Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ruben Garcia Sobrino | -- | 6985 | 2023-12-25 10:46:29 | | | |
| 2 | Lindsay Dong | -38 word(s) | 6947 | 2023-12-27 01:47:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
García-Sobrino, R.; Muñoz, M.; Rodríguez-Jara, E.; Rams, J.; Torres, B.; Cifuentes, S.C. Magnesium Bioabsorbable Materials Based on Reinforced Polymeric Matrices. Encyclopedia. Available online: https://encyclopedia.pub/entry/53114 (accessed on 07 February 2026).
García-Sobrino R, Muñoz M, Rodríguez-Jara E, Rams J, Torres B, Cifuentes SC. Magnesium Bioabsorbable Materials Based on Reinforced Polymeric Matrices. Encyclopedia. Available at: https://encyclopedia.pub/entry/53114. Accessed February 07, 2026.
García-Sobrino, Rubén, Marta Muñoz, Elías Rodríguez-Jara, Joaquín Rams, Belén Torres, Sandra C. Cifuentes. "Magnesium Bioabsorbable Materials Based on Reinforced Polymeric Matrices" Encyclopedia, https://encyclopedia.pub/entry/53114 (accessed February 07, 2026).
García-Sobrino, R., Muñoz, M., Rodríguez-Jara, E., Rams, J., Torres, B., & Cifuentes, S.C. (2023, December 25). Magnesium Bioabsorbable Materials Based on Reinforced Polymeric Matrices. In Encyclopedia. https://encyclopedia.pub/entry/53114
García-Sobrino, Rubén, et al. "Magnesium Bioabsorbable Materials Based on Reinforced Polymeric Matrices." Encyclopedia. Web. 25 December, 2023.
Copy Citation
Improvements in Tissue Engineering and Regenerative Medicine (TERM)–type technologies have allowed the development of specific materials that, together with a better understanding of bone tissue structure, have provided new pathways to obtain biomaterials for bone tissue regeneration.
bone regeneration
bioabsorbable materials
composites
1. Introduction
Economic, technological and demographic difficulties associated with conventional therapies such as organ transplantation, implants and/or surgeries have generated worldwide interest over the years in research and clinical applications in the field of Tissue Engineering and Regenerative Medicine (TERM) [1]. Over the last decades, this sector has become an emerging and multidisciplinary field that seeks the maintenance, improvement and/or reconstruction of damaged tissues or organs from the synergy formed between different disciplines such as materials science, cell biology and biochemistry [2][3]. In this way, the search for and optimization of new materials with improved performance is, therefore, of great importance in the TERM sector to address increasingly complex pathologies.
In the case of bone-type tissues, although the native bone presents intrinsic regeneration capacities after damage or fracture processes, there are lesions that do not regenerate in a natural way. These defects generally are greater than 2 cm in some anatomical points and are associated with degenerative diseases or include congenital defects related with tumor removal processes [4][5]. As a solution, surgeries incorporating implants are performed, which in the cases of elderly patients may involve high risks to their health [6]. Improvements in TERM-type technologies have allowed the development of specific materials that, together with a better understanding of the biology of bone tissue structure, have provided new opportunities for bone regeneration with in vitro [7][8] or in vivo models [9][10] and even in the clinical phase [11][12].
The journal “Biomaterials” defines the concept of biomaterial as a material designed so that, individually or as part of a system, it can direct, through interactions with living tissue, the course of any therapeutic procedure [13]. These materials, linked to the sector, have gone from being characterized as simple materials that do not interact with the original tissue (bioinert) to bioactive materials that, in addition to generating a harmonious interface with the area to be replaced, should stimulate the processes of cell adhesion, proliferation and differentiation [14]. Thus, in the continuous search for possible improvements in patients’ life, biomaterials have revolutionized modern medicine with a wide range of applications in the biomedical sector, including their use as implants (dental implants, heart valves, intraocular lenses, ligaments, vascular grafts, etc.) or equipment for medical devices (artificial hearts, biosensors, pacemakers, etc.) [15][16].
Bioabsorbable materials are temporary structures that breakdown and are cleared from the physiological environment. These materials are designed to be non-toxic and gradually degrade, allowing mechanical stresses to be transferred gradually to the healing tissue [17]. This quality makes them even more interesting for certain applications such as research in the TERM sector, since in cases such as surgical treatments, they avoid second surgery processes thanks to their degradation [18]. That is why the study of bioabsorbable materials for implants is essential at this moment; in fact, the global market for this type of implants is expected to grow annually at 10.5% between 2022 and 2030 [19].
Depending on the family of the materials used, these elements can be metals, which include magnesium (Mg), zinc (Zn) and iron (Fe) [20]; ceramic, mainly bio glasses and calcium phosphate [21][22][23]; or polymeric type. The last one can be divided according to its origin into natural polymer (collagen or cellulose) and/or synthetic (commonly based on poly glutamic acid (PGA), poly lactic acid (PLA), poly caprolactone (PCL), poly (lactid-co-glycolid) (PLGA), polyethylene terephthalate (PET) or poly urethane (PU)) [20][24]. Polymers have traditionally been the most widely used family of bioabsorbable materials, with an approximate percentage of 81.4% compared to the 18.6% represented by the use of bioabsorbable metals [25]. Most commercially available resorbable implants are based on polymers and are applied in low-load bearing applications such as ankle, knee and hand surgery or cranio-maxillofacial surgery [26]. Global manufacturers of implants are committed to the growth of bioresorbable implants’ market. Efforts are focused into expanding the range of applications (for instance, the treatment of long-bone fractures or critical size defects) and offering more comfort to the patient and the surgeon by improving the performance of the implants. These goals necessarily imply the improvement of bioresorbable materials. A fourth family of materials, the so-called composite materials, has generated special interest in the bioabsorbable field in recent years. The properties resulting from the combination of materials from different families give rise to hybrid systems that allow to obtain a composite with superior performance than each material separately. Given their characteristics and interactions in the organism, polymers are ideal matrices for the study of composite biomaterials due to their versatility in terms of geometry, morphology, microstructures and synthesis, making them systems that can perfectly mimic the original structure of an organ [27][28].
In reference to studies related to bone lines, in addition to elements that favor osteoinductive processes, the inferior mechanical properties of polymers with respect to bone make it necessary to study reinforcement processes that allow obtaining hybrid materials with optimal density and mechanical properties [29]. This mechanical compatibility requirement is highly important. Thus, significantly different elastic modulus values with respect to the host bone tissue can lead to patient problems such as osteoporosis and osteolysis (moduli above and below the native value, respectively) [30]. In short, the synergy between the polymer matrix and reinforcement in composite materials makes it possible to achieve hybrid characteristics that match the properties required for the proper reconstruction of damaged tissues [31].
Regarding the study of composite material constructions with polymeric matrix and bioabsorbable capacity, in the last decades, mainly ceramic reinforcements have been extensively studied [32], such as collagen/hydroxyapatite [33], cellulose/calcium phosphates [34], PLA/hydroxyapatite [35], among others. The study on this kind of reinforcement is due to their compositional similarity to bone tissue and high absorption rate. In addition, acceptable mechanical and physical properties such as high hardness and wear motivate their use. However, their high brittleness limits their use in certain applications when high loads are required [36]. To overcome these limitations, Mg biodegradable metal is presented as an alternative solution to ceramic reinforcements. Mg is characterized by a high osteoinductive character due in part to its high presence in tissues of a bony nature (50% of the Mg content in the human body is found in this type of tissue). Also, it is necessary to mention that Mg has an elastic modulus similar to that of native osseous tissue (3–20 GPa) [37]. In the last decade, research on Mg as a biomaterial for bone regeneration has remarkably increased, and few commercially available biodegradable devices based on Mg have already been developed. These devices are mainly applied in low-load bearing applications [38].
