Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Luísa De Sousa-Coelho | -- | 2903 | 2023-12-27 02:23:04 | | | |
| 2 | Camila Xu | Meta information modification | 2903 | 2023-12-27 02:30:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Sousa-Coelho, A.L.; Fraqueza, G.; Aureliano, M. Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/53154 (accessed on 11 January 2026).
De Sousa-Coelho AL, Fraqueza G, Aureliano M. Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/53154. Accessed January 11, 2026.
De Sousa-Coelho, Ana Luísa, Gil Fraqueza, Manuel Aureliano. "Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer" Encyclopedia, https://encyclopedia.pub/entry/53154 (accessed January 11, 2026).
De Sousa-Coelho, A.L., Fraqueza, G., & Aureliano, M. (2023, December 27). Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/53154
De Sousa-Coelho, Ana Luísa, et al. "Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer." Encyclopedia. Web. 27 December, 2023.
Copy Citation
Repurposing drugs by uncovering new indications for approved drugs accelerates the process of establishing new treatments and reduces the high costs of drug discovery and development. Metal complexes with clinically approved drugs allow further opportunities in cancer therapy. Many vanadium compounds have previously shown antitumor effects, which makes vanadium a suitable metal to complex with therapeutic drugs, potentially improving their efficacy in cancer treatment.
drug repurposing
vanadium complexes
vanadate
decavanadate
cancer treatment
1. Introduction
Several metals and metal complexes such as platinum, gold, and ruthenium, and complexes containing essential metals such as Mn, Cu, and Co, show potential for application in medicine [1][2][3]. Vanadium, although yet to be fully investigated, displays specific versatile properties that allow it to form several types of complexes and compounds with distinct biological applications. These have been investigated as potential therapeutic agents against relevant first-world diseases such as diabetes, cancer, and neurodegenerative and other aging-related diseases [4][5].
Although there has been an exponential increase in the number of papers published in the field over the last few decades, further studies are still required to fully comprehend the mechanisms of action of metal-based drugs. For vanadium compounds, several mechanisms of action have been proposed [6][7], namely the effects of vanadium on oxidative stress and lipid peroxidation [8]. In addition to being a transition metal, and thus inducing Fenton-like reactions, vanadium has the ability to form polyoxidovanadates (POVs) that can target several biomolecules and affect essential biochemical processes. Some of these processes have direct and/or indirect associations with oxidative stress, aging, and diseases, although the major mechanisms of action of vanadium and metals in general are yet to be completely understood.
Although past research has studied the application of vanadium-based compounds in clinical practice, there is a need to further explore their potential against cancer diseases. The repurposing of clinically approved drugs, complexed as metal-based drugs, may represent a simple approach to close the gap and increase the number of vanadium metallodrugs for cancer treatments.
2. Drug Repurposing in Cancer
The number of articles published related to drug repurposing increased substantially over the last decade [9]. Drug repurposing, or repositioning, refers to the identification and application of clinically approved drugs to alternative disease indications and new therapeutic purposes. Because of their established formulations, known adverse effect profiles, and defined pharmacokinetic properties, the re-use of such existing therapies for new indications can save the time and money otherwise invested for the de novo drug design and development [10]. Repurposed therapies for cancer patients allow for faster treatment with fewer restrictions due to safety concerns. Additionally, drugs that do not display direct cytotoxicity may be combined to target distinct critical pathways, potentially producing a synergistic therapeutic effect or allowing tailored regimens, increasing the number of patients who will benefit from precision medicine [11]. The re-use of existing oncological drugs for new oncological indications (i.e., different types of cancers), is referred to as “soft repurposing”, whereas the use of non-cancer drugs as anticancer medications is referred to as “hard repurposing” [12].
Drugs from many different pharmacotherapeutic classes may be amenable to repositioning. For instance, antiparasitic (ex.: mebendazole) and antiepileptic (ex.: valproate) drugs are potentially novel options for glioblastoma based on their ability to cross the blood–brain barrier [13][14][15]. However, there is greater evidence for the repurposing of antidiabetic and antihypertensive drugs [16][17], given their efficacy in chronic metabolic diseases (diabetes and cardiovascular disease), because (1) these diseases are highly prevalent and their treatments well-studied, with an increased amount of knowledge regarding their use and potential benefit; (2) there are metabolic links and shared risk factors between these diseases and cancer; and (3) drugs that can be administered chronically are normally well-tolerated [18].
Repurposed drugs are emerging as promising strategies to overcome therapy resistance, one of the greatest challenges of current cancer treatments. If the molecular pathways driving drug resistance are identified, drugs that specifically target those pathways will be a great asset. For instance, it is expected that when combined with immunotherapies, repurposed drugs that modulate the immunosuppressive tumor microenvironment (TME), will boost their effect and avoid therapeutic failure [19][20][21]. This means that optimized approaches are needed to identify “old” candidate drugs with such actions. Indeed, the range of computational predictive tools, high-throughput screening methods, machine learning algorithms, bioinformatics analysis, and artificial intelligence that facilitate the drug repurposing process, unraveling molecular signatures, and contribute to novel, affordable, and tailored treatment options, is very impressive [22][23][24][25].
3. The Potential of Vanadium for Cancer Therapeutics
Many vanadium complexes show therapeutic potential in cancer. Involved pathways for vanadium compounds may include AMP-activated protein kinase (AMPK) activation and protein tyrosine phosphatase 1B (PTP1B) inhibition pathways, as described for the treatment of breast cancer [26]. Polyoxidovanadates (POVs) inhibit the activity of P-type ATPases [27][28][29] and oxygen consumption in the mitochondria [30]. Meanwhile, changes in lipid peroxidation may be one of the mechanisms involved in the anticancer action of vanadium [8]. In melanoma, several vanadium compounds and/or materials lead to decreased cell viability, changes in cell morphology and apoptosis, cell cycle arrest, production of reactive oxygen species (ROS), inhibition of mitochondrial respiration, differential expression of proteins and signaling, and tumor regression along with increased survival rates in animal models [31]. Over the last few years, significant evidence has been gathered supporting the application of different vanadium complexes as anticancer agents, which is extensively reviewed elsewhere [32][33][34][35][36].
4. Vanadium Complexed with Marketed-Approved Drugs
Vanadium-based complexes may incorporate different ligands, ranging from chemical elements such as cobalt to plant pigments (flavonoids) [37][38], or synthetic drugs, enclosing defined pharmacological properties and indications [39]. Complexes of metal ions with free drugs may reduce the toxicity of the drugs and increase their lipophilicity, improving their transport across cell membranes [40]. Here, the effects of several vanadium compounds and complexes with distinct chemical structures are described (Figure 1).
