokine levels in the lungs. However, the pattern of cytokine production in the Euglena-fed group did not completely match that of the paramylon-fed group, suggesting the involvement of components other than the paramylon contained in Euglena. The oral intake of Euglena and paramylon can eliminate influenza viruses, mainly through the activity of β-1,3-glucan on dendritic cells and induction of CD8+ T and/or NK cells. Further research is required to gain a more comprehensive understanding of Euglena and paramylon’s functions and their potential for influenza prevention, possibly through their direct effects on the viral replication cycle.
4. Effect of Euglena on Cellular Infection with the Influenza Virus
Influenza is an infectious respiratory tract disease caused by A, B, or C influenza viruses. Type A and C are the most and least common, respectively. Influenza A and B viruses, the most prevalent types, comprise two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which are antigens against the host targets for protective immunity. The antigenic properties of these viruses differ depending on the combination of HA and NA subtypes. Viruses with various combinations of these glycoproteins exist in humans and other parts of the animal kingdom. Accumulation of mutations in HA and NA genes gradually changes the antigenicity of the virus, and new strains emerge, even of the same subtype. Influenza viruses are endemic every year because of frequent antigenic mutations. Generally, after an incubation period of 1–3 days after influenza virus infection, symptoms such as fever (usually higher than 38 °C), headache, general malaise, and muscle and joint pain suddenly occur. This is followed by upper respiratory tract inflammatory symptoms, such as cough and nasal discharge, and symptoms usually abate after one week. Patients, particularly the elderly, with chronic diseases of the respiratory, cardiovascular, and renal systems, or with metabolic diseases such as diabetes, and patients of any age with compromised immune function are more susceptible to secondary bacterial infections of the respiratory system, which can worsen the original disease and increase the risk of hospitalization or death. In children, influenza can also cause otitis media, febrile convulsions, and bronchial asthma. Currently, vaccines are used to prevent influenza virus infection, and antiviral drugs are used for treatment. However, there are concerns about the side effects of adjuvants contained in existing influenza vaccines, the inability to respond rapidly to new viruses, and the fact that these vaccines are less effective in prevention than vaccines against other viruses. Furthermore, the side effects of antiviral drugs are equally problematic, as is the emergence of drug-resistant bacteria [68][69][70][71]. Thus, there is a need to develop new preventive and therapeutic methods to overcome these issues. In light of this, researchers have focused on the functional properties of foods and investigated the mechanisms of action of components with antiviral activity. The previous section mentioned that mice fed a diet containing Euglena powder and subsequently infected with the influenza virus showed improved survival [67]. Based on the pattern of cytokine production in these mice, it was speculated that paramylon, which is mainly contained in Euglena, may have contributed to the elimination of the virus by mobilizing the systemic immune system. However, the antiviral effect of Euglena may have mechanisms other than systemic immunity. In fact, the cytokine production pattern of the Euglena-fed group in the in vivo experiment described in the previous section did not completely match that of the paramylon-fed group, suggesting that components other than the paramylon contained in Euglena may be involved. The direct mechanism by which other components of Euglena suppress viruses in cells was reported by Nakajima et al. [72]. In vitro experiments are necessary to examine the direct effect; however, the paramylon contained in Euglena is an insoluble β-glucan that is difficult to add to the culture medium, making it difficult to conduct in vitro experiments. Therefore, this section aims to clarify the antiproliferative effect of Euglena hot water extract on the influenza virus and its mechanism.
It was confirmed that Euglena hydrothermal extract suppresses virus proliferation using MDCK cells infected with various influenza virus strains. In particular, the results showed that the Euglena hot water extract was effective against oseltamivir-resistant virus strains, suggesting that the mechanism of inhibition of influenza virus growth by the extract is different from that of oseltamivir [72]. In addition, no significant difference in IC50 values was observed among the virus strains examined. Therefore, the virus replication inhibitory activity of the Euglena hot water extract does not show virus specificity [72]. This differs from amantadine, which is effective against type A influenza virus strains but ineffective against type B strains [73].