2. Polymers as Bioabsorbable Materials for Bone Tissue Engineering
2.1. Aliphatic Polyester as Bioabsorbable Material: PLA and PCL
Since the last century, research on polymers has mainly focused on healthcare applications (controlled drug release, tissue engineering, wound healing, etc.) [39]. Although it is not so often mentioned to the vast majority, this family of materials is a fundamental and inherent part of nature itself. As previously mentioned, depending on their origin, polymers can be of natural or synthetic origin. In the case of the first ones, they coexist in our daily life, since they involve protein macromolecules of our body to elements such as silk generated by certain animals, for example, silkworms or spiders. It is for this reason that the study of these materials, in relation to applications in the health field, is so important, given their inherent excellent behavior in terms of biocompatibility and bioabsorbable capacity, complementary to the fact that they lack immunogenic response. Unfortunately, for certain applications, natural polymers are limited by their low mechanical properties and their lack of versatility in terms of chemical modification, which limits their study options [40].
Among the synthetic polymers applied in TERM studies, aliphatic polyesters, a family of polymers such as PLA, PGA, PCL or PLGA, have been studied. Their bioabsorption is based on the hydrolysis of the ester groups of the polymer chain, so this behavior can be modulated with compositional modifications that alter the structure, molecular weight and composition [41][42].
2.1.1. Polylactic Acid (PLA)
PLA is the most researched and used bioabsorbable polymeric material in medical applications (bone fixation material, suture, drug delivery microspheres and tissue engineering) [43]. PLA-based materials were approved by the Food and Drug Administration (FDA) [44] for direct contact with biological fluids in the 1970s and are obtained from the polymerization process of the lactic acid monomer unit (LA or 2-hydroxypropionic acid, C3H4O2 using catalysts and rigorous conditions (temperature, pressure and pH) in a long-time process [43]. This molecule is an organic acid of natural origin that in 90% of the cases is generated from the fermentation of sugars instead of chemistry synthesis processes [45]. PLA is a chiral molecule, in which two possible enantiomeric units, L- and D-lactic acid, co-exist. Thus, through the polymerization process (by polycondensation, ring-opening polymerization or direct methods such as azeotropic dehydration or enzymatic polymerization) [46], it is possible to obtain different macromolecule conformations depending on the proportion of the repeated units: on one hand, it is possible to form macrochains composed of PLLA and PDLA, both with a high degree of isotacticity, causing a high degree of crystallinity; and on the other hand, it is possible to form macrochains using a combination of D- and L-lactic acid, generating PDLLA macromolecules of atactic structure, thus reducing the crystallinity values [47][48]. This degree of tacticity is reduced with D-isomer values higher than 8–15%, making the polymer amorphous [49]. It is well known that the higher the degree of crystallinity, the better the mechanical properties of the system.
As for the associated degradation of this aliphatic polyester, it is related to the interaction of microorganisms such as fungi, bacteria and algae [50]. In the case of physiological environments, with the intention of study in the biomedical field as a bioabsorbable material, the oxidative, enzymatic and catalytic reactions related to the pH of the aqueous medium are the causes of the degradation process mentioned. In this scenario, water penetrates more easily into the amorphous phases of the structure, causing a decrease in the molecular weight value involving the cleavage of the macromolecule and with it the consequent loss of mechanical and mass properties.
2.1.2. Polycaprolactone (PCL)
The second of the polymeric and bioabsorbable matrices to be described is PCL, which was also approved by the Food and Drug Administration (FDA) as a biocompatible element [51]. This polymer is characterized by being a polyester of synthetic origin and partially crystalline (generally up to 80% crystallinity) [52], whose glass transition temperature (Tg) and melting temperature (Tm) are around −60 and 60 °C, respectively. Its polymerization process develops from the ring opening of the cyclic monomeric unit ε-caprolactone (ε-CL, C6H10O2).
In terms of degradation and in a generic manner, being also an aliphatic polyester, PCL in the presence of water undergoes hydrolysis with subsequent degradation of the soluble oligomer generated by the cells and microorganisms in the environment [53]. The PLA mechanism process begins with a reduction in the molecular weight of the macromolecule, which implies a breakdown of the chain structure that facilitates the process of metabolization by cells and/or microorganisms [54]. In fact, it is the decrease in molecular weight itself that precisely controls the hydrolysis of the polymer. Because of the hydrolysis process, there is a loss of mechanical properties based on cleavage of the macromolecule, which finally leads to the consequent loss of mass of the bioabsorbable material. These stages do not have a fixed duration and depend on the characteristics of the medium and the structure and configuration of PCL itself, i.e., high molecular weight values impair the degradation of the material, as expected.
3. Metals as Bioabsorbable Materials: Magnesium (Mg)
Traditionally, the good mechanical properties that characterize biomaterials of metallic nature have allowed them to be highly valued options in the selection of materials for biomedical applications related to bone lesions. Thus, for decades, their behavior towards the host tissue has been characterized as a bioactive relationship with it, as observed in the literature with numerous articles based on 316 L steels (also known as surgical steel), CoCr and titanium alloys such as Ti6Al4V [55][56][57]. In the last decades, the paradigm on the corrosion of metals has changed in the biomedical field, so that the so-called corrosion process is now referred to as a process of material degradation in a physiological environment, being ideal in biomedical applications where a bioresorbable device is appropriate [20]. Thanks to its biodegradable capacity, metals cited (Fe, Zn or Mg) play interesting roles as temporary orthopedic and vascular implants [58]. In the case of Fe, although its mechanical properties are similar to 316 L alloy and it being present in preclinical studies in the literature [59], Fe presents a very low degradation rate that is a problem for the bone regeneration process target; so, there is a continuous search for new iron alloys to accelerate its degradation rate [17]. On the other hand, in the case of Zn, although there are also studies in the preclinical phase [60], this metal has an intermediate corrosion rate between Fe and Mg and lower mechanical properties than Fe [17].
Mg is a metal with a high presence in the human body, thus ensuring its biocompatibility. To be exact, it is the fourth trace element with the highest presence in the body, participating in many of the physiological functions including the absorption and metabolization of calcium itself in the bones [37]. The density value of this metal is close to that of cortical bone. Also, regarding mechanical capabilities, this metal in its pure state has elastic modulus values like those of bone, as mentioned previously. The symbiosis between low density and strength close to bone generates a value of specific strength, the results of which are interesting for tailoring properties [61].
Magnesium degradation in a physiological environment can be understood with Equations (1) and (2). It is known that the concentration of chloride ions in physiological media is high (150 mmol/L). Under these conditions, a thin layer of magnesium hydroxide is formed after the contact of magnesium with aqueous media. At this point, magnesium hydroxide reacts with the chloride anion to form magnesium chloride, soluble in water, and hydrogen in the gaseous state [49]. The rate of generation of hydrogen gas is the most common parameter to evaluate the corrosion of magnesium.
