Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gerard Campos | -- | 3435 | 2023-12-26 20:04:42 | | | |
| 2 | Mona Zou | Meta information modification | 3435 | 2023-12-27 08:39:48 | | | | |
| 3 | Mona Zou | -230 word(s) | 3205 | 2024-02-29 08:51:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Campos, G.; Sciorio, R.; Fleming, S. Healthy Live Births after Transfer of Mosaic Embryos. Encyclopedia. Available online: https://encyclopedia.pub/entry/53147 (accessed on 14 January 2026).
Campos G, Sciorio R, Fleming S. Healthy Live Births after Transfer of Mosaic Embryos. Encyclopedia. Available at: https://encyclopedia.pub/entry/53147. Accessed January 14, 2026.
Campos, Gerard, Romualdo Sciorio, Steven Fleming. "Healthy Live Births after Transfer of Mosaic Embryos" Encyclopedia, https://encyclopedia.pub/entry/53147 (accessed January 14, 2026).
Campos, G., Sciorio, R., & Fleming, S. (2023, December 26). Healthy Live Births after Transfer of Mosaic Embryos. In Encyclopedia. https://encyclopedia.pub/entry/53147
Campos, Gerard, et al. "Healthy Live Births after Transfer of Mosaic Embryos." Encyclopedia. Web. 26 December, 2023.
Copy Citation
The implementation of next generation sequencing (NGS) in preimplantation genetic testing for aneuploidy (PGT-A) has led to a higher prevalence of mosaic diagnosis within the trophectoderm (TE) sample. Regardless, mosaicism could potentially increase the rate of live-born children with chromosomic syndromes, though available data from the transfer of embryos with putative PGT-A mosaicism are scarce but reassuring. Even with lower implantation and higher miscarriage rates, mosaic embryos can develop into healthy live births. Therefore, this urges an explanation for the disappearance of aneuploid cells throughout development, to provide guidance in the management of mosaicism in clinical practice. Technical overestimation of mosaicism, together with some sort of “self-correction” mechanisms during the early post-implantation stages, emerged as potential explanations. Unlike the animal model, in which the elimination of genetically abnormal cells from the future fetal lineage has been demonstrated, in human embryos this capability remains unverified even though the germ layer displays an aneuploidy-induced cell death lineage preference with higher rates of apoptosis in the inner cell mass (ICM) than in the TE cells.
mosaicism
trophectoderm biopsy
rebiopsy
preimplantation genetic testing
self-correction
overestimation
intermediate copy number
1. Self-Correction and Embryo Plasticity
Beyond standard and well-defined embryo development, signs of embryo plasticity are increasingly evident. Although several perturbations can affect embryo viability and implantation potential, they are still proven to be compatible with implantation and live-term pregnancies. The ability to reverse binucleation at the two-cell stage in the next cell division, which potentially might alter cell compaction, the morula stage and blastocyst [1][2][3][4], and the capacity to overcome cell loss from cryopreservation and develop to term, are examples of human embryo plasticity. Several studies have reported decreasing aneuploidy rates in preimplantation embryos, suggesting their capacity to self-correct and normalize their chromosomal content. Munnè and colleagues [5] found a chromosome normalization in 23 aneuploid embryos previously diagnosed by fluorescence in situ hybridization (FISH) at day 3, as having an increase in euploid cell rate from day 6 (average of 13%) to day 12 (average of 48%). Subsequently, 7 embryos became euploid and 11 were identified as mosaic, with between 21% and 88% of normal cells. A further study based on FISH data also showed a reduction in aneuploid cells in 32.6% of the embryos diagnosed as aneuploid and mosaic at day 3 when they were reanalyzed on day 5, reporting a total normalization in 9.7% of the 83 embryos included [6]. Extended in vitro embryo culture and the introduction of NGS corroborated these findings when 71% of the embryos originally diagnosed as a mosaic at day 5 were reported to be euploid at day 12 and, following extended culture, normal profiles were identified not only in the TE-derived lineages but also in the inner cell mass (ICM) [7].