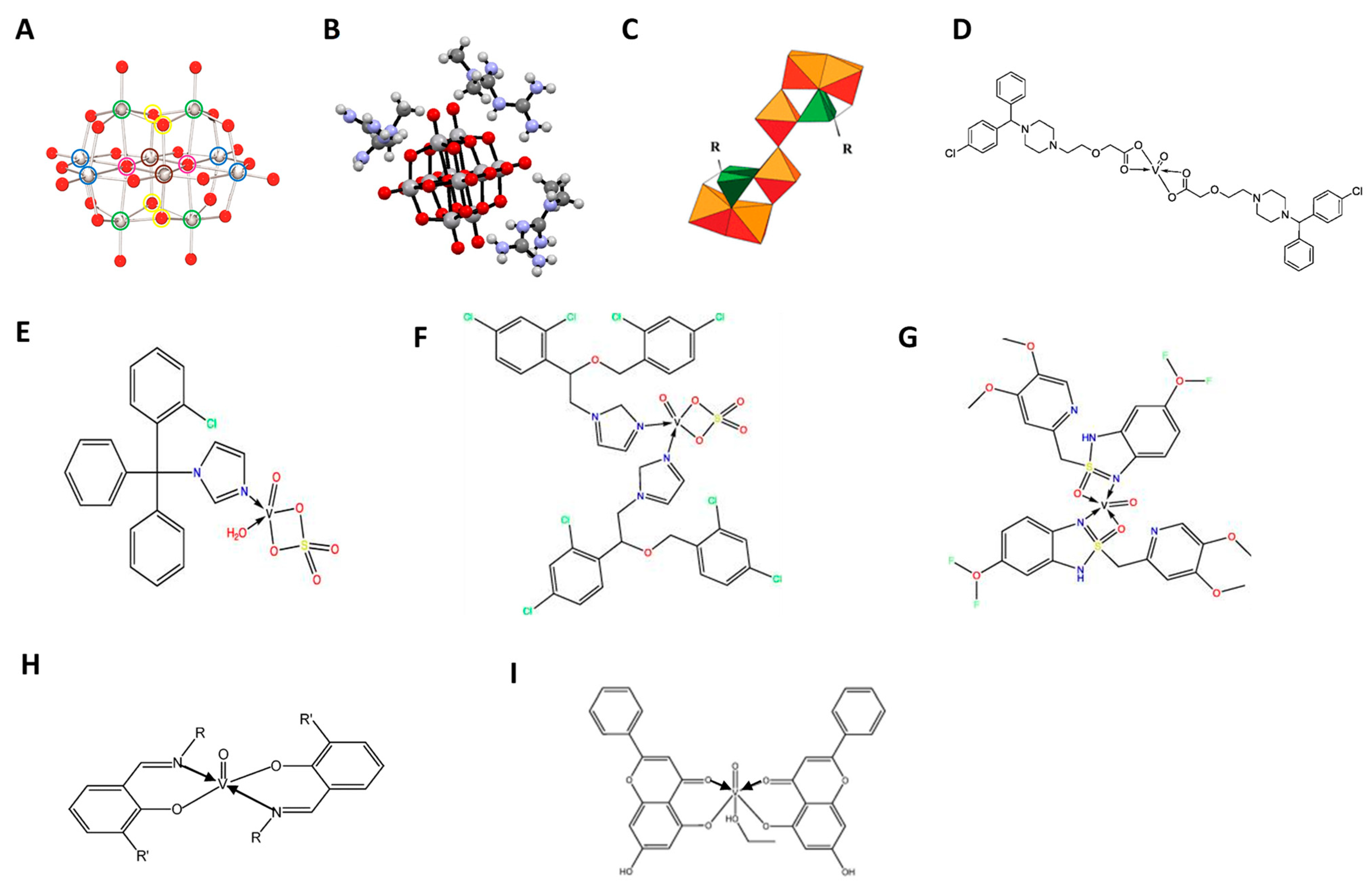
Figure 1. Structures of vanadium compounds and complexes with approved drugs. (A) Structure of the decameric species of vanadate, decavanadate, V10O286-. Color code: V, gray; O, red. The green (four), blue (four), and brown (two) circles refer to vanadium atoms with the same chemical environment [41]; (B) Ball and stick representation of metforminium decavanadate (H2Metf)3[V10O28]·8H2O. Water molecules are omitted for clarity [42]; (C) Polyhedral representation common to the Mo6L2 (where L corresponds to a ligand, either alendronate (Ale) or zoledronate (Zol)) POM frameworks, green tetrahedral = PO3C, orange polyhedra = MoO6 [43]; (D) Oxidovanadium(IV) complexes with cetirizine, [VO(CTZ)2] 2H2O [44]; (E) Clotrimazole oxidovanadium(IV) complex [VO(SO4)(CTNZ)(H2O)]H2O; (F) Miconazole oxidovanadium(IV) complex, [VO(SO4)(MNZ)2] H2O; (G) Pantoprazole oxidovanadium(IV) complex, [VO(PNZ)2]SO4.2H2O; (H) Oxidovanadium(IV) complexes with Schift based compounds, such as for ibuprofen and naproxen [45]; (I) Oxidovanadium(IV) chrysin complex [46].
4.1. Vanadyl(IV) Complexes with Non-Steroidal Anti-Inflammatory Drugs
Tumor-promoting inflammation is one of the hallmarks of cancers, along with many others, such as avoiding immune destruction [47]. The connection of inflammation with tumor development and progression may justify the interest in exploring anti-inflammatory drugs in cancer research. Studies regarding their repurposing have shown their potential as chemopreventive agents against certain types of cancer or as anticancer agents [48]. In fact, non-steroidal anti-inflammatory drugs (NSAIDs) may protect against the development of cancer, as studied for aspirin and ibuprofen at low doses [49].
Notably, such NSAIDs have a carboxylate group available for metal–ligand interaction, which has raised interest in their use in complexes with vanadium for medicinal applications [50]. Different vanadyl(IV) complexes with either ibuprofen (2-(4-isobutylphenyl)propionic acid) ([VO(Ibu)2].5CH3OH) or naproxen (6-methoxy-α-methyl-2-naphthalene acetic acid) ([VO(Nap)2].5CH3OH) have been synthesized [51]. All NSAIDs–VO2+ complexes (Figure 1H) were then characterized with respect to their potential effect on the proliferation of osteoblast-like cells [51]. The results of the mitogenic bioassay with increasing concentrations of NSAIDs-VO2+, in both tumoral UMR106 from a rat osteosarcoma (Figure 2A) and non-transformed MC3T3E1 derived from mouse calvaria (Figure 2B), showed in some cases a biphasic effect (Ibu-VO and Nap-VO in UMR106), or the inhibition of cell growth in a dose–response manner (Nap-VO in MC3T3E1 cells and UMR106 cells in high doses) (Figure 2). From all tested compounds, Nap-VO was the most potent inhibitor of cell growth, mainly in osteosarcoma cells (Figure 2A), as subsequently confirmed by the same research group [52]. By contrast, ibuprofen and naproxen alone, tested in the same concentration range of 0–100 µM, neither inhibited nor promoted cell proliferation.