Mg (s) + 2H2O (l) → Mg2+ (aq)+ 2(OH)− (aq) + H2 (g)
Mg(OH)2 (s) + 2Cl− (aq) → MgCl2 (soluble) + 2(OH)− (aq)
Although pure Mg has already been applied in the pre and clinical phase based on the literature [62], this material shows some disadvantages for which improvements are required. For instance, it is necessary to design Mg alloys with better mechanical performance, lower degradation rate and easier conformability capabilities. In this sense, research groups have redirected their efforts towards alternative solutions such as the development and optimization of new Mg alloys, coating processes or modifying the microstructure to find suitable alloys for different applications in the biomedical field. In the case of Mg alloys, presence of other elements in the structure causes variations in the target properties; so, it is necessary to emphasize that even the presence of traces or impurities in low concentrations can cause noticeable variations in the final properties of the material. For example, it is described that Fe, Ni and Cu play a detrimental effect on Mg corrosion rate [63]. Aluminum (Al), the main magnesium alloying element, is characterized by a high solubility in magnesium (12.7 wt.%); thus, the higher mechanical capacity of aluminum itself improves the strength conditions of the base metal [64].
4. Bioabsorbable Composite Materials Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg)
4.1. Bioabsorbable Hybrid Materials Based on PLA Reinforced with Mg (PLA/Mg)
Composites based on PLA and reinforced with Mg have been designed and developed using different types of reinforcement and various fabrication processes. The final biological properties of a composite and its performance in vitro and in vivo depend on the nature of the constituents, the geometry and amount of the reinforcement, the matrix–reinforcement interface and the fabrication process. Multiple combinations are possible to create composites. For clarification purposes, studies based on PLA/Mg composites in this section are organized here in three categories considering the geometry of the reinforcement and the fabrication technology. In this way, the first category deals with studies where PLA has been reinforced with Mg particles, the second category includes PLA reinforced with Mg wires and the last one includes PLA/Mg composites fabricated using 3D printing technology.
4.1.1. PLA Reinforced with Mg Particles
The study of PLA-based systems reinforced with Mg started in 2012 with the proof of the concept of the improvement of PLLA mechanical properties by incorporating 30 wt.% of Mg particles in the polymeric matrix. In the mentioned manuscript, Cifuentes, S. et al. [65] fabricated PLLA/Mg composites by solvent-casting and compression molding using Mg particles (<250 µm). PLLA/Mg showed higher compressive strength (101.3 MPa) and Young´s modulus (8 GPa) than pure PLLA (58.6 MPa and 2.86 GPa, respectively). Subsequent studies focused on developing PLA/Mg composites by different processing methods and on further improving the PLA mechanical performance by increasing Mg content. As a continuation of their research, Cifuentes, S. et al. [66] also explored the suitability of processing PLLA/Mg composites by hot extrusion. They homogeneously blended Mg particles (<50 µm) in different contents (0, 0.5, 1, 3, 5 and 7 wt.%) with a PLLA matrix (MFI: 35.8 g/10 min) using a Haake Minilab extruder. They found that Mg particles influenced PLLA thermal degradation during the extrusion process, and this led to a slight improvement in the stiffness of PLLA until 5 wt.% of Mg was achieved with a drastic drop of mechanical compressive performance in 7 wt.%. A later study on the extrusion of PLA/Mg composites achieved the incorporation of 15 wt.% Mg particles with a median particle size of 24.6 µm within polymeric matrices of PLLA (approximately 95,000 g/mol) and PDLLA (approx. 103,000 g/mol) [67]. They used a Rondol co-rotating twin-screw continuous extruder to compound the materials and incorporated Mg particles in contents of 0 as a control, 1, 5, 10 and 15 wt.%. Then, the mechanical properties of the composites were analyzed by Depth Sensing Indentation (DSI); an improvement in the modulus and hardness of composites was found when the reinforcement was higher than 5 wt.%. Mg reinforcement also influenced the creep behavior of polymeric matrices by incrementing the viscosity coefficient.
Ferrández-Montero, A. et al. [68] modified the surface of spherical Mg particles using stabilizers, polyethylenimine (PEI) and cetyltrimethylenimine (CTAB). They created colloidal suspensions with a high Mg content in deionized water at pH 12, added the stabilizer and dispersed the suspension in PLA previously dissolved in tetrahydrofuran (THF). Colloidal mixtures with a PLA/Mg ratio up to 50/50 wt./wt. were casted in films where a homogeneous distribution of Mg particles was achieved by the improvement of the particle bond to the matrix. The mechanical properties of PLA/Mg films with different Mg contents (0, 5, 10, 30 and 50 wt.%) modified with PEI and CTAB have been studied using dynamic mechanical thermal analysis (DMTA). Authors found a better interaction between PEI-modified particles with PLA than CTAB-modified particles with PLA. The elastic modulus of the composite reinforced with 10 wt.% Mg modified with PEI was higher than that of PLA and the composite modified with CTAB with the same percentage of reinforcement. However, a significant loss of modulus was observed when Mg content increased to 30 and 50 wt.% with both surface modifications. Authors explained this behavior with the effect of Mg content on the crystallinity of the films. Mg particles interfered with the crystallization of the polymer during the casting tape process. The films with 10 wt.% Mg modified with PEI presented a crystallinity of 45%, while the crystalline fraction with 30 wt.% and 50 wt.% of filler decreased to 30% and 6% respectively.
Regarding the in vitro degradation of PLA/Mg particles composites, Cifuentes, S. et al. [69] have conducted studies on the influence of Mg particle shape and the relevance of the nature and crystalline degree of the polymeric matrix [70]. The shape of Mg reinforcement played an important role in the degradation rate of PLA/Mg composites. Composites based on PLA (2002D) and reinforced with 10 wt.% Mg particles (less than 50 μm), which were spherical and irregular in shape (Mg-SPH and Mg-IRR, respectively), were fabricated and subjected to degradation studies in phosphate buffered saline (PBS) solution, at 37 °C after 7 and 28 days.
The analysis of the effect of PLA matrix crystallinity on the cytocompatibility of PLA/Mg composites has also been studied [70]. Human bone marrow-derived mesenchymal stem cells (MSCs) have been cultured in DMEM medium for up to 14 days with amorphous and crystalline PDLLA and PDLLA/Mg composites with an amorphous and a crystalline matrix. It was found that cytocompatibility increased with the addition of Mg into amorphous PDLLA. However, cytocompatibility of composites with the high crystalline matrix was considerably lower than that of composites with an amorphous polymeric matrix.
4.1.2. PLA Reinforced with Mg Wires
The improvement of PLA mechanical properties by incorporating Mg with the particle form is limited, and bone reparation required adequate and suitable mechanical properties. Compressive strength can be enhanced, but tensile strength could decrease [71]. The strengthening effect under compressive testing competes with the effect of particles on the thermal degradation of the polymer and on the interference in the crystallization of the matrix. Strategies to effectively improve the tensile and/or flexural strength of PLA/Mg composites suggest the incorporation of Mg alloys in the form of braided or unidirectional wires. The most common alloys that have been used as wire reinforcement for PLA are AZ31 and MgZn.
In this sense, Li, X. et al. [72] reinforced PLA (density of 1.24 g/cm3) with 2D braided wires of AZ31 alloy with a diameter of 0.3 mm. The effect of the braiding angle and wire content on the mechanical properties of the composite was evaluated. They studied four different braiding angles (15, 30, 45 and 60°) at 10 vol.% and four different wire contents (5, 7, 10 and 12 vol.%) at a braiding angle of 30°. The fabrication process of the composite consisted of lamina stacking. Laminas were formed by pouring a PLA solution in chloroform onto the braided wires. PLA/AZ31 composite laminas and PLA laminas were alternative stacked and blended by hot pressing. They found that the tensile and bending strength of the composite decreased proportionally with the braiding angle. The shear strength and impact strength showed a maximum value for the composites reinforced with wires braided at 45°. Regarding the effect of the volume fraction of wires, tensile, bending, shear strength and impact strength increased with AZ31 content. The manuscript also studied the effect of the modification of wires surface by micro-arc oxidation (MAO). The modification allowed a stronger filler–matrix interface that induced a positive strengthening effect on tensile, bending, shear and impact strength.
