Global warming and the continued increase in the world’s population have led to a shortage of freshwater resources and a further decline in groundwater levels, posing a major challenge to agriculture worldwide
[21]. Drought, which is defined as being in a state of water shortage for several consecutive weeks
[22], is the most common abiotic stress around the world and severely affects flowering time, flower morphological developmental processes, and the seed productivity of several plant species. It is particularly noteworthy that drought stress can also cause flower abortion and eventually plant sterility by altering the expression levels of various genes critical to flowering regulation pathways, which regulate both flowering time and response to drought stress
[23]. In the following, how plants perceive and respond to drought stress is discussed, the differential physiological phenotypes of various plant species under drought stress conditions are further summarized, and, finally, the potential molecular mechanisms underlying drought-stress-induced flowering are focused on.
2. Perception and Coping Strategies of Drought Stress
Plants perceive drought stress signals mainly through the leaves and the root system. Stomatal movement can be observed in leaves, and drought stress can lead to the accumulation of reactive oxygen species (ROS) (7,
Table 1) and of abscisic acid (ABA) (8,
Table 1) in leaves, which, in turn, regulates the movement of guard cells and ultimately determines the opening and closing state of stomata
[24]. However, it is difficult to determine how plants respond to drought stress in the root system
[25][26]. A deficiency of water can constrain the growth and development process of plants, and can even have a significant impact on plant survival
[1][27]. As a result, plants have evolved a variety of strategies to cope with damage caused by drought stress. The process by which plants sense water deficit signals and further initiate coping strategies in response to drought stress is known as drought resistance. The adaptability of plants to drought stress mainly consists of three different coping strategies, namely, drought escape, drought avoidance, and drought tolerance
[28]. Drought escape, a common strategy exploited in response to drought stress, refers to plants that accelerate flowering and shorten their entire life cycle before severe drought stress hinders their survival
[29][30]. However, in order to achieve early flowering, a drought escape strategy will terminate vegetative growth in advance, which can severely influence the growth and development of vegetative organs, and eventually lead to a dramatic decrease in seed yield. Drought avoidance (also known as drought dehydration) is another strategy for plants to cope with external drought conditions by increasing the internal water content (by reducing water loss or maximizing water uptake)
[31]. The drought tolerance strategy is the ability of plants to tolerate low internal water content and to adapt to the drought stress while initiating reproduction
[32].
Table 1. Specialized terms and their abbreviations appearing in this research.
| Number |
Abbreviations |
Full-Name |
Number |
Abbreviations |
Full-Name |
| 1 |
CO |
CONSTANS |
24 |
HDA6 |
HISTONE DEACETYLASE 6 |
| 2 |
FT |
FLOWERING LOCUS T |
25 |
FES1 |
FRI ESSENTIAL 1 |
| 3 |
LFY |
LEAFY |
26 |
FRL1 |
FRI-LIKE 1 |
| 4 |
AP1 |
APETALA1 |
27 |
FLX |
FLC EXPRESSOR |
| 5 |
GI |
GIGANTEA |
28 |
SUF4 |
SUPPRESSOR OF FRI 4 |
| 6 |
SOC1 |
SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 |
29 |
VRN1 |
VERNALIZATION1 |
| 7 |
ROS |
reactive oxygen species |
30 |
FUL |
FRUITFULL |
| 8 |
ABA |
abscisic acid |
31 |
VAL |
CAULIFLOWER |
| 9 |
SD |
short-day conditions |
32 |
PIF4 |
PHYTOCHROME-INTERACTING TRANSCRIPTION 4 |
| 10 |
LD |
long-day conditions |
33 |
MAF2 |
MADSAFFECTING FLOWERING 2 |
| 11 |
TSF |
TWIN SISTER OF FT |
34 |
FCA |
FLOWERING CONTROL LOCUS A |
| 12 |
SVP |
SHORT VEGETATIVE PHASE |
35 |
FVE |
FLOWERING LOCUS VE |
| 13 |
FLC |
FLOWERING LOCUS C |
36 |
FLM |
FLOWERING LOCUS M |
| 14 |
Hd3a |
HEADING DATE 3a |
37 |
HOS1 |
HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1 |
| 15 |
RFT1 |
RICE FLOWERING LOCUS T1 |
38 |
HSR |
heat stress response |
| 16 |
Ehd1 |
EARLY HEADING DATE 1 |
39 |
HSPs |
heat shock proteins |