Once the virus attaches to the cell membrane, it is endocytosed, and RNA is released from the viral particles into the cytoplasm, where it is transferred to the nucleus for replication and transcription, followed by the synthesis of viral proteins and the viral genome. Once the virus components are in place, the budding phase of the viral growth cycle occurs, when the viral particles aggregate near the cell membrane and are released via neuraminidase activity. One growth cycle lasts approximately eight hours, and studying the inhibition of the viral process is possible. "Relenza" and "Tamiflu," which are the mainstream anti-influenza drugs, inhibit the budding phase of the virus by blocking neuraminidase activity [74], whereas the recently launched "Xofluza" inhibits viral RNA replication [75]. Although the mechanism of inhibition by Euglena hydrothermal extract has not yet been predicted, Euglena hydrothermal extract reduced the viral titer by pretreatment or prolonged treatment of infected cells [72]. These results suggest that the Euglena hydrothermal extract activates host cell defense mechanisms.
In the mouse infection experiments described in the previous section, oral ingestion of Euglena alleviated the symptoms of influenza virus infection, mainly through the contribution of paramylon. The ingestion of paramylon by mice reduced the amount of interferon β in their blood, which the virus infection had increased, on day 3 [67]. After insoluble paramylon is recognized by Dectin-1 (a major β-glucan receptor expressed on intestinal immune cells, such as dendritic cells and macrophages) and endocytosed, the activation of tyrosine-protein kinase SYK and transcription factor nuclear factor-kappa B promote cytokine secretion [63][76]. The hydrothermal extract of Euglena consists of water-soluble components and low-molecular-weight substances, such as polyphenols, from which insoluble paramylons are thought to be excluded. The concentration of carbohydrates in the hydrothermal extract of Euglena was 0.53% using the phenol sulfate method, whereas the concentration of carbohydrates in the Euglena powder was approximately 30–40%. However, to discard the possibility that a small amount of glucan was involved, the hydrothermal extract of Euglena was treated with β-glucanase to verify if it affected its antiviral activity, and it was found to have no effect. This suggests that β-glucan is not involved in the in vitro influenza virus growth suppression observed with the hydrothermal extract of Euglena.
Recently, it has been confirmed that the hydrothermal extract of Euglena suppresses lung cancer symptoms in mice by stimulating host immunity [77]. Therefore, components other than paramylon may be involved in such immune mechanisms. Generally, polyphenols exhibit antiviral effects [78]. In the case of the hydrothermal extract of Euglena, as β-glucan and polyphenols showed no antiviral activity, the minerals were analyzed [72]. Zinc is required for the growth of Euglena and accumulates in the cells. In addition, compared to other metals, zinc has antiviral activity by promoting the induction of type 1 interferon receptors, thereby inducing the production of the antiviral protein 2’–5’ oligoadenylate synthetases [79]. In the case of SARS-CoV and equine arteritis virus (EAV), the RNA synthesis is catalyzed by an RNA-dependent RNA polymerase, which is directly inhibited by zinc [80]. Specifically, zinc blocks the initiation step of EAV RNA synthesis, whereas the RNA-dependent RNA polymerase elongation is inhibited and template binding reduced in the case of the SARS-CoV [80]. Nakashima et al. [72] showed that the addition of zinc acetate inhibited influenza virus growth. Furthermore, the addition of zinc acetate to the demineralized Euglena extract restored its influenza virus inhibitory activity. The CER (cation exchange resin)-treated Euglena extract, which has an equivalent zinc concentration, has higher anti-influenza virus inhibitory activity than zinc alone [72]. Ionophores are required for zinc uptake into cells [80]. Euglena may contain substances that act similarly to ionophores, whereas the hydrothermal extract of Euglena may contain higher concentrations. The ionophores may capture zinc, ensuring some anti-influenza virus activity of the membrane-treated extract at high concentrations. Therefore, the effect of zinc acetate was retained in the normal Euglena extract because of the presence of free zinc, but in CER-treated Euglena, the removal of free zinc by membrane treatment allowed the ionophores to exert the effect of the zinc captured by the ionophores. This suggests that substances other than zinc are also involved in the anti-influenza virus activity of Euglena.