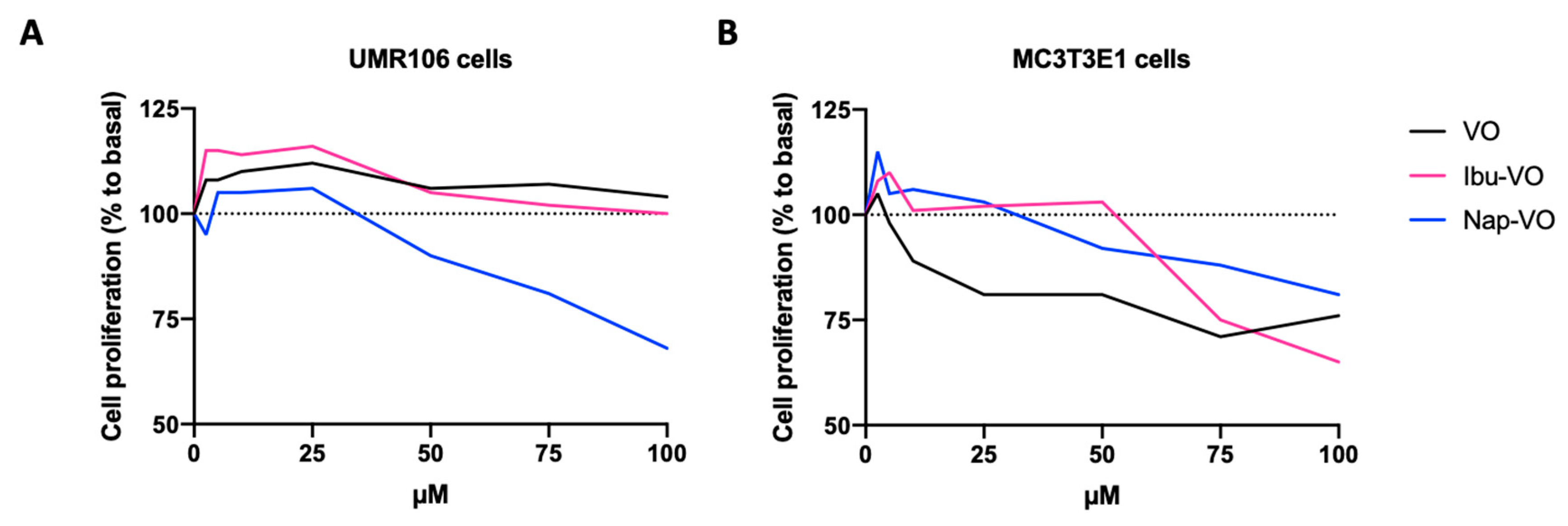
Figure 2. Effects of Ibu-VO, Nap-VO, and VO, on UMR106 (A) and MC3T3E1 (B) cell proliferation. Approximate values were extracted from [51] and are expressed as a percentage of the basal value (without treatment, 0 µM). Abbreviations: Ibu-VO, vanadyl(IV) complexes with ibuprofen; Nap-VO, vanadyl(IV) complexes with naproxen; VO, vanadyl(IV).
In the case of the vanadyl(IV)–aspirin complex (Asp-VO), the non-transformed cell line was found to be more sensitive to such derivatives when compared with the osteosarcoma cell line [53]. Nevertheless, a follow-up study showed that Asp-VO was able to inhibit cell adhesion, spreading, and migration in UMR106 cells, in a mechanism dependent on protein kinase A (PKA) activity [54]. Taken together, these results highlight the need for investigating the anticarcinogenic potential of NSAIDs–VO2+ complexes in other types of tumors.
4.2. Vanadium Compounds Bound to Bisphosphonates
Bisphosphonates (BPs) are used to treat bone resorption. Both alendronate (Ale) and zoledronate (Zol) are classed as nitrogen-containing BPs. BPs can induce apoptosis, due to the production of cytotoxic ATP analogs [55][56]. In addition, BPs can inhibit cell adhesion, invasion, and proliferation; modulate the immune system, and affect angiogenesis [57]. Because of its high affinity for bone, Zol is used in the treatment of metastatic prostate bone metastases [58][59][60]. BPs also reduce bone metastasis and mortality in patients with early-stage breast cancer [61][62]. Moreover, recent evidence suggested an association between the use of BPs and reduced risk of endometrial cancer, mainly in postmenopausal women [63]. However, the known adverse effects of BPs [64][65] justify developing safe and effective bisphosphonate conjugates for adjuvant treatment of metastatic bone cancers. Indeed, many BP-conjugates containing anticancer drugs were previously tested [66], while other authors have proposed encapsulation in liposomal nanoparticles [67] to improve uptake and efficiency, and to decrease toxicity.
Hybrid vanadium-bisphosphonates (V-BPs) (Figure 1C) showed anticancer activity [43]. BPs complexed with polyoxidovanadates with nuclearities ranging from 3 to 6, V6(Ale)4, V5(Ale)2, V5(Zol)2, and V3(Zol)3, inhibited the proliferation of different tumor cell lines, such as MCF-7 (breast cancer), NCI-H460 (lung cancer), and SF-268 (glioblastoma) (Table 1) [43]. While the calculated IC50 values were comparable with those obtained when treating cells with decavanadate (Na6[VV10O28]), they were much lower than for the ligands themselves, especially for free alendronate (Ale), which was also considerably less potent than zoledronate (Zol) (Table 1). Nevertheless, the differences between the four V-BPs were minimal, suggesting that the BPs do not play a major role in inhibiting cell viability and that most of the activity comes from the inorganic part. Compared with other hybrid BPs, polyoxidometalates (POMs) such as with MoVI and WVI, the complexes containing VIV,V cores, showed the greatest inhibitory potential [43].
