Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xin Yang | -- | 2817 | 2023-12-23 02:05:54 | | | |
| 2 | Fanny Huang | Meta information modification | 2817 | 2023-12-29 09:32:44 | | | | |
| 3 | Fanny Huang | Meta information modification | 2817 | 2024-01-02 09:44:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, H.; Li, M.; Cheng, C.; Han, Y.; Fu, S.; Li, R.; Cao, G.; Liu, M.; Cui, C.; Liu, J.; et al. Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/53086 (accessed on 07 March 2026).
Zhu H, Li M, Cheng C, Han Y, Fu S, Li R, et al. Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/53086. Accessed March 07, 2026.
Zhu, Hongwei, Minjie Li, Cuilin Cheng, Ying Han, Shiyao Fu, Ruiling Li, Gaofeng Cao, Miaomiao Liu, Can Cui, Jia Liu, et al. "Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors" Encyclopedia, https://encyclopedia.pub/entry/53086 (accessed March 07, 2026).
Zhu, H., Li, M., Cheng, C., Han, Y., Fu, S., Li, R., Cao, G., Liu, M., Cui, C., Liu, J., & Yang, X. (2023, December 23). Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors. In Encyclopedia. https://encyclopedia.pub/entry/53086
Zhu, Hongwei, et al. "Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors." Encyclopedia. Web. 23 December, 2023.
Copy Citation
The international community has been paying close attention to the issue of food safety as a matter of public health. The presence of a wide range of contaminants in food poses a significant threat to human health, making it vital to develop detection methods for monitoring these chemical contaminants. Electrochemical sensors using emerging materials have been widely employed to detect food-derived contaminants. Covalent organic frameworks (COFs) have the potential for extensive applications due to their unique structure, high surface area, and tunable pore sizes.
covalent organic frameworks
electrochemical sensors
food safety
1. Introduction
The safety of food is of vital importance to the health of people and to the long-term stability of society in general [1]. Food forms the basis of human survival and is essential for maintaining a stable and sustained existence [2]. Food safety is defined by the Food Safety Law of the People’s Republic of China as non-toxic, harmless, and meeting the nutritional requirements without causing acute, subacute, or chronic harm to humans. It is noteworthy that the European Union, the United States, and other countries have very similar definitions of food safety, even if they express it in a slightly different manner.
Globally, the top 100 food and beverage companies generated revenues of USD 1.3 trillion in 2019, equivalent to approximately CNY 9.2 trillion [3]. However, with the achievement of economic globalization, food safety issues have become a worldwide issue that has impacted more than just an individual country or region. Currently, food safety is subjected to many challenges due to differences in the natural environment in different countries and regions. It is possible for food to become unavoidably contaminated during its preparation, transportation, and storage, regardless of how rigorous and meticulous the handling procedures are. As a result of the excessive use of veterinary drugs and pesticides [4] and heavy metal ions [5] and the introduction of illegal additives [6], in particular, food can be contaminated in a variety of ways throughout the food chain [7]. In addition to these factors, hazardous food contaminants deserve special attention because even in low concentrations, they are able to cause serious diseases such as cancer [8], and furthermore, fungi that contaminate food such as aspergillus, penicillium, and neotyphodium [9] pose a serious threat to human health and safety. The food safety industry has experienced some extremely detrimental incidents in recent years, including the melamine incident at Sanlu Group in China in 2008, the salmonella-contaminated peanut butter incident at Peanut Corporation of America from 2008 to 2009, the E. coli contamination of bean sprouts in the European Union in 2011, and the contamination of milk powder in 2013 with Clostridium botulinum toxin by Fonterra in New Zealand. Due to these recent food safety incidents, the global society has been paying close attention to this issue, and many countries and regions have adjusted their policies and intensified their supervision on food regulation [10]. In addition to posing significant risks to the health and safety of the general public, these frequent food safety incidents also cause significant losses for the industries that are directly affected by the incidents.
Having a rational and effective approach for food testing is an essential component of food safety management. Conducting well-informed research on testing techniques can provide powerful assurances regarding food safety being maintained continuously.
1.1. Electrochemical Sensors and Their Role in Food Safety Analysis
The foundation of any food safety program is improved food safety testing techniques, which are key in addressing food safety. Food safety testing methods can be classified as traditional or rapid detection. Usually, conventional methods consist of using techniques such as gas chromatography–mass spectrometry [11], high-performance liquid chromatography [12], and liquid chromatography–mass spectrometry [13][14] to determine the identity of food. These methods are usually performed in laboratories with sophisticated equipment. They are frequently used as reference standards to ensure food safety because of their high sensitivity, accuracy, precision, and repeatability. However, the length of their analysis cycle and their low throughput are their limitations. Rapid detection methods, on the other hand, deliver faster results. It is common to use these methods of qualitative or semi-quantitative screening of target analytes [15]. The benefits of electrochemical detection methods are many, including affordability, simplicity, ease of operation, miniaturization, and diversification, over traditional methods such as spectroscopy and chromatography [16]. In addition, electrochemical detection is suitable for automated control as well as online sensitive and rapid analysis since it can be conducted remotely [17]. They can be applied to biomedical sciences, pharmaceuticals, environmental sciences, and food sciences, and are considered to be one of the most dynamic and promising analytical techniques [18].