| 17 |
RCN1 |
RICE CENTRORADIALIS 1 |
40 |
HSFs |
heat stress transcription factors |
| 18 |
FD |
FLOWERING LOCUS D |
41 |
BOB1 |
BOBBER1 |
| 19 |
FAC |
florigen activation complex |
42 |
FTL3 |
FLOWERING LOCUS T-like 3 |
| 20 |
FRI |
FLOWERING CONTROL LOCUS A |
43 |
PRR |
PSEUDO RESPONSE REGULATOR |
| 21 |
OST1 |
OPEN STOMATA 1 |
44 |
LUX |
LUX ARRHYTHMO |
| 22 |
VOZ1 |
VASCULAR PLANT ONE-ZINC FINGER 1 |
45 |
Eps-D1 |
Earliness per se locus |
| 23 |
RFS |
Regulator of Flowering and Stress |
46 |
EG1 |
EXTRA GLUME 1 |
Under drought stress conditions, plants can respond by early or late flowering, depending on the onset, duration, and severity of drought
[20][33]. A bibliometrics analysis showed that plant response to drought has become an important research topic
[34]. When plants are adequately supplied with water, the stomata remain open to a large extent to enable the plants to fully photosynthesize, while under mild drought stress, plants will appropriately regulate stomatal closure to minimize water loss by reducing transpiration, but this will result in a decrease in the rate of photosynthesis. When subjected to severe drought stress, the stomata are generally in a minimally open state to ensure that some photosynthesis can take place, thus guaranteeing the normal survival needs of plants
[35]. This is one of the main approaches for plants to avoid damage caused by drought stress in the short term
[36]. Influenced by geography, many terrestrial plants are frequently affected by drought stress and have developed various drought-tolerant mechanisms to adapt to or to resist the drought environment through a long-term evolutionary process
[37][38]. The adaptation of plants to the drought environment is mainly reflected both morphologically and biochemically
[39][40][41]. Morphologically, adaptation is manifest in the presence of a very thick cuticle on the leaf surface, with the fenestrated cells being tightly arranged, while some leaves have a tomentum on the surface, which can effectively control water loss, and can also absorb dew at night to replenish the plant’s own water
[42]. Generally, there is a very well-developed root structure with greater water and nutrient absorption capacity and a poorly developed aboveground branching structure with weaker transpiration and better water retention capacity
[43]. The more drought-resistant the plant, the greater the root–crown ratio. These morphological adaptations are closely related to the cell division, elongation, and differentiation of the root apex. Plant vascular tissue systems, such as the xylem and phloem, are involved in the transport of substances, while their developmental status also affects plant drought resistance
[44]. In
Arabidopsis, drought-escape-induced early flowering is associated with the phloem tissue transport of the florigen FT protein from the leaves to the shoot apical meristems
[45]. Biochemically, it is manifested in the high expression of some drought resistance genes that positively increase the content of amino acids and sugars in plants (such as proline and trehalose), the enhanced activity of antioxidant-related enzymes, and inhibition of the activity of enzymes involved in degradation pathways to ensure that normal metabolic homeostasis is maintained under drought stress condition
[46]. ROS, including superoxide radicals (O
2−), hydrogen peroxide (H
2O
2), and hydroxyl radicals (OH
−), regulate plant growth and development at lower concentrations
[47][48]. Excessive accumulation of ROS under drought stress leads to membrane lipid peroxidation
[49][50]. Previous studies have shown that excessive ROS in plants will be scavenged by antioxidant mechanisms, including the enzymatic antioxidants, SOD (superoxide dismutase), CAT (catalase), POD (peroxidase), and the non-enzymatic antioxidants, ascorbic acid, proline, flavonoids, and polyphenols, which ultimately improve the plant’s drought resistance
[51][52][53][54]. Ascorbic acid has also been reported to play a role in controlling the flowering time in plants
[55]. Understanding the perception of drought stress and the coping strategies (early or late flowering) used by plants in response to drought provides a physiological basis for subsequent studies on the molecular mechanisms of drought-stress-induced flowering.