This indicates that virus elimination is not only caused by the paramylon in Euglena eliminating viruses by activating the systemic immunity, but also by the action of other components of Euglena, including zinc, which may act directly on cellular defense mechanisms. A limitation is that the MDCK cells used in the aforementioned study are regularly used in viral research, whereas cells of other origins should also be investigated.
In conclusion, hydrothermal extracts of Euglena reduced the viral titer of various influenza viruses in vitro, and its inhibitory activity was more potent when host cells were pre-exposed to hydrothermal extracts of Euglena, suggesting that Euglena components may provide signals to enhance host cell defense mechanisms. Furthermore, the Euglena extract inhibits the growth of influenza viruses by a nonspecific mechanism different from those of existing drugs. Therefore, this extract may be a promising treatment for infections caused by newly mutated influenza virus strains.
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzuki, H.; Fujieda, S.; Masuyama, K. The Japanese Society of Allergology. 2017. Japanese guidelines for allergic rhinitis. Allergol. Int. 2017, 66, 205–219.
- Yasueda, H.; Yui, Y.; Shimizu, T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) polln. J. Allergy Clin. Immunol. 1983, 71, 77.
- Sakaguchi, M.; Inouye, S.; Taniai, M.; Ando, S.; Usui, M.; Matuhasi, T. Identification of the second major allergen of Japanese cedar pollen. Allergy 1990, 45, 309–312.
- Fujiwara, D.; Wakabayashi, H.; Watanabe, H.; Nishida, S.; Iino, H. A double-blind trial of Lactobacillus paracasei strain KW3110 administration for immunomodulation in patients with pollen allergy. Allergol. Int. 2005, 54, 143–149.
- Maeda-Yamamoto, M.; Ema, K.; Monobe, M.; Shibuichi, I.; Shinoda, Y.; Yamamoto, T.; Fujisawa, T. The efficancy of early treatment of seasonal allergic rhinitis with benifuuki green tea containing O-methylated catechin before pollen exposure: An open randomized study. Allergol. Int. 2009, 58, 437–444.
- Sato, Y.; Akiyama, H.; Sugamura, H.; Watanabe, T.; Hamano-Nagaoka, M.; Inakuma, T.; Goda, Y.; Maitani, T. The feeding of β-carotene down-regulates serum IgE levels and inhibits the type I allergic response in mice. Biol. Pharm. Bull. 2004, 27, 978–984.
- Kopf, M.; Le Gros, G.; Bachmann, M.; Lamers, M.C.; Bluethmann, H.; Köhler, G. Disruption of the murine IL-4gene blocks Th2 cytokine respnses. Nature 1993, 362, 245–248.
- Kondo, Y.; Kato, A.; Hojo, H.; Nozoe, S.; Takeuchi, M.; Ochi, K. Cytokine-related immunopotentiating activities of paramylon, a β-(1→3)-D-glucan from Euglena gracilis. J. Pharmacobio.-Dyn. 1992, 15, 617–621.
- Sugiyama, A.; Suzuki, K.; Mitra, S.; Arashida, R.; Yoshida, E.; Nakano, R.; Yabuta, Y.; Takeuchi, T. Hepatoprotective effects of pramylon, a β-1,3-D-glucan isolated from Euglena gracilis Z, on acute liver injury induced by carbon tetrachloride in rats. J. Vet. Med. Sci. 2009, 71, 885–890.
- Koizumi, N.; Sakagami, H.; Utsumi, A.; Fujinaga, S.; Takada, M.; Asano, K.; Sugawara, I.; Ichikawa, S.; Kondo, H.; Mori, S.; et al. Anti-HIV (human immunodeficiency virus) activity of sulfated paramylon. Antivir. Res. 1993, 21, 1–14.
- Sakagami, H.; Kikuchi, K.; Takeda, M.; Sato, T.; Ichikawa, S.; Fujimaki, M.; Wada, C.; Komatsu, N. Macrophage stimulation activity of antimicrobial N, N-dimethylaminoethyl paramylon. In Vivo 1991, 5, 101–105.