2. The Mortality Model (Apoptosis and Depletion)
Aneuploid cells may be eliminated and progressively depleted from a mosaic embryo through selective apoptosis and reduced proliferation, with slower cell cycles of the abnormal cells [4]. This self-correcting ability to eliminate genetically abnormal cells from the future fetal lineage has been demonstrated in mice [8]. To investigate the fate of aneuploid cells during pre- and post-implantation development, a mouse model of euploid–aneuploid mosaicism was generated using the drug Reversine, an inhibitor of monopolar spindle 1-like 1 kinase [9], which inactivated the spindle assembly checkpoint and induced high rates of chromosomal segregation errors. This study reported a significant decrease in the percentage of altered blastomeres in each mosaic (1:1 Reversine-treated and control blastomeres) embryo from the early-stage to late-stage blastocyst (53% to 47%, p < 0.01), mainly due to a reduction of abnormal cells from 55.8% to 44.2% (p < 0.05) in the ICM but not a significant reduction in the TE. The preferential allocation of abnormal blastomeres to different cell lineages was tracked using high-resolution time-lapse imaging. Importantly, not only was apoptosis reported to be present in most mosaic embryos at the blastocyst stage, with apoptotic features detected in 30.9% of the ICM cells and fewer (2%) in the TE, but also the frequency of apoptosis was significantly higher in the aneuploid group of cells than in the euploid group, both in the ICM (41.4% vs. 19.5%, p < 0.001) and the TE (3.3% vs. 0.6%, p < 0.001). Hence, apoptosis proved to be a mechanism to eliminate chromosomally abnormal cells in mice, especially in the ICM, while TE cells were progressively depleted because of increased cell cycle length and senescence. Cell cycle lengths were significantly longer in TE aneuploid cells (not in ICM abnormal cells) than in normal cells (9.2% of the TE abnormal slower cells versus 1.4% of the TE cells from the control, p < 0.001). Progressive depletion of aneuploid cells in the preimplantation embryo was established through different mechanisms related to specific cell lineages, with apoptosis as the primarily responsible means in the ICM, while in the TE, the aneuploid cells would be negatively selected and reduced in relation to their euploid counterparts due to their longer cell cycles. This asynchrony in eliminating abnormal cells could explain the differences between the ICM and TE, especially when high rates of mosaicism are present. Even though it has been demonstrated in animal models, differences in cell cycle regulation and the timing of development between mice and humans [10], together with the limitations regarding the methodology and reagents permitted for human use, make it difficult to confirm this hypothesis in human embryos. However, the use of human “grastruloids”, created from RUES2 (NIHhESC-09-0013) human embryonic stem cells treated with Reversine, recently provided a human model for aneuploid cell fate in preimplantation human development [11]. A significant enhancement of apoptosis in the embryonic germ layer displayed an aneuploidy-induced cell death lineage preference, showing that pluripotent epiblast and TE cells were remarkably resilient to aneuploidy with significantly lower levels of apoptosis. These findings support a similar underlying mechanism in humans [11]. Furthermore, in human blastocysts, Victor and colleagues [12], using immunofluorescent markers of mitosis (Phosphohistone 3) and apoptosis (Caspase-3), were able to report differential dynamics of cell proliferation and death between euploid, mosaic, and aneuploid embryos. Abnormal blastocysts showed significantly higher rates of apoptosis (ICM, p < 0.05; TE, p < 0.001) and increased rates of cell division (ICM, p < 0.05; TE, p < 0.01), presumably compensating for the slower proliferation of aneuploid cells or their loss by programmed cell death [12]. An alternative approach based on gene expression profiles of embryos from day 4 morulae to day 7 blastocysts showed upregulated immune response genes and, more importantly, the downregulation of genes involved in proliferation and metabolism in the aneuploid cells of mosaic embryos [13]. Data from human blastocoel fluid (BF) support selective apoptosis as a self-correction mechanism that may rescue embryos from aneuploidy (Figure 1) [14]. The presence of cell-free DNA (cfDNA) and other molecular remnants in BF may suggest an apoptotic origin during early embryonic development. Though the biological mechanisms by which embryonic DNA from lysed or partially lysed cells is released into the blastocoel are difficult to define, it may potentially originate from cells undergoing apoptosis [15][16]. Testing the blastocoel cfDNA of euploid blastocysts developed from embryos previously diagnosed as aneuploid on day 3, Tobler and collaborators [17] found that 86% (12 of 14) of normalized embryos on day 5 still showed aneuploid results within the BF, suggesting that abnormal cells may be marginalized during blastulation. Recent data support the extrusion of this aneuploid embryonic DNA; specifically, when several structures of the blastocyst derived from day 3 aneuploid embryos were analyzed, the rate of aneuploidy was found to be significantly higher (p < 0.0001) in the BF (78%) than in the ICM (39%) and TE (49%), with nearly all abnormalities concordant with day 3 diagnosis [18]. Blastocoel fluid has been reported to contain cfDNA as well as mRNAs encoding apoptotic genes [19][20]. The identification of these pro-apoptotic gene products together with extracellular vesicles also found within the BF, provides additional support for the hypothesis of apoptosis as a mechanism that purges the preimplantation embryo of aneuploid cells [21].