3. Flowering Time of Various Plant Species in Response to Drought Stress
Most plants have evolved and adapted to the frequent fluctuations in the natural environment, especially the severe damage caused by droughts due to water deficit. Drought stress can lead to alterations in the flowering time of various plants, effects on flower development (including reduced flower number, restricted filament elongation, and delayed anther development), immature seed development, and reduced yields
[23][56][57]. Therefore, the flowering time is an important agricultural characteristic for the development of adaptation to drought stress in a wide range of plants (
Table 2). The discrepancy between early and late flowering resulting from the effects of drought stress depends on the plant species
[20][58]. For example, forage and biofuel crops generally have delayed flowering as a desirable target for them due to the importance of the plant vegetative biomass. Cereal crops, by contrast, usually exhibit early flowering as an ideal trait to shorten the harvest time, and, thus, increase the number of plantings during the growing season, while growing as fast as possible to minimize damage from drought stress caused by environmental fluctuations
[56][59]. Drought stress caused by water deficit delays the flowering time of
Arabidopsis under short-day (SD) (9,
Table 1) conditions but accelerates flowering time under long-day (LD) (10,
Table 1) conditions
[11][29]. Drought stress causes premature bolting of Chinese cabbage in the growing season, which leads to insufficient vegetative growth and influences yield and quality
[60]. By studying the effect of drought stress on flowering time in
Brassica rapa offspring, it was found that materials with seeds collected after drought flowered earlier than those collected before drought, suggesting that
Brassica rapa responds to drought stress and evolves towards earlier flowering
[61]. Studies of drought-induced flowering in the chickpea suggest that drought stress generally accelerates the flowering time of temperate grain legumes
[62][63]. The role of drought in influencing the flowering time varies among plant species and with environmental conditions, so that drought regulation of flowering is the result of multiple factors.
Recently, there have been studies on drought-induced flowering in several other plant species
[12][64][65][66][67]. In contrast to early flowering induced by drought stress, the flowering time of rice is delayed under water deficit conditions to avoid reproductive growth in unfavorable environmental conditions, but water shortage still results in the retardation of plant growth and spikelet development, which leads to reduced crop yield and ultimately economic losses
[68][69][70]. Water deficiency can also delay the first flowering of
Medicago polymorpha. The effect of drought stress on early and late flowering in plants is closely related to the intensity and duration of the water deficit, in addition to varying by plant species. The artificial control of the duration and intensity of drought treatment in agricultural production plays an important role in accelerating plant development, especially regarding flowering. It was found that the response of rice to drought stress was dependent on the intensity of drought, and mild water deficit in the early development stage triggered a drought escape response with accelerated flowering and reduced tillering
[71][72]. In a study of wheat response to drought stress, it was also found that the flowering time showed a nonlinear relationship with the plant water content status, with mild water deficit shortening the flowering time, while severe drought stress delayed flowering
[73].
Drought-induced flowering is a phenomenon more common in annual plants. The drought stress regulation of plant flowering has been less well studied in woody plants and remains poorly understood, mainly due to the long duration of vegetative growth in perennials and the excessive long period of research. Drought stress is one of the major environmental factors inducing flowering in adult citrus in subtropical regions. Different citrus species are induced to flower by different environmental conditions, with lemon, four-season orange, and kumquat being mainly affected by drought stress, while sweet orange, trifoliate orange, mandarin orange, grapefruit, and tangerine are mainly affected by seasonal low temperature
[22][74]. Notably, drought-induced flowering in citrus was also accompanied by the upregulation of the
CiFT expression level
[74][75]. Earlier studies have also shown that
Citrus latifolia flowering is also induced by drought stress, and that all citrus plant species share this same flowering mechanism
[76][77]. Additionally, the perennial woody plant
Sapium sebiferum takes 3–5 years to flower normally, but one-year-old seedlings under drought stress flower early, which provides a feasible way to shorten the vegetative growth years of woody plants in genetic research and breeding efforts
[78]. These indicate that the effect of drought stress on the flowering time is not specific to a particular plant species but is conserved in annuals as well as perennials. There are few studies on the regulation of flowering time by drought stress in perennials due to the long study time and the lack of phenotypes, but the available evidence supports the feasibility of researching this topic in perennials. Moreover, studies on drought-induced flowering in perennial plants can be carried out on the basis of sufficient theoretical evidence in annual plants.