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arashida, R.; Asayama, Y.; Yabuta, Y.; Takeuchi, T. Oral administration of paramylon, a β-1,3-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763.
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466.
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999, 120, 70–75.
- Iida, M.; Desamero, M.J.; Yasuda, K.; Nakashima, A.; Suzuki, K.; Chambers, J.K.; Uchida, K.; Ogawa, R.; Hachimura, S.; Nakayama, J.; et al. Effect of orally administered Euglena gracilis and its reserve polysaccharide, paramylon, on gastric dysplasia in A4gnt knockout mice. Sci. Rep. 2021, 11, 13640.
- Watanabe, T.; Shimada, R.; Matsuyama, A.; Yuasa, M.; Sawamura, H.; Yoshida, E.; Suzuki, K. Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. Food Funct. 2013, 4, 1685–1690.
- Kimura, Y.; Sumiyoshi, M.; Suzuki, T.; Sakanaka, M. Inhibitory effects of water-soluble low-molecular-weight beta-(1,3-1,6) d-glucan purified from Aureobasidium pullulans GM-NH-1A1 strain on food allergic reactions in mice. Int. Immunopharmacol. 2007, 7, 963–972.
- Yamada, J.; Hamuro, J.; Hatanaka, H.; Hamabata, K.; Kinoshita, S. Alleviation of seasonal allergic symptoms with super fine beta-1,3-glucan: A randomized study. J. Allergy Clin. Immunol. 2007, 119, 1119–1126.
- Koizumi, M.; Yoshii, Y.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Yoshida, E.; Suzuki, K. Effect of euglena on cedar pollen allergies in cry j1-sensitized mice. J. Integr. Study Diet. Habits 2013, 24, 171–176.
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious disease, immunopathology, and cancer. Front. Immunol. 2018, 9, 1830.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514.
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225.
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493.
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721.
- Hague, A.; Elder, D.J.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406.
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829.
- Schneider, S.M.; Le Gall, P.; Girard-Pipau, F.; Piche, T.; Pompei, A.; Nano, J.L.; Hébuterne, X.; Rampal, P. Total artificial nutrition is associated with major changes in the fecal flora. Eur. J. Nutr. 2000, 39, 248–255.
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164.
- Sasaki, K.; Inoue, J.; Sasaki, D.; Hoshi, N.; Shirai, T.; Fukuda, I.; Azuma, T.; Kondo, A.; Osawa, R. Construction of a model culture system of human colonic microbiota to detect decreased Lachnospiraceae abundance and butyrogenesis in the feces of ulcerative colitis patients. Biotechnol. J. 2019, 14, e1800555.
- Nakashima, A.; Sasaki, K.; Sasaki, D.; Yasuda, K.; Suzuko, K.; Kondo, A. The alga Euglena gracilis stimulates Faecalibacterium in the gut and contributes to increased defecation. Sci. Rep. 2021, 11, 1074.
- Miquel, S.; Martin, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261.
- Gao, Z.; Yin, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517.
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci. Rep. 2017, 7, 7046.
- Shimada, R.; Fujita, M.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Nakashima, A.; Suzuki, K. Oral administration of green algae, Euglena gracilis, inhibits hyperglycemia in OLETF rats, a model of spontaneous type 2 diabetes. Food Funct. 2016, 7, 4655–4659.
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts from Euglena gracilis: Synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 108.
- Sugimoto, R.; Ishibashi-Ohgo, N.; Atsuji, K.; Miwa, Y.; Iwata, O.; Nakashima, A.; Suzuki, K. Euglena extract suppresses adipocyte-differentiation in human adipose-derived stem cells. PLoS ONE 2018, 13, e0192404.
- Okouchi, R.E.S.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K.; Tsuduki, T. Simultaneous intake of Euglena gracilis and vegetables exerts synergistic anti-obesity and anti-inflammatory effects by modulating the gut microbiota in diet-induced obese mice. Nutrients 2019, 11, 204.