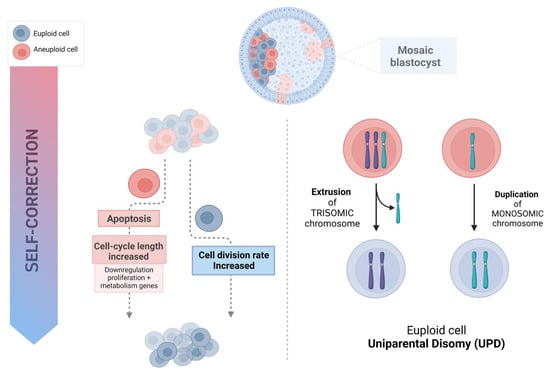
Figure 1. Possible mechanism of human embryo self-correction.
3. Trisomy/Monosomy Rescue Model
Correction of aneuploidy by extruding a trisomy chromosome or by monosomic chromosome duplication could theoretically represent a mechanism to explain success after the transfer of “mosaic” embryos [4][22], leading to UPD. In such a case, two copies of the chromosome are inherited from the same parent with no representative copy from the other; therefore, if imprinted genes or harmful recessive alleles are involved, this may result in syndromic newborns. Nevertheless, the low prevalence of UPD (from 0% to 0.06%) found in human embryos [22][23] suggests that, though possible, it is an extremely rare phenomenon. These results were recently corroborated by data from the general population, where the UPD rate was estimated to be 1 in 2000 euploid, liveborn individuals (rate: 0.05%; 99% CI: [0.04–0.06%]) [24], which would hardly explain the rescue of mosaic embryos into healthy euploid babies. Moreover, Scuffins and co-workers [25] reported the presence of UPD involving imprinted chromosomes in only one out of 320 syndromic infants born.
4. Misinterpretations of PGT-A Results
4.1. Technical Accuracy
The technical limitations of PGT-A may lead to inaccuracy in embryo diagnoses, identifying as “mosaic”, embryos that are in fact uniformly euploid or aneuploid [12][26][27]. PGT-A using NGS is usually performed as a method of quantifying chromosomes to profile the karyotype following TE biopsy, using chromosome copy number thresholds to predict the euploid, aneuploid, or mosaic status of the embryo. Briefly, 24-chromosome copy number analysis by NGS involves fragmenting the whole-genome amplified DNA sample into hundreds of thousands of small fragments (100–200 base pairs) that are sequenced in parallel. Sequencing of each fragment, which requires the addition of fluorescent nucleotides and ultrahigh-resolution imaging technology, continues until a sufficient “read depth” (the number of sequence reads for the same genomic region) is acquired. Then, these sequences are compared with the reference genome and counted using specific software. As a result, a specific number of reads from a given chromosome is proportional to the copy number in case of euploidy, while greater or lower read depth would entail trisomy or monosomy, respectively. Far from being analyzed and karyotyped individually, NGS collectively analyses the amount of DNA for each chromosome from a group of cells (multicellular TE biopsy) using a bioinformatics algorithm to compare it with a normal copy number reference value. The NGS approach requires whole genome amplification (WGA) followed by a library construction, sequencing, and alignment of readings with the human reference genome. More precisely, this necessary DNA extraction and amplification, known to be susceptible to errors, together with other methodological issues (i.e., undetected sample contamination, suboptimal polyploidy, or the bioinformatic algorithms used) may affect the level of noise observed, which may result in artifactual intermediate copy numbers and contribute to an overestimation of chromosomal mosaicism in clinical practice [7][28][29][30]. In addition to the DNA standard amplification technologies’ propensity for errors, the high variability of TE biopsies, in terms of quantity and quality (intact cells together with fragmented cellular remnants) may lead to intermediate copy number results and therefore to false-positive mosaicism profiles. An insufficient or excessive number of cells may have a significant impact on the PCR amplification plot at the time of quantification, which could result in an underestimate of the relative amount of DNA [31].