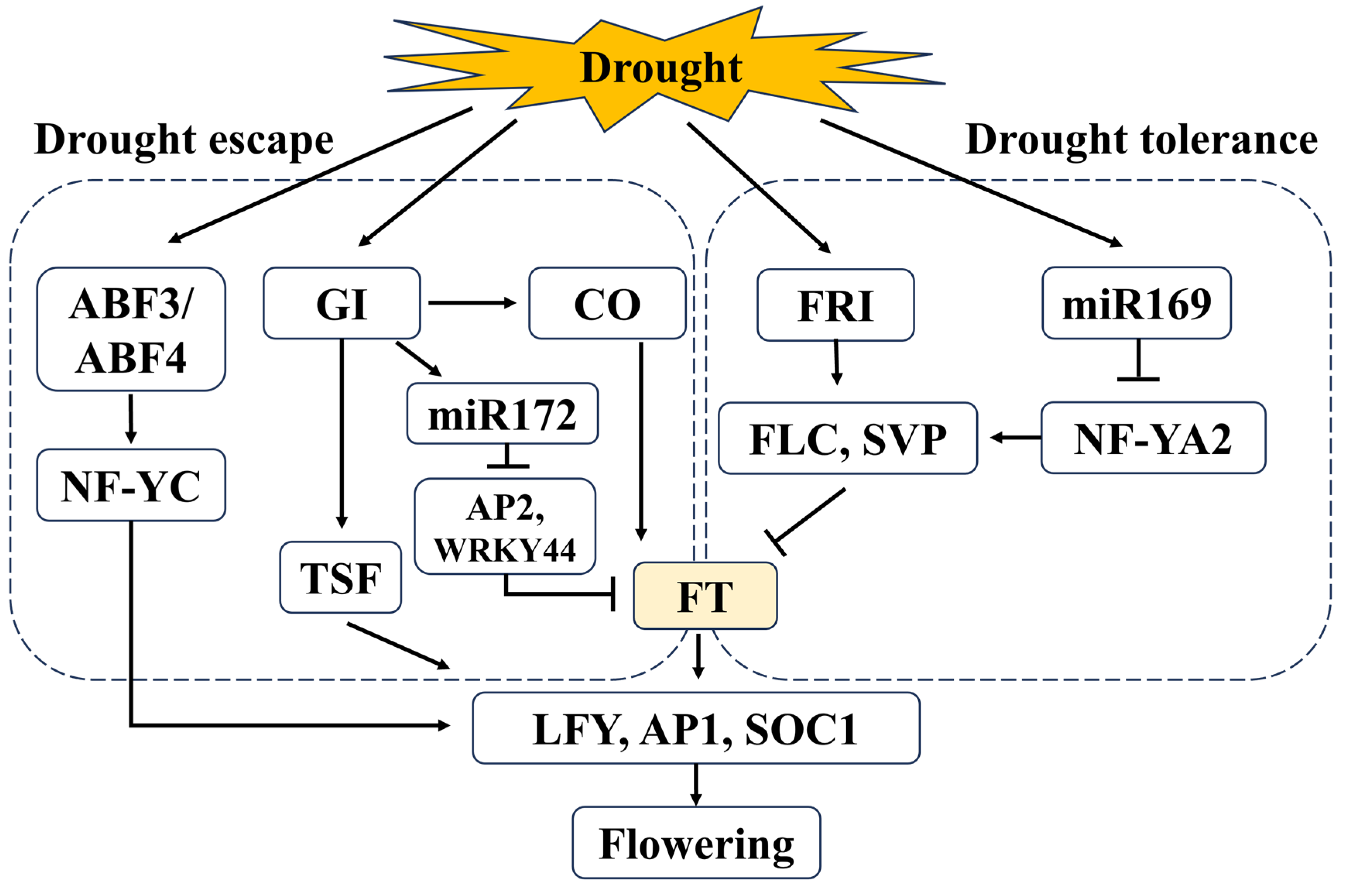
4. Molecular Regulatory Mechanisms of Flowering Involved in Drought Stress
Drought-stress-induced flowering, as well as the traditional flower formation pathway, accomplish the same flowering purpose, but the traditional pathway is the primary option for flowering under normal environmental conditions, whereas drought-induced flowering is an emergency response under stressful conditions
[20][79]. Compared with the in-depth studies of the flower formation regulatory pathways in plants, the molecular regulatory mechanisms of flowering involved in drought stress are still obscure. Drought stress triggers the differential expression of a variety of genes, including flowering time regulation genes and transcription factors associated with the stress response. Among them, the key genes of flowering regulation in response to drought stress tolerance are
FT,
CO,
LFY,
GI,
SOC1, and
TWIN SISTER OF FT (TSF) (11,
Table 1)
[11][23][29][80] (
Figure 1).