Ali, W. et al. [73] compared the degradation of the mechanical properties of a PLA composite reinforced with 50 vol.% of AZ31 wires vs. pure PLA. They performed the degradation tests in PBS at 37 and 50 °C and studied the tensile strength and flexural strength at certain immersion times (0,1, 4, 7, 14, 21, 28 and 35 days). They found that the elastic and flexural moduli of the composite were stable during the first four weeks, which is an important property for load-bearing applications. They also found that the degradation of the composite caused an increment in pH, which at 37 °C stayed below 8 for the first 21 days and increased up to 9 at the end of the experiment (35 days). At 50 °C, pH remained below 8 during the first 2 days and reached the value of 11 after 14 days. The authors stated the importance of controlling the degradation rate of the composite by different surface treatments. Therefore, further research works from Ali, W. et al. focused on the effect of surface treatments of wires on the degradation behavior of PLA/Mg wire composites.
The effect of fluoride coating on the degradation behavior of a PLA/AZ31 composite reinforced with 50 vol.% of wires has been studied [74]. The mechanical performance of a composite without the surface treatment and another coated with fluoride has been compared. Degradation tests were performed at 37 °C in PBS solution for 8 weeks (56 days). Composites were tested under tensile and flexural loading. Surprisingly and in contrast with the studies of Cai, H. et al. [75], fluoride coating did not improve the initial mechanical properties of PLA reinforced with untreated wires. However, the fluoride coating was effective in controlling the decrement of mechanical properties during the first 4 weeks. Tensile strength slightly dropped during the first day (163.7 MPa to 156.5 MPa), but then it remained at stable values until day 28 and further decreased till day 56. The composite reinforced with untreated AZ31 wires suffered a drastic decrement in tensile strength and lost its mechanical integrity after 35 days immersed in PBS. Fluoride treatment was also effective in maintaining the elastic modulus of the composite for 4 weeks and in slowing down the loss of flexural strength with time.
4.1.3. 3D Printing of PLA/Mg Particle Composites
Additive manufacturing (AM) technologies have been a breakthrough since their introduction due to their potential advantages, such as the customization of designs and the ability to create complex structures in one piece and without assemblies, reasons that favor the fabrication of complex geometry as interconnected scaffolds, ideal in TERM studies [76][77][78]. Methods of obtaining PLA/Mg composites by 3D printing can also be found in the literature. In fact, PLA is one of the most used and favorable polymers for 3D printing based on FFF technology due, among other things, to its low melting point [79]. For example, Bakhshi, R. et al. [80] describes the fabrication of scaffolds by FFF technology of PLA matrix and reinforced with magnesium (≤100 µm, irregular flake) for percentages of 0 as the control, 2, 4, 6, 8 and 10 wt.%. The mixing process was developed by dissolving the polymer together with the reinforcement in dichloromethane (DCM) with stirring for 5 min. Then, the mixture was heated in an oven for 12 h at 80 °C and then passed through an extruder to promote homogeneity (rotation at 30 rpm and 190 °C).
Similarly, Ali, F. et al. [81] complemented the study of Bakhshi, R. et al. [82] of composite printing under FFF technology but in this case with magnesium alloy reinforcement (AZ61). The PLA matrix used (3–5 mm pellet of approx. 68,000 g/mol) was diluted in chloroform at 20 g/L ratio and 400 rpm for 4 h at room temperature. The alloy was added to this mixture as reinforcement in different percentages: 0 as the control sample, 5, 10 and 15 wt.% (diameters <100 µm). Then, after drying at room temperature for 24 h, the authors passed the mixture through the extruder (175 °C), obtaining filaments of 2.75 mm diameter. From SEM images, the fabricated composites showed good distribution and no observable defects. On the other hand, as in previous studies, the presence of the metallic reinforcement increases the Tg value as it is an impediment in terms of intermolecular mobility. In terms of degradation, the samples were immersed for 2–4 weeks in PBS, and degradation was observed in proportion to the percentage of reinforcement incorporated and associated with an opportune increase in the pH of the medium (values of 8.5 with only 5 wt.% of reinforcement). The in vitro qualitative study developed on MC7s epithelial line showed improvements in terms of cell adhesion under magnesium alloy reinforcement.
Finally, printing processes that respond to the limitations of traditional FFF technology have also been addressed. Schmidt, F. et al. [83] presented a preliminary study on powder bed fusion-laser based (PBF-LB) technology. This technology, unlike FFF, allows the printing of geometries with better resolution and without the requirement of supports [84]. Thus, PBF-LB technology is based on laser sintering of polymer powders, whereby the final characteristics are dependent on the type of laser applied and the powder particle of origin [85].
In a complementary manner and in order to facilitate the information provided to the reader, a brief summary of PLA with Mg particle reinforcement is incorporated in Table 1.
Table 1. Biological studies on PLA/Mg bioabsorbable composites with application in bone tissue engineering.