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176.
- Asayama, Y.; Suzuki, K.; Nakashima, A.; Shioya, N.; Sugimura, H. The study of the effects of food containing Euglena gracilis on improvement in constipation. Jpn. Pharmacol. Ther. 2017, 45, 1359–1364.
- Kawano, Y.; Nakano, Y.; Kitaoka, S.; Katou, K.; Shigeoka, S.; Ohnishi, T. Effects of Euglena cells on the absorption and tissue distribution of dietary cholesterol in rats. J. Jpn. Soc. Nutr. Food. Sci. 1987, 40, 193–198.
- Yang, J.; Wang, H.-P.; Zhou, L.; Xu, C.-F. Effect of dietary fiber on constipation: A meta analysis. World J. Gastroenterol. 2012, 18, 7378–7383.
- Pituch, A.; Walkowiak, J.; Banaszkiewicz, A. Butyric acid in functional constipation. Prz. Gastroenterol. 2013, 8, 295–298.
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary fiber confers protection against flu by shaping Ly6c– patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018, 48, 992–1005.e8.
- Taubenberger, J.K.; Morens, D.M. Influenza: The once and future pandemic. Public Health Rep. 2010, 125, 16–26.
- McCaughey, C. Influenza: A virus of our times. Ulster Med. J. 2010, 79, 46–51.
- Van Reeth, K.; Nauwynck, H.; Pensaert, M. Bronchoalveolar interferon-α, tumornecrosis factor-α, interleukin-1, and inflammation during acute influenza inpigs: A possible model for humans. J. Infect. Dis. 1998, 177, 1076–1079.
- Julkunen, I.; Sareneva, T.; Pirhonen, J.; Ronni, T.; Melén, K.; Matikaine, S. Molecular pathogenesis of influenzaA virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001, 12, 171–180.
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom pathogenesis during acuteinfluenza: Interleukin-6 and other cytokine responses. J. Med. Virol. 2001, 64, 262–268.
- Mok, K.P.; Wong, C.H.K.; Cheung, C.Y.; Chan, M.C.; Lee, S.M.Y.; Nicholls, J.M.; Peiris, J.S.M. Viral genetic determinants of H5N1 influenza viruses that contribute to cytokine dysregulation. J. Infect. Dis. 2009, 200, 1104–1112.
- Arimori, Y.; Nakamura, R.; Yamada, H.; Shibata, K.; Maeda, N.; Kase, T.; Yoshikai, Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antivir. Res. 2013, 99, 230–237.
- Arimori, Y.; Nakamura, R.; Yamada, H.; Shibata, K.; Maeda, N.; Kase, T.; Yoshikai, Y. Type I interferon plays opposing roles in cytotoxicity and interferon-γ production by natural killer and CD8 T cells after influenza A virus infection in mice. J. Innate Immun. 2014, 6, 456–466.
- Muramatsu, D.; Iwai, A.; Aoki, S.; Uchiyama, H.; Kawata, K.; Nakayama, Y.; Nikawa, Y.; Kusano, K.; Okabe, M.; Miyazaki, T. β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 2012, 7, e41399.
- Vetvicka, V.; Vetvickova, J. Glucan supplementation enhances the immune response against an influenza challenge in mice. Ann. Transl. Med. 2015, 3, 22.
- Hassanzadeh-Kiabi, N.; Yáñez, A.; Dang, I.; Martins, G.A.; Underhill, D.M.; Goodridge, H.S. Autocrine type I IFN signaling in dendritic cells stimulated with fungal β-glucans or lipopolysaccharides promotes CD8 T cell activation. J. Immunol. 2017, 198, 375–382.
- Gibbert, K.; Schlaak, J.F.; Yang, D.; Ditmer, U. IFN-α subtypes: Distinct biological activities in anti-viral therapy. Br. J. Pharmacol. 2013, 168, 1048–1058.
- Hunter, C.A.; Chizzonite, R.; Remington, J.S. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 1995, 155, 4347–4354.