Different genetic testing laboratory practices (i.e., cut-off values used) may entail different levels of accuracy (sensitivity and specificity) and may therefore have significant impact upon the mosaicism rate reported [26]. Most validation studies are based on models employing mixtures of euploid and aneuploid cell lines at different ratios that intend to mimic the variation found in in vivo samples. These cell mixes have been mostly developed from the genomic DNA of cell lines with different well-defined chromosome complements [32] or, alternatively, by merging well-defined proportions of euploid and aneuploid cells [33]. However, these models represent a highly stable scenario, with no variation in the quantity or quality of cells analyzed, which certainly differs from blastocyst biopsy specimens, characterized by an uncertain number of intact cells together with fragmented cellular remnants derived from technical procedures. Consequently, any extrapolation of mosaicism from these idealistically stable models might constrain the diagnostic efficiency of NGS in diagnosing it [26].
The cut-off values represent analytical noise levels and specific technological variations [34]. Some laboratories employ more dynamic ranges (i.e., 20–80% thresholds), in which chromosome copy number deviations less than 20% (1.8≥ and ≤2.2) are reported as euploidy and greater than 80% as aneuploid (i.e., 0.8≥ and ≤1.2 for aneuploidy; 2.8≥ and ≤3.2 for triploidy) while others accept more conservative cut-off values, i.e., from 30% to 70%, and consequently higher analytical noise levels [28][35]. Data analysis may reveal intermediate copy numbers outside these ranges for the two normal copies and full monosomies or trisomies. These results are presumably consistent with the presence of both euploid and aneuploid cells among the biopsied TE and are therefore profiled as mosaics [29]. However, different factors may affect the level of noise observed (i.e., undetected sample contamination, suboptimal DNA amplification, polyploidy, or the bioinformatic algorithms used), which may result in artifactual intermediate copy numbers and contribute to an overestimation of chromosomal mosaicism in clinical practice [7][29][30]. Nevertheless, data from one systematic review involving reanalysis of embryos deemed mosaic demonstrated a high discordance when mosaicism was diagnosed using NGS testing based upon intermediate copy numbers [35]. Embryo reanalysis included TE rebiopsies, ICM sampling, whole embryo screening and blastocyst outgrowths analysis. The accuracy of embryo diagnosis was reported to be lower with NGS, displaying an euploidy concordance of 92.2% compared to 97.1% (p = 0.0053) when NGS was not used in the original biopsy, and showed even less concordance in cases with a full aneuploidy diagnosis: 75.9% with NGS and 94.8% without NGS (p < 0.0001). Particularly poor was the predictive value for the embryos placed in the mosaic range by NGS, as the concordance of mosaic aneuploidy with the remaining embryo was only 42.6%. Consequently, these data suggested that even if NGS technology accurately detects euploidy, it is significantly inaccurate for aneuploidy, and highly inefficient in diagnosing mosaicism. Moreover, the reanalysis of the mosaic embryos revealed that most of them (57.4%) were unlikely to present mosaicism, reporting euploidy in 29% of embryos and 28.4% of full aneuploids. This lack of specificity of mosaicism predictions was corroborated recently by Handyside and co-authors [29], who performed SNP genotyping and karyomapping to follow-up embryos identified by NGS-based PGT-A as mosaic. In addition to detecting overestimation of mosaicism, they were able to identify a significant proportion of embryos with meiotic aneuploidies. Only 1 out of 21 (4.8%) cases diagnosed as putative mosaic was confirmed, 42.8% were euploid, and 47.6% were found to be aneuploid, including embryos with meiotic trisomies, monosomies, and triploidies [29]. The significant discordance upon reanalysis would lead to the consideration of false-positive mosaic classification as an alternative hypothesis for the clinical outcomes found after transferring embryos diagnosed as putative “mosaic” by NGS and chromosome copy number analysis. Therefore, uniform aneuploid embryos misdiagnosed as mosaic would lead to negative reproductive consequences [36], while the healthy deliveries reported would be the result of transferring truly euploid embryos misdiagnosed as mosaic [31][35].