Table 1. Human tumor cell growth inhibition upon vanadium complexes with approved drugs and for decavanadate. IC50 (µM) determined by MTT ((3-(4,5-dimethylthiazole-2-yl)-2,5-diphenylte-trazolium bromide) cell proliferation assay. The values were collected from [43].
| Treatment | NCI-H460 Cells | MCF-7 Cells | SF-268 Cells |
|---|---|---|---|
| V6(Ale)4 | 0.4 ± 0.0 | 0.5 ± 0.3 | 0.5 ± 0.2 |
| V5(Ale)2 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.2 |
| Ale | 200 ± 43 | 130 ± 2.2 | 140 ± 13 |
| V5(Zol)2 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.0 |
| V3(Zol)3 | 0.3 ± 0.2 | 0.3 ± 0.0 | 0.3 ± 0.2 |
| Zol | 8.1 ± 1.7 | 7.7 ± 2.6 | 12.4 ± 1.4 |
| Na6[V10O28] | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 |
Interestingly, both V5(Ale)2 and V3(Zol)3 complexes also showed antiparasitic potential, reducing the viability of Leishmania tarentolae cultures, while the ligands alone (i.e., alendronate (Ale) or zoledronate (Zol)) did not show activity against these parasites [68], highlighting the therapeutic potential of such vanadium-based compounds.
4.3. Metformin-Decavanadate
Metformin belongs to the biguanide group of antidiabetic drugs that have been widely used for many years [69][70]. Based on its safety profile and the current knowledge of its mechanisms of action, metformin has additional approved medical off-label indications (namely obesity and polycystic ovary syndrome) and has accumulated evidence to be repositioned for the treatment of age-related diseases (such as sarcopenia), inflammatory diseases, and cancer [71][72][73]. Almost two decades ago, the first epidemiological evidence revealed that diabetic patients taking metformin were less prone to developing cancer [74]. Metformin is, by far, the most frequently studied antidiabetic agent in clinical trials (typically combined with chemotherapy) [75]. However, it is currently debatable whether metformin as a cancer therapeutic is truly effective [73][76][77], despite new evidence regarding its potential benefits when combined with immunotherapy [78][79].
After its synthesis and characterization [80], metformin-decavanadate (Metf-V10) (Figure 1B) was proposed for the treatment of diabetes mellitus, and found to have hypoglycemic properties and an excellent safety profile in animal models [42][81][82]. Recently, it was further tested for its potential anticancer action in hepatoma and melanoma cell lines [83][84]. When compared to the decavanadate sodium salt (V10), a higher concentration was needed to induce 50% inhibition of Ca2+-ATPase enzyme activity (IC50) (around six-fold), although similar IC50 values were obtained in UACC-62 melanoma cells viability (1.3-fold higher in V10 (Figure 1A) compared to Metf-V10) [84]. In the hepatoma HepG2 cells, by contrast, a 3-fold higher IC50 was observed for Metf-V10 compared to V10 [83]. Despite these inconsistencies, both studies showed PI3K/AKT signaling pathways were activated by both Metf-V10 and V10 in a dose-dependent manner [83][84], suggesting that AKT hyperactivation could be one of the mechanisms of action involved, independent of the cancer cellular context.
4.4. Cetirizine-Based Oxidovanadium (IV) Complex
Cetirizine (CTZ) is an antihistamine medicine commonly used for treating allergic diseases. Other antihistaminic drugs showed antitumoral potential, particularly in colorectal cancer, associated with enhanced immune response [85]. Improved cancer survival was associated with the administration of the antihistamine desloratadine, specifically in patients with tumors that respond to therapy with immune checkpoint inhibitors, while lower evidence was found for CTZ, which was only observed in gastric, pancreatic, and ovarian cancer [86]. However, others showed that the concomitant use of CTZ and anti-PD-1 monoclonal antibodies led to increased progression-free survival in patients with stage IIIb-IV melanoma, suggesting that the effect of CTZ may synergize with immunotherapies enhancing its efficacy [87].
Recently, the propensity for DNA binding and biological potency of different VO2+ complexes was evaluated by absorption titration and electrophilicity, respectively. Their behavior on a specific protein in colon cancer cells was also studied using molecular docking [44]. The cetirizine-based oxidovanadium(IV) complex ([VO(CTZ)2].2H2O) (Figure 1D) showed enhanced binding affinity to the studied protein when compared with the free ligand (i.e., CTZ). Based on the quantitative structure–activity relationships (QSAR) model, a prediction of effective activity against colon cancer was obtained for the CTZ complex (PRED IC50 = 1.45 μM) (Figure 3). When performing cellular in vitro experiments of cytotoxicity (sulforhodamine B method), the IC50 of [VO(CTZ)2].2H2O was comparable to the predicted value for the human colon cancer cell line HCT116 (2.11 μM) (Figure 3), and over 300 times higher for the normal cell line LLC-MK2 (649.8 μM). Interestingly, when compared to cisplatin (2.13 μM), the [VO(CTZ)2].2H2O complex showed similar IC50 values, other than presenting the highest Kb value (1.40 × 106 M−1) upon DNA interaction, which implies that the compound has a better binding ability compared with other vanadium compounds and its ligands [44].
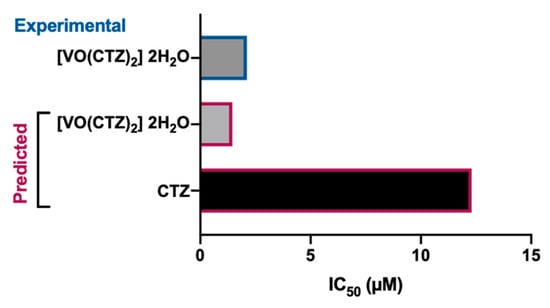
Figure 3. Predicted and experimental anticancer activity of cetirizine (CTZ) and [VO(CTZ)2] 2H2O in colon cancer. The IC50 values were extracted from [44].
The authors of the above study also synthesized and characterized other drug-based oxidovanadium(IV) complexes, namely with carbimazole ([VO(SO4)(CBZ)] 8H2O), lornoxicam ([VO(LOR)2] SO4) and sulfonamide ([VO(SO4)(SCZ)] 7H2O), though those were considered with lower biological potency and less capacity as anticancer agents, compared to the cetirizine complex [44].
4.5. Clotrimazole (CTNZ), Miconazole (MNZ), and Pantoprazole (PNZ) Vanadyl-Based Complexes
Imidazole derivates are used as anticancer agents, namely dacarbazine and temozolomide, or zoledronic acid (referred to in Section 4.2), among many other drugs [88][89]. Additional examples of medicines comprising this five-member ring molecule containing a nitrogen atom include clotrimazole (CTNZ), miconazole (MNZ), and pantoprazole (PNZ), which are traditional antifungal (CTNZ, MNZ) and proton pump inhibitor (PNZ) medications. Nevertheless, there is experimental evidence they may be repositioned to treat cancers, such as hepatocellular carcinoma [90], bladder cancer [91], breast cancer [92], glioblastoma [93], gastric cancer [94], and others.