| Sample | Preclinical Phase | Cell Line | Comments | Year/Reference |
|---|---|---|---|---|
| PDLLA with 0, 0.2 and 1 wt.% Mg | In vitro | hMSCs | Low presence of magnesium resulted in improved cell viability and macrophage responses | 2016, [71] |
| PLLA and PDLLA with 10 wt.% Mg | In vitro | hMSCs | Mg favored cytocompatibility evaluation. On the other hand, the structures with higher amorphous content (PDLLA) showed better cellular response | 2019, [70] |
| PLA with 0, 2 and 5 wt.% Mg | In vitro | MC3T3−E1 | Improved cell viability | 2017, [86] |
| PLA with 0, 15 and 30 vol.% Mg | In vitro | MC3T3−E1 | Improved cell activity in terms of cell adhesion and proliferation compared to control and 30 vol.%. This later was associated with an increase in pH | 2023, [87] |
| Mg ion antibacterial properties with respect to E. Coli and S. Aureus | Antibacterial capacity of magnesium ions under near infrared (NIR) emission (808 nm) | |||
| PLA (AZ31 wires with Ca-P) | In vitro | ADSCs | Mg improves cell adhesion and proliferation processes | 2023, [88] |
| PLA with 0, 2, 4, 6, 8 and 10 wt.% Mg | In vitro | Human fibroblast (L929) | Up to 6% positive effect in terms of bone cell culture | 2021, [80] |
| PLA with 0, 5, 10 and 15 wt.% (AZ61) | In vitro | MC7s epithelial line | Improved cell viability | 2023, [81] |
4.2. Bioabsorbable Hybrid Materials Based on PCL Reinforced with Mg (PCL/Mg)
4.2.1. PCL Reinforced with Mg Particles
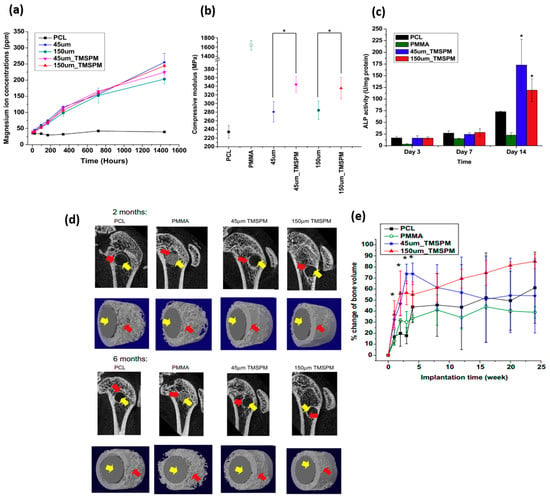
The effect of Mg particle size and surface modification on the release of Mg ions, compressive mechanical properties and cytocompatibility in in vitro and in vivo studies of PCL/Mg composites has been addressed by Wong, H. M. et al. [89] (see Figure 1). They developed their study systems based on PCL (approx. 80,000 g/mol) and reinforced it with Mg microparticles with different sizes (45 and 150 µm). The surface modification of the reinforcement was developed with the aim to improve the bonding generated between the metal and the polymeric matrix. The particles were coated with silane-based common coupling agents such as 3-(Trimethoxy silyl) propyl methacrylate (TMSPM) [90]. Already coated, particles were mixed in a Mg/PCL ratio of 0.1:1 (60 °C) with the polymeric matrix. The presence of silane and the variability in the size of the reinforcement did not show significant differences in the release of magnesium ions (see Figure 1a). In terms of stiffness under compression testing, the silane-reinforced samples also showed better mechanical performance, around 20% higher than their counterparts (Figure 1b), thus demonstrating their efficiency in the application of coupling agents between both systems and no influences on size reinforcement. To analyze the cell viability of these materials, pre-myoblast cell line MC3T3−E1 was evaluated and showed in Figure 1c. All the materials studied, including the control, showed cytocompatibility, with a 40% improvement when the samples were reinforced with magnesium. In relation to the presence of magnesium ions and capacity to promote bone tissue synthesis, the protein marker alkaline phosphatase (ALP) was evaluated, showing significant differences with respect to the control evaluation (PCL pure and PMMA) in the assays analyzed at 14 days. The highest ALP activity was shown by the composite reinforced with particles of 45 µm and modified with TMSPM. In the case of in vivo evaluation, the researchers based their study on the use of 2-month-old female Sprague–Dawley rats (SD rats), which were implanted with the new material in the lateral epicondyle of the animal. Figure 1d shows the influence of Mg particle incorporation and their surface modification on bone formation (red arrow in the image) quantified by micro-CT. Greater osteoconduction was shown in magnesium-reinforced samples compared to the proposed control systems without any associated inflammatory response (Figure 1e).

Figure 1. Images adapted from H.M. Wong et al. [89], where magnesium–silane reinforced PCL-based systems were analyzed. (a) Influence on the release of Mg2+ ions. (b) Elastic modulus values (MPa) in compression tests. (c) ALP analysis of magnesium-loaded samples in in vitro bone studies with MC3T3−E1 compared to control systems based on PMMA and PCL. (d) Images and analysis from micro-CT of the percentage of bone obtained also compared to control systems as a function of implantation time (yellow and red arrows are graft and new bone regenerated, respectively). (e) Quantified volume bone regeneration; difference significantly higher * (p < 0.05).
4.2.2. 3D Printing of PCL/Mg Composites
As was the case for using PLA as a matrix, different studies based on AM can be found in the literature. The effect of Mg content on the surface roughness, cell adhesion and proliferation in in vitro and in vivo studies in PCL/Mg composites has been addressed by Zhao, S. et al. [91]. They used FFF technology to manufacture polymeric scaffolds based on PCL (Mn approximately 68,000 g/mol), reinforced with Mg particles of 45 µm size. In order to obtain a uniform mixture, the precursors were heated at 100 °C in a biaxial roller mixer. Scaffolds with three percentages of reinforcement (5, 10 and 15 wt.%) together with a comparative control were evaluated and printed under the following conditions: temperature 110 °C, gas pressure 0.6 MPa and printing speed of 6–8 mm/s. All the proposed scaffolds presented the desired printability; however, according to the images analyzed by SEM, the presence of magnesium in the PCL causes surface modifications that affect the final roughness of the part. The authors attributed the heterogeneities on the surface to the dissimilar melting points and cooling capacities of PCL and Mg. In fact, the contact angle studies showed that the presence of magnesium in the filament favors the surface hydrophilicity of the system with more than favorable consequences in biological studies, where it is recognized that hydrophobic systems tend to hinder elementary processes such as adhesion and cell proliferation in TERM studies [92][93]. On the other hand, the release of magnesium ions showed that the samples with a higher presence of magnesium released a greater number of ions with a consequent increase in pH medium, reaching in samples with 15 wt.% alkaline values of pH 8.3 after three days of study. This result was supported by the in vitro assay developed from the rat bone marrow mesenchymal stem cells (rBMSCs) cell line, where samples with 10 wt.% of reinforcement showed the highest capacity for cell adhesion and proliferation.
The effect of Mg content on the mechanical properties, in vitro cytocompatibility studies and in vivo bone regeneration capacity has been also studied by Dong, Q. et al. [94]. They fabricated scaffolds using FFF technology. They developed their material based on PCL (80,000 g/mol) at 200 °C blended with magnesium particles (28.6 µm) in different percentages (0, 1, 3, 5, 7 and 9 wt.%) for 60 min. The printing conditions were a temperature of 160 °C and printing velocity of 1.5 mm/s. The scaffolds obtained showed a 66% porosity with a porosity size of 480 ± 25 µm. The analyses referred to DSC studies showing a reduction in the degree of crystallinity of PCL with the increment of Mg content especially from 3 wt.%. Also, an increase in hydrophilicity was observed with Mg contents higher than 3%. In terms of compression studies, scaffolds with 3, 5 and 7 wt.% of Mg exhibited higher compressive resistance than the control or with low content reinforcement. The appropriate Mg incorporation has a reinforcing effect on mechanical properties, but excessive amounts of Mg micro-particles weaken the mechanical performance. Cytocompatibility studies were based on the rBMSC cell line. A progressive improvement in cell adhesion and proliferation capacity was observed up to 5%. Higher content of Mg reinforcement implied an excessive release of magnesium ions, which reduced the effectiveness of the scaffold. Under cell quantification with ALP, it was observed that percentages of 3% generated greater apatite synthesis capacity, showing significant improvements with respect to the control system. Finally, in vivo studies, based on white rabbits of about 3–5 months, were evaluated in localization on the medial tibial tubercle and confirmed that the 3% reinforcement systems had greater bone regeneration capacity than the control systems. Table 2 shows the preclinical work developed on this bioabsorbable composite for bone tissue engineering applications.