4.2. Concordance between TE and ICM
Estimates based on a small biopsy (5–10 cells) could not possibly be representative of the whole embryo if aneuploid cells are not distributed evenly. Biologically, the moment when mosaicism arises influences the location and distribution of aneuploid cells. Importantly, when performing PGT-A, the biopsy takes a few cells from the TE, the precursor to the placenta, but not from the ICM, the cell lineage from which the fetus arises. Thus, it remains an indirect estimation of the entire embryo’s status and does not prove the surrounding TE cells’ karyotype. Indeed, at least theoretically, according to mathematical modelling, a single TE biopsy could not reliably determine the genetic status of the remaining embryo, so its clinical utility would be questionable [37]. Data on the distribution of aneuploid cells within mosaic embryos are scarce; most studies have reported a poor concordance between the TE and the rest of the embryo [27][38][39][40], while others have found that the TE karyotype is a relatively accurate predictor of ICM chromosomal status [12][41][42][43]. In one study, five blastocysts initially classified as mosaics were re-analyzed using ICM biopsy and one additional sample from the TE [27]. Even with the limited sample size, they identified as euploid the ICM of 3/5 blastocysts and the subsequent TE biopsy in 2/5 embryos, demonstrating that despite being classified as mosaic, the embryo can be euploid in other regions and that clinically diagnosed mosaicism from a single biopsy is not a good predictor of the whole embryo’s karyotype. Nevertheless, the same group reported 96.8% (n = 93) of clinical TE-ICM concordance in blastocysts classified as “uniform aneuploids”, highlighting that this experimental evidence does not apply to mosaic diagnoses [12]. Similarly, Huang and colleagues [43] analyzed 51 donated abnormal blastocysts and reported TE aneuploidy as an outstanding predictor of ICM imbalance but showed a much lower correlation for mosaicism. The ICM and three different separated TE regions (opposite, upper right, and lower right of the ICM) were biopsied and analyzed by aCGH, reporting 84.3% of embryos with consistent results in all four biopsies. Interestingly, when discordance was identified between one of the TE regions and the rest of the samples (mosaicism), these aneuploid cells had no special location among the areas biopsied, being not limited to a specific region of the TE. A similar result was reported after reanalysis via FISH of previous genetic diagnosis obtained by TE aCGH analysis, showing no preferential allocation of abnormal cells in euploid/aneuploid mosaics, which were evenly distributed across the blastocyst [42][44]. In contrast, recent conclusions from a dataset of disaggregated human blastocysts are distinctly noteworthy [38]. The prevalence and distribution of aneuploid cells was studied by NGS in 91 blastocysts, from which the ICM was isolated, and the TE was divided into four pieces, with the ICM labelled separately. When the concordance between the reference TE and the rest of the portions was analyzed, they found significant differences depending on the chromosomal content of the baseline TE biopsy. Interestingly, when high level mosaicism (50–70%) was reported in the diagnostic TE sample, 65% of blastocysts were uniformly aneuploid in the ICM and the rest of the analyzed pieces. However, when aneuploid cells represented less than 50% in the TE biopsy, meaning they had been classified as euploid or low-medium level mosaics, chromosomal abnormalities were extremely rare (1% of cases) across the ICM or affected other portions of the embryo. Indeed, the distribution of aneuploid cells and their impact on the embryo were equivalent between euploid and low-medium level mosaics (p = 0.14). They concluded that low-medium level mosaicism diagnoses would be consistent with postzygotic errors in chromosome segregation that emerge after TE and ICM differentiation, which would confine aneuploidy to a specific area rather than evenly across the whole blastocyst [38]. Consistent with these results, true incidence of mosaicism was also confirmed by Wu and colleagues [40] when they reanalyzed 101 mosaic embryos and found a concordance with the ICM of only 27.5%, while high rates of euploidy (63.7%) and low rates of aneuploidy (8.8%) were disclosed. Additionally, increased rates of full aneuploidy (≥37.5%) were reported after the rebiopsy of high-level mosaics (≥60%) compared with the 2.6% of true aneuploids found when low-level mosaicism was initially diagnosed. This study also revealed differences related to the type of mosaicism, displaying a lower ICM concordance for segmental-chromosome mosaicism than for results implying whole chromosomes [40]. More recently, another study has shed additional light on the competence of the TE biopsy to reflect any part of the remaining embryo [45], after splitting the entire embryo into four pieces and analyzing their genetic content. An extremely low confirmation rate in all the rebiopsies was reported for mosaicism, both for whole-chromosome (2.29%) and segmental aneuploidy (2.15%), while the partial embryo concordance (diagnoses confirmed in at least one sample) were 39.08% and 41.94%, respectively. By extension, a scattered but not uniform distribution of mosaicism was inferred by the authors, although it was a general model because they did not distinguish different patterns between low, medium, and high levels of mosaicism.