The aforementioned imidazole molecules were reacted with oxidovanadium(IV) salt and the following complexes were obtained: [VO(SO4)(CTNZ)(H2O)]H2O (Figure 1E), [VO(SO4)(MNZ)2] H2O (Figure 1F), [VO(PNZ)2]SO4.2H2O (Figure 1G) [95]. After treating the hepatocellular carcinoma HepG2 and the breast adenocarcinoma MCF-7 human cell lines for 24 h, all oxidovanadium(IV)-based imidazole drug complexes showed either comparable (MCF-7 cells) or lower (HepG2 cells) IC50 values compared to cisplatin (Figure 4), evaluated by the MTT metabolic assay [95]. When analyzing their binding affinities as targeted drug molecules with specific hepatocellular carcinoma and breast cancer proteins, the authors of the latter study obtained higher molecular docking scores for all three complexes compared to those for the free imidazole ligands [95].
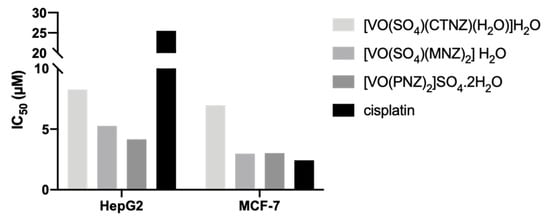
Figure 4. Experimental anticancer activity of the imidazole-based oxidovanadium(IV) complexes [VO(SO4)(CTNZ)(H2O)]H2O, [VO(SO4)(MNZ)2] H2O, [VO(PNZ)2]SO4.2H2O in HepG2 and MCF-7 cell lines. The IC50 values were obtained from [95]. Abbreviations: CTNZ, clotrimazole; MNZ, miconazole; PNZ, pantoprazole.
Over the last 25 years, different approved drugs were used as ligands in different vanadium complexes (Figure 5), highlighting novel potential therapeutic candidates based on drug repurposing.
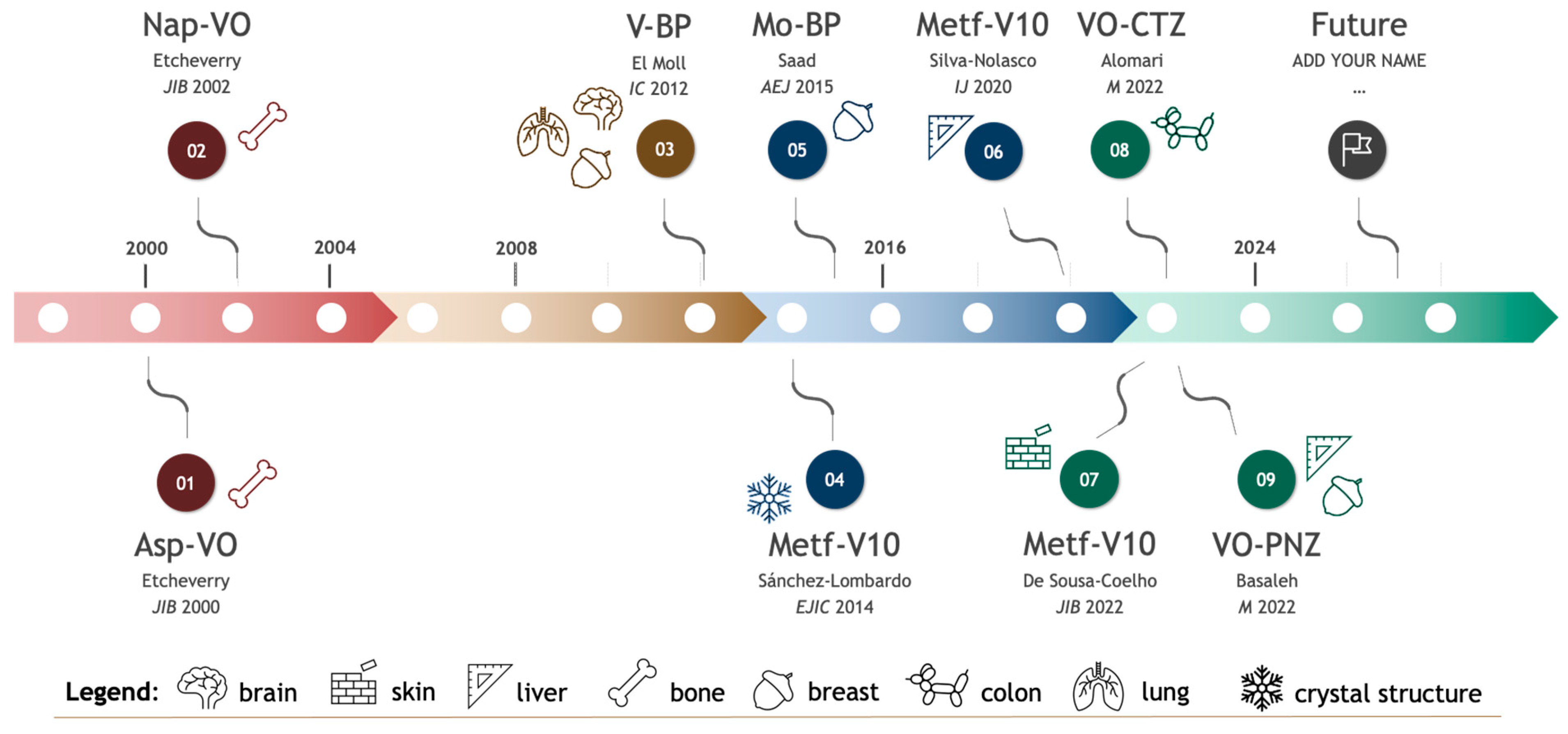
Figure 5. Timeline of selected complexes of marketed-approved drugs with transition metals, synthesized and characterized over the past 25 years. Chronological representation of each significant publication. For each complex represented, the last name of the first author and year of publication is shown [43][44][51][53][80][83][84][95][96]. Abbreviations: Asp-VO, vanadyl(IV)–aspirin complex; Nap-VO, vanadyl(IV) complex with naproxen; V-BP, hybrid vanadium-bisphosphonates; Metf-V10, metformin-decavanadate; Mo-BP, polyoxidomolybdate-bisphosphonates; VO-CTZ, cetirizine-based oxidovanadium(IV) complex; VO-PNZ, oxidovanadium(IV)-based pantoprazole complex.
References
- Sales, T.A.; Prandi, I.G.; de Castro, A.A.; Leal, D.H.S.; da Cunha, E.F.F.; Kuca, K.; Ramalho, T.C. Recent Developments in Metal-Based Drugs and Chelating Agents for Neurodegenerative Diseases Treatments. Int. J. Mol. Sci. 2019, 20, 1829.