- Sabel, M.S.; Arora, A.; Su, G.; Mathiowitz, E.; Reineke, J.J.; Chang, A.E. Synergistic effect of intratumoral IL-12 and TNF-alpha microspheres: Systemic anti-tumor immunity is mediated by both CD8+ CTL and NK cells. Surgery 2007, 142, 749–760.
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37.
- Kiss, J.Z.; Roberts, E.M.; Brown, R.M.; Triemer, R.E. X-ray and dissolution studies of paramylon storage granules from Euglena. Protoplasma 1988, 146, 150–156.
- Chuah, C.T.; Sarko, A.; Deslandes, Y.; Marchessault, R.H. Packing analysis of carbohydrates and polysaccharides. Part 14. Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 1983, 16, 1375–1382.
- Ujita, M.; Nagayama, H.; Kanie, S.; Koike, S.; Ikeyama, Y.; Ozaki, T.; Okumura, H. Carbohydrate binding specificity of recombinant human macrophage β-glucan receptor dectin-1. Biosci. Biotechnol. Biochem. 2009, 73, 237–240.
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2016, 5, 205–214.
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular interactions of β-(1→3)-glucans with their receptors. Molecules 2015, 20, 9745–9766.
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-D-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14.
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383.
- Belshe, R.B.; Burk, K.; Newman, F.; Cerruti, R.L.; Sim, I.S. Resistance of influenza A virus to amantadine and rimantadine: Results of one decade of surveillance. J. Infect. Dis. 1989, 159, 430–435.
- Bloom, J.D.; Gong, L.I.; Baltimore, D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 2010, 328, 1272–1275.
- Le, Q.M.; Kiso, M.; Someya, K.; Sakai, Y.T.; Nguyen, T.H.; Ngyuen, K.H.; Pham, N.D.; Ngyen, H.H.; Yamada, S.; Muramoto, Y.; et al. Avian flu: Isolation of drug resistant H5N1 influenza A virus. Nature 2005, 437, 1108.
- Seibert, C.W.; Rahmat, S.; Krammer, F.; Palese, P.; Bouvier, N.M. Efficient transmission of pandemic H1N1 influenza viruses with high-level oseltamivir resistance. J. Virol. 2012, 86, 5386–5389.
- Nakashima, A.; Horio, Y.; Suzuki, K.; Isegawa, Y. Antiviral activity and underlying action mechanism of Euglena extract against influenza virus. Nutrients 2021, 13, 3911.
- Barr, I.G.; Hurt, A.C.; Iannello, P.; Tomasov, C.; Deed, N.; Komadina, N. Increased adamantane resistance in influenza A(H3) viruses in Australia and neighbouring countries in 2005. Antivir. Res. 2007, 73, 112–117.
- Nitsch-Osuch, A.; Brydak, L.B. Influenza viruses resistant to neuraminidase inhibitors. Acta Biochim. Pol. 2014, 61, 505–508.
- Todd, B.; Tchesnokov, E.P.; Götte, M. The active form of the influenza cap-snatching endonuclease inhibitor baloxavir marboxil is a tight binding inhibitor. J. Biol. Chem. 2021, 296, 100486.
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in foods and its physiological functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17.
- Ishiguro, S.; Upreti, D.; Robben, N.; Burghart, R.; Loyd, M.; Ogun, D.; Le, T.; Delzeit, J.; Nakashima, A.; Thakkar, R.; et al. Water extract from Euglena gracilis prevents lung carcinoma growth in mice by attenuation of the my-eloid-derived cell population. Biomed. Pharmacother. 2020, 127, 110166.
- Yang, Z.-F.; Bai, L.-P.; Huang, W.-B.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia 2014, 93, 47–53.
- Nagamine, T.; Nakajima, K.; Takada, H.; Sekine, Y.; Suzuki, K. Induction of type 1 interferon receptor by zinc in U937 cells. Cytokine 2009, 46, 346–350.
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176.
More
Information
Subjects:
Food Science & Technology; Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
565
Revisions:
4 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