References
- Barrie, A.; Homburg, R.; McDowell, G.; Brown, J.; Kingsland, C.; Troup, S. Preliminary investigation of the prevalence and implantation potential of abnormal embryonic phenotypes assessed using time-lapse imaging. Reprod. Biomed. Online 2017, 34, 455–462.
- Lagalla, C.; Tarozzi, N.; Sciajno, R.; Wells, D.; Di, S.M.; Nadalini, M.; Distratis, V.; Borini, A. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod. Biomed. Online 2017, 34, 137–146.
- Coticchio, G.; Lagalla, C.; Sturmey, R.; Pennetta, F.; Borini, A. The enigmatic morula: Mechanisms of development, cell fate determination, self-correction and implications for ART. Hum. Reprod. Update 2019, 25, 422–438.
- Coticchio, G.; Barrie, A.; Lagalla, C.; Borini, A.; Fishel, S.; Griffin, D.; Campbell, A. Plasticity of the human preimplantation embryo: Developmental dogmas, variations on themes and self-correction. Hum. Reprod. Update 2021, 27, 848–865.
- Munne, S.; Velilla, E.; Colls, P.; Bermudez, M.G.; Vemuri, M.C.; Steuerwald, N.; Garrisi, J.; Cohen, J. Self Correction of chromosomally abnormal embryos in culture and implications or stem cell production. Fertil. Steril. 2005, 845, 1328–1334.
- Barbash-Hazan, S.; Frumkin, T.; Malcov, M.; Yaron, Y.; Cohen, T.; Azem, F.; Amit, A.; Ben-Yosef, D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil. Steril. 2009, 92, 890–896.
- Popovic, M.; Dhaenens, L.; Taelman, J.; Dheedene, A.; Bialecka, M.; De Sutter, P.; Chuva de Sousa Lopes, S.M.; Menten, B.; Heindryckx, B. Extended in vitro culture of human embryos demonstrates the complex nature of diagnosing chromosomal mosaicism from a single trophectoderm biopsy. Hum. Reprod. 2019, 34, 758–769.
- Bolton, H.; Graham, S.J.L.; Van der Aa, N.; Kumar, P.; Theunis, K.; Gallardo, E.F.; Voet, T.; Zernicka-Goetz, M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploidy cells and normal developmental potential. Nat. Commun. 2016, 7, 665–666.
- Santaguida, S.; Tighe, A.; D’Alise, A.M.; Taylor, S.S.; Musacchio, A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 2010, 190, 73–87.
- Rossant, J.; Tam, P.L. New insights into early human development: Lessons for stem cell derivation and differentiation. Cell Stem Cell 2017, 20, 18–28.
- Yang, M.; Rito, T.; Metzger, J.; Naftaly, J.; Soman, R.; Hu, J.; Albertini, D.F.; Barad, D.H.; Brivanlou, A.H.; Gleicher, N. Depletion of aneuploid cells in human embryos and gastruloids. Nat. Cell Biol. 2021, 23, 314–321.
- Victor, A.R.; Griffin, D.K.; Brake, A.J.; Tyndall, J.C.; Murphy, A.E.; Lepkowsky, L.T.; Lal, A.; Zouves, C.G.; Barnes, F.L.; McCoy, R.C.; et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum. Reprod. 2019, 34, 181–192.
- Starostik, M.R.; Sosina, O.A.; McCoy, R.C. Single-cell analysis of human embryos reveals diverse patterns of aneuploidy and mosaicism. Genome Res. 2020, 30, 814–825.
- Lal, A.; Roudebush, W.E.; Chosed, R.J. Embryo Biopsy can offer more information than just ploidy status. Front. Cell Dev. Biol. 2020, 8, 78.
- Palini, S.; Galluzzi, L.; De Stefani, S.; Bianchi, M.; Wells, D.; Magnani, M.; Bulletti, C. Genomic DNA in human blastocoele fluid. Reprod. Biomed. Online 2013, 26, 603–610.