Figure 1. Simplified regulatory pathways linking drought stress and flowering in
Arabidopsis thaliana. Drought escape:
ABF3/
ABF4 further activates the expression of
LFY,
AP1, and
SOC1 by targeting
NF-YC [81].
GI accelerates flowering under drought conditions by positively regulating the expression of
CO and
miR172 [80], which, in turn, activate the expression of
FT, or directly activate the transcription of
TSF, which ultimately upregulates the expression levels of
LFY,
AP1, and
SOC1 [11][29][82]. Drought tolerance:
miR169 targets
NF-YA2 to reduce its transcriptional abundance
[83], which attenuates the repressive effect on downstream genes
FLC and
SVP [84], while
FRI positively regulates the expression of
FLC and
SVP, resulting in the repression of
FT transcription and delayed flowering under drought conditions
[85]. Solid lines indicate identified associations, arrows indicate positive regulation, and horizontal bars indicate negative regulation.
The molecular mechanisms by which drought stress regulates flowering time in
Arabidopsis have been partially elucidated. Emerging evidence suggests that
GI, a photoperiodic pathway gene that promotes flowering, is a pivotal regulator of the abiotic stress response and can influence plant tolerance to abiotic stresses, especially drought
[11][86]. Under long-day environmental conditions, water deprivation achieves drought-induced early flowering in
Arabidopsis through ABA-dependent control of
GI signaling that activates expression of the florigen genes
FT and
TSF. Under short-day conditions, the drought and plant stress hormone ABA is considered to inhibit the transcription of
FT and
TSF via activating repressors of floral formation, which, in turn, leads to late flowering in Arabidopsis
[11][20][29][82][87]. It has been confirmed that the
GI-miR172 pathway is involved in drought-induced early flowering by downregulating
WRKY44 (directly repressed by miR172)
[80] (
Figure 1). Several other flowering inhibition genes are also induced by drought stress. For example, water deficit induces the flowering repressor gene
SHORT VEGETATIVE PHASE (SVP) (12,
Table 1), which represses the transcription of genes related to ABA catabolism, and increases ABA accumulation, which improves drought tolerance in
Arabidopsis, but flowering is delayed
[84]. Similarly,
FLOWERING LOCUS C (FLC) (13,
Table 1), a flowering suppressor gene, also plays a role in the drought stress pathway, and the loss of
FLC function leads to early flowering and decreased drought tolerance in
Arabidopsis [88]. In rice (
Oryza sativa), activation of the florigen genes
HEADING DATE 3a (Hd3a) (14,
Table 1), flowering integration factor
OsMADS50 (an orthologue gene of
SOC1 in
Arabidopsis), and
RICE FLOWERING LOCUS T1 (
RFT1) (15,
Table 1) (
AtFT-like gene) coordinates the modulation of the drought escape response
[71]. Meanwhile, the CCT domain protein Ghd7 plays an important role in delaying the rice heading date and regulating drought stress tolerance under long-day conditions
[89][90]. The transcription levels of
Hd3a,
RFT1,and
EARLY HEADING DATE 1 (
Ehd1, upstream of the florigen genes) (16,
Table 1) are drastically decreased under drought environmental conditions, which eventually leads to delayed floral transition
[69].
RICE CENTRORADIALIS 1 (
RCN1, an orthologue of
TFL in
Arabidopsis) (17,
Table 1) is reported in rice as a flowering time regulation gene in the pathway of drought-regulated floral transition that interacts with the 14-3-3 protein and OsFD1 to repress Hd3a protein function but not its transcriptional level, causing delayed flowering in rice under drought stress
[68][91] (
Table 2). These results suggest that when plants are subjected to drought stress, a large number of genes are induced to be expressed, including genes critical to the flowering pathway, and that differences in the expression of these genes between species ultimately lead to different flowering outcomes.
Table 2. Some examples of stress-induced flowering associated with flowering pathway genes.