- Cirri, D.; Bartoli, F.; Pratesi, A.; Baglini, E.; Barresi, E.; Marzo, T. Strategies for the Improvement of Metal-Based Chemotherapeutic Treatments. Biomedicines 2021, 9, 504.
- Marzo, T.; Messori, L. A Role for Metal-Based Drugs in Fighting COVID-19 Infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068.
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98.
- Gonzalez-Cano, S.I.; Flores, G.; Guevara, J.; Morales-Medina, J.C.; Treviño, S.; Diaz, A. Polyoxidovanadates a new therapeutic alternative for neurodegenerative and aging diseases. Neural Regen Res. 2024, 19, 571–577.
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143.
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344.
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382.
- Sun, G.; Dong, D.; Dong, Z.; Zhang, Q.; Fang, H.; Wang, C.; Zhang, S.; Wu, S.; Dong, Y.; Wan, Y. Drug repositioning: A bibliometric analysis. Front. Pharmacol. 2022, 13, 974849.
- Hernandez, J.J.; Pryszlak, M.; Smith, L.; Yanchus, C.; Kurji, N.; Shahani, V.M.; Molinski, S.V. Giving Drugs a Second Chance: Overcoming Regulatory and Financial Hurdles in Repurposing Approved Drugs as Cancer Therapeutics. Front Oncol. 2017, 7, 273.
- To, K.K.W.; Cho, W.C.S. Drug Repurposing for Cancer Therapy in the Era of Precision Medicine. Curr. Mol. Pharmacol. 2022, 15, 895–903.
- Pantziarka, P.; Bouche, G.; André, N. “Hard” Drug Repurposing for Precision Oncology: The Missing Link? Front. Pharmacol. 2018, 14, 9.
- Meco, D.; Attinà, G.; Mastrangelo, S.; Navarra, P.; Ruggiero, A. Emerging Perspectives on the Antiparasitic Mebendazole as a Repurposed Drug for the Treatment of Brain Cancers. Int. J. Mol. Sci. 2023, 24, 1334.
- Natale, G.; Fini, E.; Calabrò, P.F.; Carli, M.; Scarselli, M.; Bocci, G. Valproate and lithium: Old drugs for new pharmacological approaches in brain tumors? Cancer Lett. 2023, 560, 216125.
- Hosseinalizadeh, H.; Ebrahimi, A.; Tavakoli, A.; Monavari, S.H. Glioblastoma as a Novel Drug Repositioning Target: Updated State. Anticancer Agents Med. Chem. 2023, 23, 1253–1264.
- Pillai, U.J.; Ray, A.; Maan, M.; Dutta, M. Repurposing drugs targeting metabolic diseases for cancer therapeutics. Drug Discov. Today 2023, 28, 103684.
- Doumat, G.; Daher, D.; Zerdan, M.B.; Nasra, N.; Bahmad, H.F.; Recine, M.; Poppiti, R. Drug Repurposing in Non-Small Cell Lung Carcinoma: Old Solutions for New Problems. Curr Oncol. 2023, 30, 704–719.
- Hijazi, M.A.; Gessner, A.; El-Najjar, N. Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp. Cancers 2023, 15, 3199.
- To, K.K.W.; Cho, W.C. Drug Repurposing to Circumvent Immune Checkpoint Inhibitor Resistance in Cancer Immunotherapy. Pharmaceutics 2023, 15, 2166.
- Spitschak, A.; Gupta, S.; Singh, K.P.; Logotheti, S.; Pützer, B.M. Drug Repurposing at the Interface of Melanoma Immunotherapy and Autoimmune Disease. Pharmaceutics 2022, 15, 83.
- Tajaldini, M.; Poorkhani, A.; Amiriani, T.; Amiriani, A.; Javid, H.; Aref, P.; Ahmadi, F.; Sadani, S.; Khori, V. Strategy of targeting the tumor microenvironment via inhibition of fibroblast/fibrosis remodeling new era to cancer chemo-immunotherapy resistance. Eur. J. Pharmacol. 2023, 957, 175991.
- Badwan, B.A.; Liaropoulos, G.; Kyrodimos, E.; Skaltsas, D.; Tsirigos, A.; Gorgoulis, V.G. Machine learning approaches to predict drug efficacy and toxicity in oncology. Cell Rep. Methods 2023, 3, 100413.
- Ahmed, F.; Samantasinghar, A.; Soomro, A.M.; Kim, S.; Choi, K.H. A systematic review of computational approaches to understand cancer biology for informed drug repurposing. J. Biomed. Inform. 2023, 142, 104373.
- Dalwadi, S.M.; Hunt, A.; Bonnen, M.D.; Ghebre, Y.T. Computational approaches for drug repurposing in oncology: Untapped opportunity for high value innovation. Front. Oncol. 2023, 18, 13.
- Zhou, H.; Liu, H.; Yu, Y.; Yuan, X.; Xiao, L. Informatics on Drug Repurposing for Breast Cancer. Drug Des. Devel. Ther. 2023, 17, 1933–1943.
- Uprety, B.; Abrahamse, H. Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights. Cells 2022, 11, 576.
- Aureliano, M.; Madeira, V.M.C. Interactions of vanadate oligomers with sarcoplasmic reticulum Ca2+-ATPase. Biochim. Biophys. Acta Mol. Cell Res. 1994, 1221, 259–271.
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89.
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700.
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796.
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828.
- Carvalho, F.; Aureliano, M. Polyoxometalates Impact as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 5043.
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. Engl. 2019, 58, 2980–2999.
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757.
- Vlasiou, M.C.; Pafiti, K.S. Cell Arrest and Apoptosis Induced by the Next Generation of Vanadium Based Drugs: Action Mechanism to Structure Relation and Future Perspectives. Anticancer Agents Med. Chem. 2021, 21, 2111–2116.
- Hashmi, K.; Satya Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal applications of vanadium complexes with Schiff bases. J. Trace Elem. Med. Biol. 2023, 79, 127245.
- Selvaraj, S.; Krishnan, U.M. Vanadium–Flavonoid Complexes: A Promising Class of Molecules for Therapeutic Applications. J. Med. Chem. 2021, 64, 12435–12452.
- Ścibior, A. Overview of Research on Vanadium-Quercetin Complexes with a Historical Outline. Antioxidants 2022, 11, 790.
- Aureliano, M.; Mal, S.S.; Fraqueza, G.; De Sousa-Coelho, A.L.; Faleiro, M.L.; Gumerova, N.I. Polyoxovanadates: Catalysis, pharmacology, antibacterial and anticancer activities. In Synthesis and Applications in Chemistry and Materials; Pombeiro, A.J.L., Mahmudov, K.T., Guedes da Silva, M., Eds.; World Scientific: Singapore, 2024.