- Capalbo, A.; Romanelli, V.; Patassini, C.; Poli, M.; Girardi, L.; Giancani, A.; Stoppa, M.; Cimadomo, D.; Ubaldi, F.M.; Rienzi, L. Diagnostic efficacy of blastocoel fluid and spent media as sources of DNA for preimplantation genetic testing in standard clinical conditions. Fertil. Steril. 2018, 110, 870–879.
- Tobler, K.J.; Zhao, Y.; Ross, R.; Benner, A.T.; Xu, X.; Du, L.; Broman, K.; Thrift, K.; Brezina, P.R.; Kearns, W.G. Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertil. Steril. 2015, 104, 418–425.
- Griffin, D.K.; Brezina, P.R.; Tobler, K.; Zhao, Y.; Silvestri, G.; Mccoy, R.C.; Anchan, R.; Benner, A.; Cutting, G.R.; Kearns, W.G. The human embryonic genome is karyotypically complex, with chromosomally abnormal cells preferentially located away from the developing fetus. Hum. Reprod. 2023, 38, 180–188.
- Athavale, D.M.; Barré, A.; Kranyak, A.C.; Lal, A.; Blalock, J.L.; Zimmerman, S.; Chang, T.A.; Robinson, R.D.; Wininger, J.D.; Roudebush, W.E.; et al. Pro-apoptotic gene expression in blastocoel fluid from euploid day-5 embryos is associated with negative pregnancy outcomes. Fertil. Steril. 2019, 112, e261.
- Kranyak, A.C.; Barré, A.; Athavale, D.M.; Lal, A.; Blalock, J.L.; Zimmerman, S.; Chang, T.A.; Robinson, R.D.; Wininger, J.D.; Roudebush, W.E.; et al. Are there any similarities in gene expression between euploid embryos and aneuploid embryos compatible with life? Fertil. Steril. 2019, 112, e259.
- Battaglia, R.; Palini, S.; Vento, M.E.; La Ferlita, A.; Lo Faro, M.J.; Caroppo, E.; Borzì, P.; Falzone, L.; Barbagallo, D.; Ragusa, M.; et al. Identification of extracellular vesicles and characterization of miRNAexpression profiles in human blastocoel fluid. Sci. Rep. 2019, 9, 84.
- Gueye, N.A.; Devkota, B.; Taylor, D.; Pfundt, R.; Scott, R.T., Jr.; Treff, N.R. Uniparental disomy in the human blastocyst is exceedingly rare. Fertil. Steril. 2014, 101, 232–236.
- Northrop, L.E.; Treff, N.R.; Levy, B.; Scott, R.T., Jr. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol. Hum. Reprod. 2010, 16, 590–600.
- Nakka, P.; Smith, S.P.; O’Donnell-Luria, A.; McManus, K.F. Characterization of Prevalence and Health Consequences of Uniparental Disomy in Four Million Individuals from the General Population. Am. J. Hum. Genet. 2019, 105, 921–932.
- Scuffins, J.; Keller-Ramey, J.; Dyer, L.; Douglas, G.; Torene, R.; Gainullin, V.; Juusola, J.; Meck, J.; Retterer, K. Uniparental disomy in a population of 32,067 clinical exome trios. Genet. Med. 2021, 23, 1101–1107.
- Girardi, L.; Figliuzzi, M.; Poli, M.; Serdarogullari, M.; Patassini, C.; Caroselli, S.; Pergher, I.; Cogo, F.; Coban, O.; Boynukalin, F.K.; et al. The use of copy number loads to designate mosaicism in blastocyst stage PGT-A cycles: Fewer is better. Hum. Reprod. 2023, 16, dead049.
- Victor, A.R.; Tyndall, J.C.; Brake, A.J.; Lepkowsky, L.T.; Murphy, A.E.; Griffin, D.K.; McCoy, R.C.; Barnes, F.L.; Zouves, C.G.; Viotti, M. One hundred mosaic embryos transferred prospectively in a single clinic: Exploring when and why they result in healthy pregnancies. Fertil. Steril. 2019, 111, 280–293.
- Palmerola, K.L.; Vitez, S.F.; Amrane, S.; Fischer, C.P.; Forman, E.J. Minimizing mosaicism: Assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A). J. Assist. Reprod. Genet. 2019, 36, 153–157.
- Handyside, A.H.; McCollin, A.; Summers, M.C.; Ottolini, C.S. Copy number analysis of meiotic and postzygotic mitotic aneuploidies in trophectoderm cells biopsied at the blastocyst stage and arrested embryos. Prenat. Diagn. 2021, 41, 525–535.