Table 2. Biological studies on PCL/Mg bioabsorbable composites with application in bone tissue engineering.
| Sample | Preclinical Phase | Cell line or Animal Model (Injury) | Comments | Year/Reference |
|---|---|---|---|---|
| PCL with 10 wt.% of Mg (treated with TMSPM) | In vitro | MC3T3−E1 | ALP protein marker showed better differentiation towards bone lineage in the presence of Mg | 2013, [89] |
| In vivo | SD rats (lateral epicondyle) | Micro-CT analysis demonstrated increased bone formation in the presence of Mg | ||
| PCL with 0, 5, 10 and 15 wt.% of Mg (treated with TMSPM) | In vitro | MC3T3−E1 | High Mg levels favored stages such as cell adhesion and proliferation with respect to the control sample. In addition, ALP marker showed greater capacity for bone differentiation | 2014, [95] |
| In vivo | SD rats (lateral epicondyle) | Micro-CT analysis demonstrated increased bone formation with Mg presence | ||
| PCL with 0, 5, 10 and 15 wt.% of Mg | In vitro | rBMSCs | Improvement of cellular processes up to 10 wt.%; from this percentage, a reduction in efficiency was observed due to an increase in the pH of the medium | 2020, [91] |
| In vivo | SD rats (skull model) | Micro-CT and X-ray measurements showed that the reinforced sample was significantly improved over the control sample | ||
| PCL with 0, 1, 3, 5, 7 and 9 wt.% of Mg | In vitro | rBMSCs | Improvement of cellular processes such as cell adhesion and proliferation effective up to 5 wt.%. In addition, ALP marker showed that 3 wt.% exhibited better differentiation capacity towards bone lineage | 2021, [94] |
| In vivo | Rabbits (medial tibial tubercle) | Analysis of 3 wt.% Mg improved in terms of bone repair with respect to the control sample | ||
| PCL with 0, 5 and 20 wt.% of Mg(OH)2 | In vitro | hOBs | Improved cell adhesion and proliferation with the presence of Mg. On the other hand, ALP also showed a greater capacity for differentiation towards bone lineage | 2020, [18] |
| PCL with 0, 10, 20 and 30 wt.% of CS-Mg | In vitro | hMSCs | Alizarin red staining and osteocalein showed higher bone matrix mineralization at 20 wt.% with respect to the control system. Synergy formed with the corresponding Mg and release of silicate ions | 2017, [96] |
4.2.3. Mg-Reinforced PCL-Based Copolymers
Finally, it is worth mentioning other studies in which the polymeric matrix is based on PCL copolymers. In the case of Shen, J. et al. [97], they developed a 3D printed material with a polymeric matrix based on polycaprolactone-co-poly(ethyleneglycol)-co-polycaprolactone (PCL-PEG-PCL) copolymers with a surface modified by the presence of magnesium oxide (MgO) nanoparticles. This reinforcement improved the wettability of the control system and its resistance capacity in compression tests. In terms of in vitro analysis, under the MC3T3−E1 cell line, previous works described the presence of magnesium that also represented improvements in cell adhesion, proliferation and differentiation towards the bone lineage with respect to the control systems. Animal studies were performed in thirty female Sprague–Dawley, which were 12-weeks-old. The presence of MgO stimulated the regeneration process, but the excess of metal reinforcement (10:1 wt. ratio between copolymer and MgO) limited the efficiency of the process.
5. Conclusions
The enhancement of mechanical properties depends on the balance between the strengthening effect of Mg particles and their influence on the thermal degradation and crystallization of the polymeric matrix. Particles can improve the behavior of the matrix under compression but deteriorate the mechanical performance under tensile or flexural stresses. The amount of Mg particles that can be incorporated into the polymeric matrix depends on the manufacturing technology and the matrix–filler interaction. Metal–polymer bonding can be improved by surface treatments on the reinforcement or by adding coupling agents in order to enhance the stiffness of the material under compression testing. Nonetheless, excessive amounts of Mg microparticles weaken the mechanical performance, and a strengthening effect is obtained only with an appropriate amount of reinforcement.
The stability of the compressive strength with time of particulate composites depends on the shape of the reinforcement. Irregularly shaped particles degrade faster than spherical ones. Composites reinforced with irregularly shaped particles lose their mechanical integrity in a week, while composites reinforced with spherical ones retain 60% of their compressive strength after 4 weeks. Relevant for load bearing applications is the capacity of the material to retain its mechanical properties during the first 4 weeks in physiological conditions. Therefore, for particulate composites to be suitable for load-bearing applications, further studies are needed to control the loss of their compressive strength.
On the other hand, to effectively improve the tensile strength and bending strength of composites, the reinforcement with Mg wires has been proposed. This strategy has been considered mainly with PLA; there are no studies on PCL reinforced with Mg wires. The surface modification of wires allows a stronger filler–matrix interface improving the mechanical performance of the material. MAO and HF treatments are the most effective. Tensile and bending strength can be tailored by changing the Mg wire content and surface treatment. PLA/Mg composites reinforced with wires have the capacity to retain their elastic and flexural moduli during the first 4 weeks in physiological conditions. This implies that this type of composite could provide enough mechanical stability for successful bone fracture healing.
Concerning composite degradation, it has been confirmed that the polymeric matrix plays an important role in controlling the degradation rate in physiological environments of Mg reinforcement. Regarding the effect of the crystalline degree on the degradation rate of composites, contradictory results have been obtained between composites reinforced with particles and composites reinforced with wires. While a high crystalline matrix accelerates the degradation rate of particulate composites, a polymeric matrix with higher orientation and crystallinity slows down the degradation rate of Mg wires in composites reinforced with them. Then, further research on the role of the crystallinity of the polymeric matrix in polymer/Mg composites is needed to elucidate the reasons behind these contradictory results. It is known that high molecular weight polymers degrade at a slower rate than low molecular weight polymers. Therefore, it is expected that the molecular weight of the polymeric matrix (PLA or PCL) influences the bioabsorption rate of composites reinforced with Mg.
Regarding in vivo evaluation, it has been confirmed that the presence of Mg in a polymer stimulates the bone formation process, accelerates the synthesis of bone apatite, reduces the inflammatory response and enhances osteoconduction in comparison to systems without reinforcement. However, these results have only been validated for PCL/Mg scaffolds. There is a lack of in vivo evaluation for PLA/Mg systems. Further research on the in vivo performance of PLA/Mg composite systems is needed to validate this material for future clinical applications in bone therapy. In the case of load-bearing applications, polymeric matrix reinforced with Mg wires look more appropriate (although to this date, only studies based on PLA/Mg were found), while particulate composites could be useful for tissue engineering and low load-bearing applications.
References
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng.—Part C Methods 2020, 26, 91–106.
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926.
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430.
- Annamalai, R.T.; Hong, X.; Schott, N.G.; Tiruchinapally, G.; Levi, B.; Stegemann, J.P. Injectable Osteogenic Microtissues Containing Mesenchymal Stromal Cells Conformally Fill and Repair Critical-Size Defects. Biomaterials 2019, 208, 32–44.
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22.
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603.
- García-Sobrino, R.; Casado-Losada, I.; Bruno-Pérez, L.; García, C.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A.; Martínez-Campos, E. Thermosensitive Hydrogels Functionalized with PH Sensitive COOH Groups for Bone Cell Harvesting. Eur. Polym. J. 2022, 169, 111131.
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO Coatings Design for Mg-Ca Alloy for Cardiovascular Stent and Bone Regeneration Applications. Mater. Sci. Eng. C 2019, 105, 110026.
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222.
- Andalib, N.; Kehtari, M.; Seyedjafari, E.; Motamed, N.; Matin, M.M. In Vivo Bone Regeneration Using a Bioactive Nanocomposite Scaffold and Human Mesenchymal Stem Cells. Cell Tissue Bank. 2021, 22, 467–477.
- Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Kakiuchi, Y.; Nakano, M.; Suzuki, S.; Handa, K.; Saito, M. Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells 2021, 10, 2687.
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162.
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909.
- dos Santos, V.; Brandalise, R.N.; Savaris, M. Composite Biomaterials. In Engineering of Biomaterials. Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2017.
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492.
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2018; pp. 85–98.