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, M.J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244.
- Marques, M.P.M.; Gianolio, D.; Ramos, S.; Batista de Carvalho, L.A.E.; Aureliano, M. An EXAFS Approach to the Study of Polyoxometalate–Protein Interactions: The Case of Decavanadate–Actin. Inorg. Chem. 2017, 56, 10893–10903.
- Treviño, S.; Velázquez-Vázquez, D.; Sánchez-Lara, E.; Diaz-Fonseca, A.; Flores-Hernandez, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 6058705.
- El Moll, H.; Zhu, W.; Oldfield, E.; Rodriguez-Albelo, L.M.; Mialane, P.; Marrot, J.; Vila, N.; Mbomekallé, I.M.; Rivière, E.; Duboc, C.; et al. Polyoxometalates Functionalized by Bisphosphonate Ligands: Synthesis, Structural, Magnetic, and Spectroscopic Characterizations and Activity on Tumor Cell Lines. Inorg. Chem. 2012, 51, 7921–7931.
- Alomari, F.Y.; Sharfalddin, A.A.; Abdellattif, M.H.; Domyati, D.; Basaleh, A.S.; Hussien, M.A. QSAR Modeling, Molecular Docking and Cytotoxic Evaluation for Novel Oxidovanadium(IV) Complexes as Colon Anticancer Agents. Molecules 2022, 27, 649.
- Barfeie, H.; Grivani, G.; Eigner, V.; Dusek, M.; Khalaji, A.D. Copper(II), nickel(II), zinc(II) and vanadium(IV) Schiff base complexes: Synthesis, characterization, crystal structure determination, and thermal studies. Polyhedron 2018, 146, 19–25.
- Naso, L.G.; Martínez Medina, J.J.; Okulik, N.B.; Ferrer, E.G.; Williams, P.A.M. Study on the cytotoxic, antimetastatic and albumin binding properties of the oxidovanadium(IV) chrysin complex. Structural elucidation by computational methodologies. Chem. Biol. Interact. 2022, 351, 109750.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Sousa, S.M.; Xavier, C.P.R.; Vasconcelos, M.H.; Palmeira, A. Repurposing some of the Well-known Non-steroid Anti-inflammatory Drugs (NSAIDs) for Cancer Treatment. Curr. Top. Med. Chem. 2023, 23, 1171–1195.
- Harris, R.E.; Beebe-Donk, J.; Doss, H.; Burr Doss, D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncol. Rep. 2005, 13, 559–583.
- Yasir Khan, H.; Parveen, S.; Yousuf, I.; Tabassum, S.; Arjmand, F. Metal complexes of NSAIDs as potent anti-tumor chemotherapeutics: Mechanistic insights into cytotoxic activity via multiple pathways primarily by inhibition of COX–1 and COX–2 enzymes. Coord. Chem. Rev. 2022, 453, 214316.
- Etcheverry, S.; Barrio, D.; Cortizo, A.; Williams, P.A. Three new vanadyl(IV) complexes with non-steroidal anti-inflammatory drugs (Ibuprofen, Naproxen and Tolmetin). Bioactivity on osteoblast-like cells in culture. J. Inorg. Biochem. 2002, 88, 94–100.
- Molinuevo, M.S.; Barrio, D.A.; Cortizo, A.M.; Etcheverry, S.B. Antitumoral properties of two new vanadyl(IV) complexes in osteoblasts in culture: Role of apoptosis and oxidative stress. Cancer Chemother. Pharmacol. 2004, 53, 163–172.
- Etcheverry, S.; Williams, P.A.; Barrio, D.; Sálice, V.; Ferrer, E.; Cortizo, A. Synthesis, characterization and bioactivity of a new VO2+/Aspirin complex. J. Inorg. Biochem. 2000, 80, 169–171.
- Molinuevo, M.S.; Cortizo, A.M.; Etcheverry, S.B. Vanadium(IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells. Cancer Chemother. Pharmacol. 2008, 61, 767–773.
- Mönkkönen, H.; Auriola, S.; Lehenkari, P.; Kellinsalmi, M.; Hassinen, I.E.; Vepsäläinen, J.; Mönkkönen, J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br. J. Pharmacol. 2006, 147, 437–445.
- Lehenkari, P.P.; Kellinsalmi, M.; Näpänkangas, J.P.; Ylitalo, K.V.; Mönkkönen, J.; Rogers, M.J.; Azhayev, A.; Väänänen, H.K.; Hassinen, I.E. Further Insight into Mechanism of Action of Clodronate: Inhibition of Mitochondrial ADP/ATP Translocase by a Nonhydrolyzable, Adenine-Containing Metabolite. Mol. Pharmacol. 2002, 61, 1255–1262.
- Teixeira, S.; Branco, L.; Fernandes, M.H.; Costa-Rodrigues, J. Bisphosphonates and Cancer: A Relationship Beyond the Antiresorptive Effects. Mini-Rev. Med. Chem. 2019, 19, 988–998.
- Finianos, A.; Aragon-Ching, J.B. Zoledronic acid for the treatment of prostate cancer. Expert Opin. Pharmacother. 2019, 20, 657–666.
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134.
- Goode, E.A.; Wang, N.; Munkley, J. Prostate cancer bone metastases biology and clinical management (Review). Oncol. Lett. 2023, 25, 163.
- Brufsky, A.; Mathew, A. Adjuvant bisphosphonate therapy in early-stage breast cancer—Treating the soil to kill the seed. Breast J. 2020, 26, 65–68.
- Coleman, R. Bisphosphonates and breast cancer—From cautious palliation to saving lives. Bone 2020, 140, 115570.
- Zhang, X.-S.; Zhang, Y.-M.; Li, B.; Fan, B.; Zhao, Y.; Yang, S.-J. Risk reduction of endometrial and ovarian cancer after bisphosphonates use: A meta-analysis. Gynecol. Oncol. 2018, 150, 509–514.
- Wu, S.; Dahut, W.L.; Gulley, J.L. The use of bisphosphonates in cancer patients. Acta Oncol. 2007, 46, 581–591.
- Jara, M.A.; Varghese, J.; Hu, M.I. Adverse events associated with bone-directed therapies in patients with cancer. Bone 2022, 158, 115901.
- Mbese, Z.; Aderibigbe, B.A. Bisphosphonate-Based Conjugates and Derivatives as Potential Therapeutic Agents in Osteoporosis, Bone Cancer and Metastatic Bone Cancer. Int. J. Mol. Sci. 2021, 22, 6869.