- Xiong, S.; Liu, W.; Wang, J.; Liu, J.; Gao, Y.; Wu, L.; Zhu, J.; Hao, X.; Li, J.; Liu, D.; et al. Trophectodrm biopsy protocolsmay impact the rate of mosaic blastocysts in cycles with preimplantation genetic testing foraneuploidy. J. Assist. Reprod. Genet. 2021, 38, 1153–1162.
- Treff, N.R.; Marin, D. The “mosaic” embryo: Misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil. Steril. 2021, 116, 1205–1211.
- García-Pascual, C.M.; Navarro-Sánchez, L.; Navarro, R.; Martínez, L.; Jiménez, J.; Simón, C.; Rubio, C. Optimized NGS Approach for Detection of Aneuploidies and Mosaicism in PGT-A and Imbalances in PGT-SR. Genes 2020, 11, 724.
- Goodrich, D.; Xing, T.; Tao, X.; Lonczak, A.; Zhan, Y.; Landis, J.; Zimmerman, R.; Scott, R.T., Jr.; Treff, N.R. Evaluation of comprehensive chromosome screening platforms for the detection of mosaic segmental aneuploidy. J. Assist. Reprod. Genet. 2017, 34, 975–981.
- Munne, S.; Blazek, J.; Large, M.; Martinez-Ortiz, P.A.; Nisson, H.; Liu, E.; Tarozzi, N.; Borini, A.; Becker, A.; Zhang, J.; et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of highresolution next-generation sequencing. Fertil. Steril. 2017, 108, 62–71.
- Marin, D.; Xu, J.; Treff, N.R. Preimplantation genetic testing for aneuploidy: A review of published blastocyst reanalysis concordance data. Prenat. Diagn. 2021, 41, 545–553.
- Wasielak-Politowska, M.; Kordowitzki, P. Chromosome Segregation in the Oocyte: What Goes Wrong during Aging. Int J Mol Sci. 2022, 23, 2880.
- Gleicher, N.; Orvieto, R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review. J. Ovarian Res. 2017, 10, 21.
- Capalbo, A.; Poli, M.; Rienzi, L.; Girardi, L.; Patassini, C.; Fabiani, M.; Cimadomo, D.; Benini, F.; Farcomeni, A.; Cuzzi, J.; et al. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am. J. Hum. Genet. 2021, 108, 2238–2247.
- Liu, J.; Wang, W.; Sun, X.; Liu, L.; Jin, H.; Li, M.; Witz, C.; Williams, D.; Griffith, J.; Skorupski, J.; et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol. Reprod. 2012, 87, 148.
- Wu, L.; Jin, L.; Chen, W.; Liu, J.M.; Hu, J.; Yu, Q.; Ren, X.L.; Huang, B.; He, H. The true incidence of chromosomal mosaicism after preimplantation genetic testing is much lower than that indicated by trophectoderm biopsy. Hum. Reprod. 2021, 36, 1691–1701.
- Fragouli, E.; Lenzi, M.; Ross, R.; Katz-Jaffe, M.; Schoolcraft, W.B.; Wells, D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum. Reprod. 2008, 23, 2596–2608.
- Capalbo, A.; Wright, G.; Elliott, T.; Ubaldi, F.M.; Rienzi, L.; Nagy, Z.P. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum. Reprod. 2013, 28, 2298–2307.
- Huang, J.; Yan, L.; Lu, S.; Zhao, N.; Qiao, J. Re-analysis of aneuploidy blastocysts with an inner cell mass and different regional trophectoderm cells. J. Assist. Reprod. Genet. 2017, 34, 487–493.
- Capalbo, A.; Rienzi, L. Mosaicism between trophectoderm and inner cell mass. Fertil. Steril. 2017, 107, 1098–1106.
- Kim, J.; Tao, X.; Cheng, M.; Steward, A.; Guo, V.; Zhan, Y.; Scott, R.T., Jr.; Jalas, C. The concordance rates of an initial trophectoderm biopsy with the rest of the embryo using PGTseq, a targeted next-generation sequencing platform for preimplantation genetic testing-aneuploidy. Fertil. Steril. 2022, 117, 315–323.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
787
Revisions:
3 times
(View History)
Update Date:
29 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