| Abiotic Stress Factors |
Species |
Flowering Response |
Related Flowering Pathway Genes |
References |
| Drought (LD) |
Arabidopsis |
early flowering |
FT, GI, SOC1, TSF |
[11] |
| Drought (SD) |
Arabidopsis |
delayed flowering |
FT, TSF |
[29] |
| Drought |
Rice |
early flowering |
Hd3a (AtFT), OsMADS50 (AtSOC1), RFT1, Ehd1, OsTIR1, OsABF2, OsmiR393 |
[69][71][92] |
| delayed flowering |
RCN1 (AtFT) |
[68] |
| Maize |
early flowering |
ZmNF-YA3 |
[93] |
| Barley |
early flowering |
miR172, AP2-like |
[94] |
| Citrus |
induction |
CiNF-YA1 |
[77] |
| CiFD |
[13] |
| Brachypodium |
delayed flowering |
BdRFS |
[17] |
| Solanum lycopersicum |
early flowering |
SlOST1, SlVOZ1 |
[18] |
| Arabidopsis |
delayed flowering |
OXS3, AP1 |
[32] |
| OXS2, SOC1 |
[66] |
| early flowering |
ABF3/4, NF-YC, SOC1 |
[81] |
| miR169d, AtNF-YA2 |
[83] |
| Sapium sebiferum |
induction |
GA1, AP2, CYR2 |
[78] |
Low
temperature |
Arabidopsis |
delayed flowering |
FCA, FVE, SVP, FLM |
[95][96] |
| MAF2 |
[97][98] |
| HOS1, CO, FLC |
[99][100][101] |
| HOS15, GI |
[102] |
| early flowering |
miR169d, AtNF-YA2 |
[83] |
| Phaibitis |
induction |
PnFT1, PnFT2 |
[64][103] |
| Chrysanthemum |
induction |
MAF2 (AtFLC) |
[98] |
| Poplar |
induction |
FT1 |
[104] |
| Citrus |
induction |
CiNF-YA1, CiFT |
[77] |
| CiFD |
[13] |
| CiFT, CsFT |
[22][105] |
| Medicago sativa |
delayed flowering |
MsFRI-L |
[106] |
| Barley/Wheat |
induction |
VRN1, VIN2, VRN3 |
[107][108][109] |
| Cymbidium goeringii |
induction |
CgSVP |
[110] |
| Heat stress |
Arabidopsis |
early flowering |
FT |
[111] |
| PIF4, PIF5 |
[112][113] |
| Soybean |
induction |
GmFT2a, GmFT5a |
[114] |
| Barley |
delayed flowering |
FLC gene family |
[115] |
| Rice |
early flowering |
EG1, OsGI |
[116] |
| Maize |
early flowering |
ZmNF-YA3, ZmFTL12 |
[93] |
| Chrysanthemum |
delayed flowering |
FTL3 (AtFT) |
[117] |
| Brassica rapa |
delayed flowering |
H2A.Z, FT |
[118] |
A nuclear factor-Y (NF-Y) transcription factor,
ZmNF-YA3, has the dual function of promoting maize flowering while increasing plant drought tolerance, but there is a lack of evidence on the specific mechanism of
ZmNF-YA3 in drought-affected maize flower formation
[93]. Also,
Arabidopsis ABF3 and
ABF4 act with
NF-YCs to mediate drought-accelerated flowering by regulating
SOC1 [81] (
Figure 1). In citrus,
CiNF-YA1 was also found to promote drought-induced flowering by forming a complex with
CiNF-YB2 and
CiNF-YC2 to activate
CiFT expression, and overexpression of
CiNF-YA1 in citrus increased plants drought-sensitivity
[77]. It is evident that
NF-YAs are likely to be functionally conserved in regulating flowering in annual and perennial plants, with functional diversity resulting from physiological differences in response to stress. These studies support a critical role for NF-YAs in promoting not only the flowering time but also drought response (tolerance/sensitivity). However, future studies are needed to clarify whether NF-YAs are directly involved in regulating drought-affected flowering. The bZIP transcription factor
FLOWERING LOCUS D (FD) (18,
Table 1), together with FT and the 14-3-3 proteins, is the florigen activation complex (FAC) (19,
Table 1) that regulates plant flowering
[119].
CiFD was found to form two distinct proteins through alternative splicing,
CiFDα and
CiFDβ, that both initiate flowering in citrus. Among them,
CiFDα was induced by low temperature while
CiFDβ was induced by drought stress. The regulatory mechanism of
CiFDβ promoting drought-induced flowering is independent of FAC and interacts directly with
AP1 [13] (
Table 2).
FRIGIDA (FRI) (20,
Table 1) is an essential regulator of flowering in various plant species, including
Populus balsamifera [120],
Medicago sativa [106],
Brassica napus [121], and
Vitis vinifera [122]. Importantly,
FRI modulates drought tolerance through the
FLC–OST1 regulatory module
[88] (
Figure 1).