- Khan, H.; Barkham, B.; Trompeter, A. The Use of Bioabsorbable Materials in Orthopaedics. Orthop. Trauma 2021, 35, 289–296.
- Abdal-hay, A.; Raveendran, N.T.; Fournier, B.; Ivanovski, S. Fabrication of Biocompatible and Bioabsorbable Polycaprolactone/Magnesium Hydroxide 3D Printed Scaffolds: Degradation and in Vitro Osteoblasts Interactions. Compos. Part B Eng. 2020, 197, 108158.
- DataIntelo. Bioabsorbable Implants Market Report|Global Forecast from 2022 to 2030. Available online: https://dataintelo.com/report/bioabsorbable-implants-market/ (accessed on 1 April 2023).
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable Materials. Orthop. Trauma 2017, 31, 316–320.
- Pearson, J.J.; Gerken, N.; Bae, C.; Lee, K.B.; Satsangi, A.; McBride, S.; Appleford, M.R.; Dean, D.D.; Hollinger, J.O.; Ong, J.L.; et al. In Vivo Hydroxyapatite Scaffold Performance in Infected Bone Defects. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2020, 108, 1157–1166.
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019, 23, 4.
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A New Ag-Nanostructured Hydroxyapatite Porous Scaffold: Antibacterial Effect and Cytotoxicity Study. Mater. Sci. Eng. C 2021, 118, 111394.
- Balcon, R.; Beyar, R.; Chierchia, S.; De Scheerder, I.; Hugenholtz, P.G.; Kiemeneij, F.; Meier, B.; Meyer, J.; Monassier, J.P.; Wijns, W. Recommendations on Stent Manufacture, Implantation and Utilization. Eur. Heart J. 1997, 18, 1536–1547.
- Prasad, A. Bioabsorbable Polymeric Materials for Biofilms and Other Biomedical Applications: Recent and Future Trends. Mater. Today Proc. 2021, 44, 2447–2453.
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346.
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63.
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2014.
- Bonilla, C.E.P.; Perilla, J.E. The Past, Present and near Future of Materials for Use in Biodegradable Orthopaedic Implants. Ing. Investig. 2011, 31, 124–133.
- Pawelec, K.M.; White, A.A.; Best, S.M. Properties and Characterization of Bone Repair Materials, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018.
- Verdier, C. Rheological Properties of Living Materials. From Cells to Tissues. J. Theor. Med. 2003, 5, 67–91.
- Palmero, P. Ceramic-Polymer Nanocomposites for Bone-Tissue Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2016.
- Liu, C. Collagen–Hydroxyapatite Composite Scaffolds for Tissue Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2015.
- Salama, A. Cellulose/Calcium Phosphate Hybrids: New Materials for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2019, 127, 606–617.
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333.
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites. Handb. Bioceram. Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–1386.
- Multigner, M.; Muñoz, M.; Pulido-González, N.; Torres, B.; Cifuentes, S.C. Mg as Bioabsorbable Material. Encycl. Mater. Met. Alloy. 2021, 1, 113–122.
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.C.; Seok, H.K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today 2019, 23, 57–71.
- Chen, W.H.; Chen, Q.W.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S.; et al. Biomedical Polymers: Synthesis, Properties, and Applications. Sci. China Chem. 2022, 65, 1010–1075.
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-Based Materials with Novel Characteristics for Tissue Engineering Applications—A Review. Int. J. Biol. Macromol. 2017, 98, 837–846.
- Hakkarainen, M.; Albertsson, A.C. Degradation Products of Aliphatic and Aliphatic-Aromatic Polyesters. Adv. Polym. Sci. 2008, 211, 85–116.
- Cameron, D.J.A.; Shaver, M.P. Aliphatic Polyester Polymer Stars: Synthesis, Properties and Applications in Biomedicine and Nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776.
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413.
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626.
- Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Grabar, T.B.; Ingram, L.O. Fermentation of 12% (w/v) Glucose to 1.2 M Lactate by Escherichia Coli Strain SZ194 Using Mineral Salts Medium. Biotechnol. Lett. 2006, 28, 663–670.
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84.
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501.
- Cifuentes, S.C.; Benavente, R.; Lieblich, M.; González-Carrasco, J.L. Biodegradable and Bioabsorbable Materials for Osteosynthesis Applications: State-of-the-Art and Future Perspectives. Handb. Compos. from Renew. Mater. 2017, 1–8, 109–143.
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers—A Review. Polish J. Environ. Stud. 2010, 19, 255–266.
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric Materials for Bone and Cartilage Repair. Prog. Polym. Sci. 2010, 35, 403–440.
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20.
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455.
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-Based (Bio)degradable Polymers as Environmentally Friendly Materials for Sustainable Development. Int. J. Mol. Sci. 2015, 16, 564–596.
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089.
- Odaira, T.; Xu, S.; Hirata, K.; Xu, X.; Omori, T.; Ueki, K.; Ueda, K.; Narushima, T.; Nagasako, M.; Harjo, S.; et al. Flexible and Tough Superelastic Co–Cr Alloys for Biomedical Applications. Adv. Mater. 2022, 34, e2202305.
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2020, 39, 84–90.
- Ryu, H.; Seo, M.H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc. Mater. 2021, 10, 2002236.
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962.
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current Status and Perspectives of Zinc-Based Absorbable Alloys for Biomedical Applications. Acta Biomater. 2019, 97, 1–22.
- Witte, F. Reprint of: The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2015, 23, S28–S40.
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In Vitro and in Vivo Studies on the Degradation of High-Purity Mg (99.99 wt.%) Screw with Femoral Intracondylar Fractured Rabbit Model. Biomaterials 2015, 64, 57–69.
- Jamel, M.M.; Jamel, M.M.; Lopez, H.F. Designing Advanced Biomedical Biodegradable Mg Alloys: A Review. Metals 2022, 12, 85.
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The Role of Solute in Grain Refinement of Magnesium. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2000, 31, 2895–2906.
- Cifuentes, S.C.; Frutos, E.; González-Carrasco, J.L.; Muñoz, M.; Multigner, M.; Chao, J.; Benavente, R.; Lieblich, M. Novel PLLA/Magnesium Composite for Orthopedic Applications: A Proof of Concept. Mater. Lett. 2012, 74, 239–242.
- Cifuentes, S.C.; Lieblich, M.; López, F.A.; Benavente, R.; González-Carrasco, J.L. Effect of Mg Content on the Thermal Stability and Mechanical Behaviour of PLLA/Mg Composites Processed by Hot Extrusion. Mater. Sci. Eng. C 2017, 72, 18–25.
- Cifuentes, S.C.; Frutos, E.; Benavente, R.; Lorenzo, V.; González-Carrasco, J.L. Assessment of Mechanical Behavior of PLA Composites Reinforced with Mg Micro-Particles through Depth-Sensing Indentations Analysis. J. Mech. Behav. Biomed. Mater. 2017, 65, 781–790.
- Ferrández-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. Study of the Matrix-Filler Interface in PLA/Mg Composites Manufactured by Material Extrusion Using a Colloidal Feedstock. Addit. Manuf. 2020, 33, 101142.
- Cifuentes, S.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L. In Vitro Degradation of Biodegradable Polylactic Acid/Magnesium Composites: Relevance of Mg Particle Shape. Acta Biomater. 2016, 32, 348–357.
- Cifuentes, S.C.; Lieblich, M.; Saldaña, L.; González-Carrasco, J.L.; Benavente, R. In Vitro Degradation of Biodegradable Polylactic Acid/Mg Composites: Influence of Nature and Crystalline Degree of the Polymeric Matrix. Materialia 2019, 6, 100270.