- La-Beck, N.M.; Liu, X.; Shmeeda, H.; Shudde, C.; Gabizon, A.A. Repurposing amino-bisphosphonates by liposome formulation for a new role in cancer treatment. Semin. Cancer Biol. 2021, 68, 175–185.
- Christensen, A.T.; McLauchlan, C.C.; Dolbecq, A.; Mialane, P.; Jones, M.A. Studies of the Effectiveness of Bisphosphonate and Vanadium-Bisphosphonate Compounds In Vitro against Axenic Leishmania tarentolae. Oxid. Med. Cell. Longev. 2016, 2016, 1–12.
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576.
- Schernthaner, G.; Schernthaner, G.-H. The right place for metformin today. Diabetes Res. Clin. Pract. 2020, 159, 107946.
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Devel. Ther. 2017, 11, 2421–2429.
- Du, Y.; Zhu, Y.-J.; Zhou, Y.-X.; Ding, J.; Liu, J.-Y. Metformin in therapeutic applications in human diseases: Its mechanism of action and clinical study. Mol. Biomed. 2022, 3, 41.
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476.
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. J. 2005, 330, 1304–1305.
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 403.
- Lord, S.R.; Harris, A.L. Is it still worth pursuing the repurposing of metformin as a cancer therapeutic? Br. J. Cancer 2023, 128, 958–966.
- Yu, O.H.Y.; Suissa, S. Metformin and Cancer: Solutions to a Real-World Evidence Failure. Diabetes Care 2023, 46, 904–912.
- Panaampon, J.; Zhou, Y.; Saengboonmee, C. Metformin as a booster of cancer immunotherapy. Int. Immunopharmacol. 2023, 121, 110528.
- Papadakos, S.P.; Ferraro, D.; Carbone, G.; Frampton, A.E.; Vennarecci, G.; Kykalos, S.; Schizas, D.; Theocharis, S.; Machairas, N. The Emerging Role of Metformin in the Treatment of Hepatocellular Carcinoma: Is There Any Value in Repurposing Metformin for HCC Immunotherapy? Cancers 2023, 15, 3161.
- Sánchez-Lombardo, I.; Sánchez-Lara, E.; Pérez-Benítez, A.; Mendoza, Á.; Bernès, S.; González-Vergara, E. Synthesis of Metforminium(2+) Decavanadates—Crystal Structures and Solid-State Characterization. Eur. J. Inorg. Chem. 2014, 2014, 4581–4588.
- Chatkon, A.; Chatterjee, P.B.; Sedgwick, M.A.; Haller, K.J.; Crans, D.C. Counterion Affects Interaction with Interfaces: The Antidiabetic Drugs Metformin and Decavanadate. Eur. J. Inorg. Chem. 2013, 2013, 1859–1868.
- Treviño, S.; Sánchez-Lara, E.; Sarmiento-Ortega, V.E.; Sánchez-Lombardo, I.; Flores-Hernández, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3 ·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J. Inorg. Biochem. 2015, 147, 85–92.
- Silva-Nolasco, A.M.; Camacho, L.; Saavedra-Díaz, R.O.; Hernández-Abreu, O.; León, I.E.; Sánchez-Lombardo, I. Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin- Like Activity. Inorganics 2020, 8, 67.
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915.
- Lin, X.; Zhang, J.; Wang, X.; Lin, G.; Chen, T. Pre-activation with TLR7 in combination with thioridazine and loratadine promotes tumoricidal T-cell activity in colorectal cancer. Anticancer. Drugs 2020, 31, 989–996.
- Fritz, I.; Wagner, P.; Olsson, H. Improved survival in several cancers with use of H1-antihistamines desloratadine and loratadine. Transl. Oncol. 2021, 14, 101029.
- Mallardo, D.; Simeone, E.; Vanella, V.; Vitale, M.G.; Palla, M.; Scarpato, L.; Paone, M.; De Cristofaro, T.; Borzillo, V.; Cortellini, A.; et al. Concomitant medication of cetirizine in advanced melanoma could enhance anti-PD-1 efficacy by promoting M1 macrophages polarization. J. Transl. Med. 2022, 20, 436.
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. Med. Chem. Commun. 2017, 8, 1742–1773.
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as Potential Anticancer Agents: An Update on Recent Studies. Molecules 2021, 26, 4213.
- Liu, X.; Gao, J.; Sun, Y.; Zhang, F.; Guo, W.; Zhang, S. Clotrimazole Inhibits HCC Migration and Invasion by Modulating the ERK-p65 Signaling Pathway. Drug Des. Devel. Ther. 2022, 16, 863–871.
- Ho, C.; Chang, A.; Hsu, C.; Tsai, T.; Lin, Y.; Chou, K.; Chen, H.; Lin, J.; Chen, P.; Hwang, T.I. Miconazole induces protective autophagy in bladder cancer cells. Environ. Toxicol. 2021, 36, 185–193.
- Chengzhu, W.U.; Gao, M.; Shen, L.; Bohan, L.I.; Bai, X.; Gui, J.; Hongmei, L.I.; Huo, Q.; Tao, M.A. Miconazole triggers various forms of cell death in human breast cancer MDA-MB-231 cells. Pharmazie 2019, 74, 290–294.
- Jung, H.-J.; Seo, I.; Jha, B.; Suh, S.-I.; Baek, W.-K. Miconazole induces autophagic death in glioblastoma cells via reactive oxygen species-mediated endoplasmic reticulum stress. Oncol. Lett. 2021, 21, 335.
- Zhang, B.; Ling, T.; Zhaxi, P.; Cao, Y.; Qian, L.; Zhao, D.; Kang, W.; Zhang, W.; Wang, L.; Xu, G.; et al. Proton pump inhibitor pantoprazole inhibits gastric cancer metastasis via suppression of telomerase reverse transcriptase gene expression. Cancer Lett. 2019, 452, 23–30.
- Basaleh, A.S.; Alomari, F.Y.; Sharfalddin, A.A.; Al-Radadi, N.S.; Domyati, D.; Hussien, M.A. Theoretical Investigation by DFT and Molecular Docking of Synthesized Oxidovanadium(IV)-Based Imidazole Drug Complexes as Promising Anticancer Agents. Molecules 2022, 27, 2796.
- Saad, A.; Zhu, W.; Rousseau, G.; Mialane, P.; Marrot, J.; Haouas, M.; Taulelle, F.; Dessapt, R.; Serier-Brault, H.; Rivière, E.; et al. Polyoxomolybdate Bisphosphonate Heterometallic Complexes: Synthesis, Structure, and Activity on a Breast Cancer Cell Line. Chem. Eur. J. 2015, 21, 10537–10547.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
588
Revisions:
2 times
(View History)
Update Date:
27 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