CiFRI, a homologue of
FRI in citrus, was drought-induced, and overexpression of
CiFRI enhanced drought tolerance in
Arabidopsis and citrus, whereas silenced plants showed drought sensitivity, and the ectopic expression in
Arabidopsis exhibited late flowering. The citrus dehydrogenase gene
CiDHN may maintain the stability of the CiFRI protein during drought-induced degradation
[14]. Therefore, the drought-induced flowering regulation genes are conserved between annual and perennial plants. Together, these studies strongly support the pivotal roles of flowering-time-regulated genes in drought stress response and tolerance. The main challenge in woody plants is that the regulatory role of genes in drought-induced flowering can be demonstrated, but there is no phenotypic evidence of flowering, which is related to its own longer developmental process.
In addition, drought-induced transcription factors are closely related to the existing flowering regulatory pathways, and these TFs affect the flowering process of plants by regulating the transcription level of flowering-regulated genes
[12][17][123]. The tomato
OPEN STOMATA 1 (SlOST1) (21,
Table 1) loss-of-function mutant causes reduced drought tolerance in plants, and the
slost1 mutant exhibits a late-flowering phenotype under both normal and drought environmental conditions.
SlOST1 combines with the flowering integrated gene
VASCULAR PLANT ONE-ZINC FINGER 1 (SlVOZ1) (22,
Table 1) to form a regulatory module, and then interacts with the promoter of
SINGLE FLOWER TRUSS to regulate tomato flowering under drought stress
[18]. A conserved and specific gene family in plants, the
Regulator of Flowering and Stress (RFS) family (23,
Table 1), produces dramatic alterations in transcriptional levels in response to drought environmental stimuli. Overexpression of
BdRFS in
Brachypodium distachyon not only substantially delayed flowering but also promoted drought tolerance. The
rfs mutants in
Arabidopsis and
Brachypodium distachyon displayed an early flowering phenotype and were susceptible to water deprivation
[17].
Studies have reported that epigenetic mechanisms, including histone acetylation as well as methylation, are involved in the plant stress response and flowering time regulation. Histone deacetylase
HISTONE DEACETYLASE 6 (HDA6)-deficient mutant plants (24,
Table 1) exhibited a phenotype of reduced drought stress tolerance and delayed flowering with the repression of
FLC expression
[124][125][126]. The histone H4 gene
BrHIS4.A04, which interacts with
BrVIN3.1, is overexpressed in Chinese cabbage and reduces plant susceptibility to drought stress and accelerates flowering under normal growth conditions, whereas under water deficit environmental conditions, the histone H4 gene represses the expression of photoperiodic flowering genes to prevent premature bolting
[60]. The regulation of flowering time under drought stress is also related to microRNAs (miRNAs). miRNAs are considered to be important suppressors of gene expression at the transcriptional and post-transcriptional levels. The involvement of miRNAs in regulating drought-stress-induced plant flowering responses has been found in many species, such as the annual plants
Arabidopsis [127], rice
[128], wheat
[129], and maize
[130], as well as in perennial plant species
[131]. miR172 acts in the process of drought tolerance and flowering time regulation. miR172b-3p and miR172b-5p, derived from a common precursor, promoted flowering and enhanced drought tolerance in barley (
Table 2). miR172b-3p expression was upregulated under drought stress treatment, which suppressed the four
AP2-like transcription factors in barley to accelerate flowering. The expression of miR172b-5p was inhibited under drought conditions; thus, trehalose-6-phosphate synthase (TPS), a key enzyme for trehalose biosynthesis targeted by miR172b-5p, was significantly accumulated to enhance drought tolerance in barley
[94]. miR156, which is in the same age pathway regulating plant flowering as miR172, is also induced by drought stress and delays flowering of
Arabidopsis and tobacco
[127][132]. miR169 family members play an important role in stress-induced flowering by inhibiting
NF-YA2, which, in turn, decreases
FLC expression, allowing the promotion of flowering
[83] (
Figure 1). In OsmiR393-overexpressing rice plants, miR393 responds to drought stress by targeting and, thus, repressing the expression of the auxin receptor genes
OsTIR1 and
OsAFB2 for early flowering
[92]. In addition, differential expression of key proteins and post-translational modifications, such as SUMOylation, act in regulating plants’ flowering processes under drought stress conditions
[133]. Taken together, when plants are subjected to drought, a variety of molecular regulatory mechanisms can be activated, suppressed, and integrated to maintain survival through the adjustment of flowering time.