- Cifuentes, S.C.; Bensiamar, F.; Gallardo-Moreno, A.M.; Osswald, T.A.; González-Carrasco, J.L.; Benavente, R.; González-Martín, M.L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Incorporation of Mg Particles into PDLLA Regulates Mesenchymal Stem Cell and Macrophage Responses. J. Biomed. Mater. Res.—Part A 2016, 104, 866–878.
- Li, X.; Chu, C.; Zhou, L.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. Fully Degradable PLA-Based Composite Reinforced with 2D-Braided Mg Wires for Orthopedic Implants. Compos. Sci. Technol. 2017, 142, 180–188.
- Ali, W.; Mehboob, A.; Han, M.G.; Chang, S.H. Experimental Study on Degradation of Mechanical Properties of Biodegradable Magnesium Alloy (AZ31) Wires/Poly(Lactic Acid) Composite for Bone Fracture Healing Applications. Compos. Struct. 2019, 210, 914–921.
- Ali, W.; Mehboob, A.; Han, M.G.; Chang, S.H. Effect of Fluoride Coating on Degradation Behaviour of Unidirectional Mg/PLA Biodegradable Composite for Load-Bearing Bone Implant Application. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105464.
- Cai, H.; Zhang, Y.; Meng, J.; Li, X.; Xue, F.; Chu, C.; Tao, L.; Bai, J. Enhanced Fully-Biodegradable Mg/PLA Composite Rod: Effect of Surface Modification of Mg-2Zn Wire on the Interfacial Bonding. Surf. Coat. Technol. 2018, 350, 722–731.
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554.
- Haleem, A.; Javaid, M. Additive Manufacturing Applications in Industry 4.0: A Review. J. Ind. Integr. Manag. 2019, 4, 1930001.
- Garcia, C.; Gallardo, A.; López, D.; Elvira, C.; Azzahti, A.; Lopez-Martinez, E.; Cortajarena, A.L.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Smart PH-Responsive Antimicrobial Hydrogel Scaffolds Prepared by Additive Manufacturing. ACS Appl. Bio Mater. 2018, 1, 1337–1347.
- Wang, S.; Daelemans, L.; D’hooge, D.R.; Couck, L.; Van Den Broeck, W.; Cornillie, P.; Gou, M.; De Clerck, K.; Cardon, L. Lifting the Quality of Fused Filament Fabrication of Polylactic Acid Based Composites. Compos. Part B Eng. 2021, 210, 108613.
- Bakhshi, R.; Mohammadi-Zerankeshi, M.; Mehrabi-Dehdezi, M.; Alizadeh, R.; Labbaf, S.; Abachi, P. Additive Manufacturing of PLA-Mg Composite Scaffolds for Hard Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105655.
- Ali, F.; Kalva, S.N.; Mroue, K.H.; Keyan, K.S.; Tong, Y.; Khan, O.M.; Koç, M. Degradation Assessment of Mg-Incorporated 3D Printed PLA Scaffolds for Biomedical Applications. Bioprinting 2023, 35, e00302.
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993.
- Schmidt, F.; Weishaupt, O.; Radwan, M.; Willeke, M.; Frerich, S. PLA-Mg Composites by Laser-Based Powder Bed Fusion—A Preliminary Study. Addit. Manuf. Lett. 2023, 6, 100148.
- Karl, D.; Jastram, B.; Kamm, P.H.; Schwandt, H.; Gurlo, A.; Schmidt, F. Evaluating Porous Polylactide-Co-Glycolide/Bioactive Glass Composite Microsphere Powders for Laser Sintering of Scaffolds. Powder Technol. 2019, 354, 289–300.
- Kusoglu, I.M.; Doñate-buendía, C.; Barcikowski, S.; Gökce, B. Laser Powder Bed Fusion of Polymers: Quantitative Research Direction Indices. Materials 2021, 14, 1169.
- Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Zhang, X. Development of PLA/Mg Composite for Orthopedic Implant: Tunable Degradation and Enhanced Mineralization. Compos. Sci. Technol. 2017, 147, 8–15.
- Lee, H.; Shin, D.Y.; Na, Y.; Han, G.; Kim, J.; Kim, N.; Bang, S.J.; Kang, H.S.; Oh, S.K.; Yoon, C.B.; et al. Antibacterial PLA/Mg Composite with Enhanced Mechanical and Biological Performance for Biodegradable Orthopedic Implants. Biomater. Adv. 2023, 152, 213523.
- Sun, S.; Gao, L.; Liang, B.; Yin, Z.; Pan, S.; Shi, C.; Guo, C.; Huang, Z.; Chu, C.; Dong, Y. Long-Term and Uniform Release of Magnesium Ions from PLA Porous Composite Materials Oriently Reinforced by Mg Wires for Potential Bone Repair Application. Surf. Interfaces 2023, 40, 103018.
- Wong, H.M.; Wu, S.; Chu, P.K.; Cheng, S.H.; Luk, K.D.K.; Cheung, K.M.C.; Yeung, K.W.K. Low-Modulus Mg/PCL Hybrid Bone Substitute for Osteoporotic Fracture Fixation. Biomaterials 2013, 34, 7016–7032.
- Cui, H.; Kessler, M.R. Pultruded Glass Fiber/Bio-Based Polymer: Interface Tailoring with Silane Coupling Agent. Compos. Part A Appl. Sci. Manuf. 2014, 65, 83–90.
- Zhao, S.; Xie, K.; Guo, Y.; Tan, J.; Wu, J.; Yang, Y.; Fu, P.; Wang, L.; Jiang, W.; Hao, Y. Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 5120–5131.
- Wolfenson, H.; Lavelin, I.; Geiger, B. Review Dynamic Regulation of the Structure and Functions of Integrin Adhesions. Dev. Cell 2013, 24, 447–458.
- Hoshiba, T.; Yoshikawa, C.; Sakakibara, K. Characterization of Initial Cell Adhesion on Charged Polymer Substrates in Serum-Containing and Serum-Free Media. Langmuir 2018, 34, 4043–4051.
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-Printed Mg-Incorporated PCL-Based Scaffolds: A Promising Approach for Bone Healing. Mater. Sci. Eng. C 2021, 129, 112372.
- Wong, H.M.; Chu, P.K.; Leung, F.K.L.; Cheung, K.M.C.; Luk, K.D.K.; Yeung, K.W.K. Engineered Polycaprolactone-Magnesium Hybrid Biodegradable Porous Scaffold for Bone Tissue Engineering. Prog. Nat. Sci. Mater. Int. 2014, 24, 561–567.
- Tsai, K.Y.; Lin, H.Y.; Chen, Y.W.; Lin, C.Y.; Hsu, T.T.; Kao, C.T. Laser Sintered Magnesium-Calcium Silicate/Poly-ε-Caprolactone Scaffold for Bone Tissue Engineering. Materials 2017, 10, 65.
- Shen, J.; Wang, W.; Zhai, X.; Chen, B.; Qiao, W.; Li, W.; Li, P.; Zhao, Y.; Meng, Y.; Qian, S.; et al. 3D-Printed Nanocomposite Scaffolds with Tunable Magnesium Ionic Microenvironment Induce in Situ Bone Tissue Regeneration. Appl. Mater. Today 2019, 16, 493–507.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
568
Revisions:
2 times
(View History)
Update Date:
27 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