The primary objective of electrochemical detection techniques is to qualitatively or quantitatively analyze and measure target substances based on their electrical and electrochemical properties through the use of electrochemical sensors [19]. There are two main components of electrochemical sensors: a molecular recognition system and a system for converting information into electrical signals (the principle of electrochemical sensors is illustrated in Figure 1). Based on the measured chemical parameters, response signals are generated in the form of voltages, currents, or changes in light intensity. These signals are then amplified, converted, and finally transformed into analyzable signals that indicate the amount of target analyte present in the sample using electronic systems [20]. It is widely known that sensors with electrochemical integration are widely used in a variety of fields, including industry, transportation, environmental monitoring, and medical surveillance. Sensors based on electrochemical reactions play an important role in combining sensing technology and electrochemical analysis technology. Electrochemical sensors have been widely applied and developed since the 1960s, with electrodes serving as the basic component [21]. The electrodes play an integral role in the overall performance of electrochemical sensors due to their functionality and interfacial performance. However, one of the main challenges is making electrodes more responsive and selective to desired reactions. Nanotechnology has made rapid progress since the 1980s, resulting in many nanomaterials with exceptional performance and unique structures, and these materials have excellent biocompatibility, a high surface chemical activity, a large specific surface area, and a high electron transfer efficiency, thus facilitating the use of nanomaterials (for instance, COF [22][23][24], MOF [25][26], MIP [27][28][29], among others [30][31]) in electrochemical sensing). New types of electrochemical sensors have been developed as a result of the convergence of nanotechnology and sensing technology, which has attracted increasing attention. In addition to providing the rapid identification of basic food components, electrochemical sensors are capable of detecting harmful substances such as heavy metal ions [32], foodborne pathogens [33], pesticide residues [34], and food additives [35].
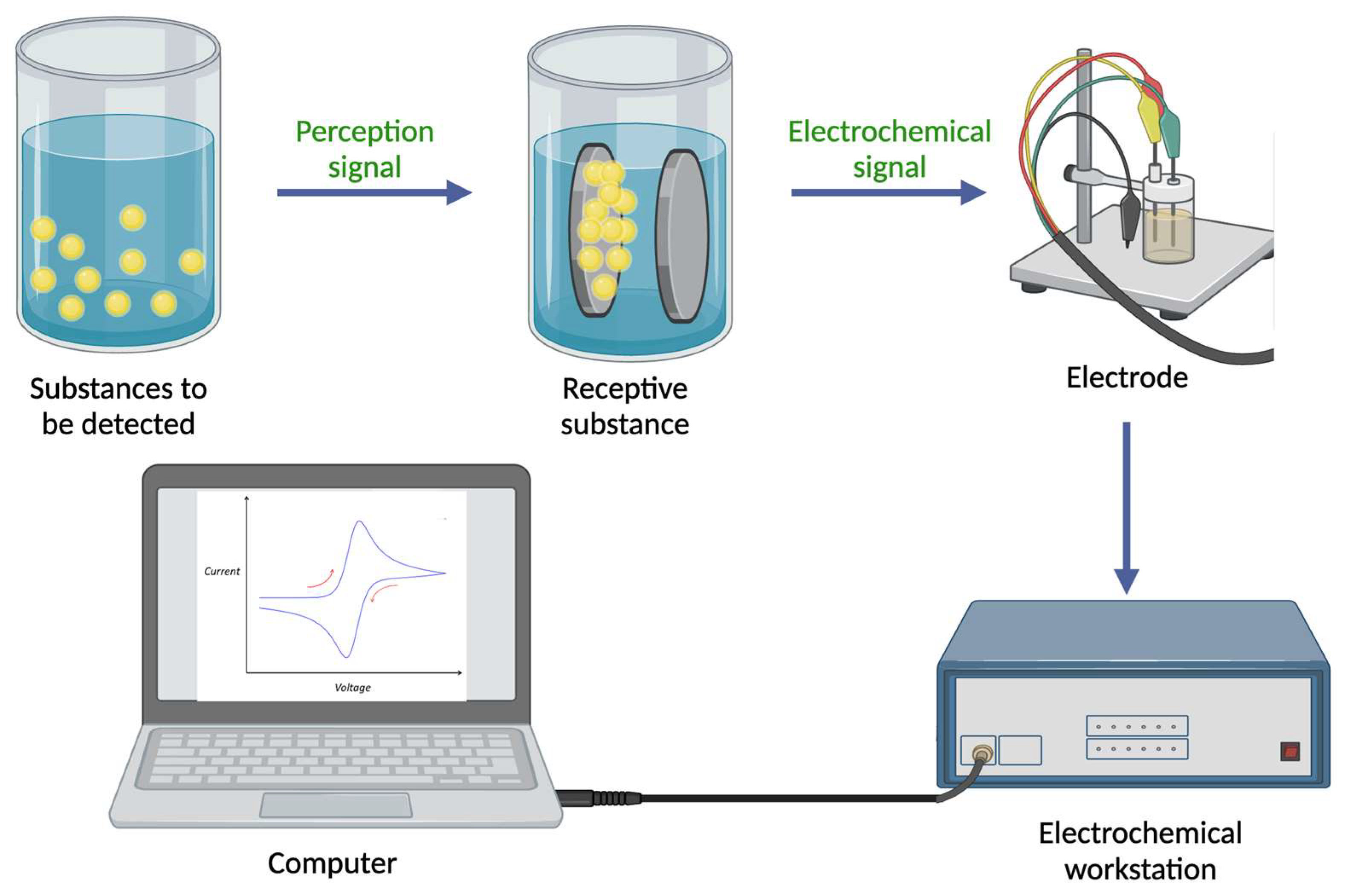
Figure 1. Diagram of an electrochemical sensor. (The mechanism map was created with BioRender.com.)
1.2. Covalent Organic Frameworks (COFs) and Their Potential Applications in Sensor Technology
As a general rule, nanomaterials are materials with at least one dimension out of the three dimensions within the nanometer size range (1–100 nm), or they are formed from basic constituents with such dimensions. Nanomaterials have unique physicochemical properties in optics, electronics, magnetism, heat, mechanics, and other fields as a result of their unique surface effects, small size effects, quantum effects, and macroscopic quantum tunneling effects [36]. The use of these technologies is widespread in fields such as food technology, electronics manufacturing, chemical engineering, and many others [37][38][39]. Moreover, nanomaterials have a small size effect, leading to a large specific surface area and a high surface energy, and they have abundant surface-active sites and exhibit an ease of functionalization. This contributes to a high catalytic efficiency as well as an excellent biocompatibility, greatly enhancing their potential of being electrochemically research [40][41]. Furthermore, nanocomposites are composed of materials in which nanoparticles are uniformly dispersed within the matrix material. It is important to note that unlike traditional single-phase nanomaterials, nanocomposites can consist of a combination of metal nanoparticles with resins or gels, polymer materials, porous inorganic materials, porous organic materials, and various types of metal nanoparticles. There have been several developments in nanomaterials so far, including carbon materials (graphene, carbon nanotubes, carbon foam, carbon fibers, carbon spheres, porous carbon materials, etc.), metal–organic frameworks (MOFs), zeolitic imidazolate frameworks (ZIFs), covalent organic frameworks (COFs), among others [42][43][44][45].
The covalent organic frameworks (COFs) represent a new class of organic porous materials [46]. In 2005, Yaghi and colleagues were successful in synthesizing two-dimensional COFs, COF-1 and COF-5, via the condensation reaction between phenylboronic acid and 2,3,6,7,10,11-hexahydroxytriphenylene. These COFs with high surface areas (711 and 1590 m2 g−1, respectively), high thermal stability, and permanent porosity were compared [47]. Following this, Yaghi proposed three-dimensional COFs in 2007, including COF-102, COF-105, and COF-108 [48]. There has been a great deal of interest in COFs since their introduction, and they have been applied in a variety of fields. As a result of their flexible polygonal frameworks that are easy to design and control, COFs have been widely applied for a variety of purposes, such as catalysis, energy storage, water treatment, drug delivery, among others [49]. Compared to conventional electronic components, COF and overoxidized PEDOT or PEDOT/PSS have better electrical signal transduction. Controlling the electrical properties of overoxidized PEDOT and PEDOT/PSS is the primary method of actuating the device. A change in the electrical conductivity of PEDOT/PSS can be achieved by applying an electric field or conducting an electrochemical reaction, thus allowing the material to be controlled in terms of its properties and functions [50]. In contrast, the COF undergoes physical or chemical changes through the adsorption or desorption of internal molecules, which cause changes in the signaling pathway. It is estimated that over 10,000 papers (from WOS) have been published over the past five years due to the wide potential applications of this unique material across many fields.
2. Fundamentals of Covalent Organic Frameworks Based Electrochemical Sensors
2.1. COFs with Different Chemical Structure Types
It is well known that covalent organic frameworks (COFs) are porous organic materials that are constructed by self-assembling materials linked together by covalent bonds [51][52]. Therefore, they possess unmatched biocompatibility and chemical stability, as well as high surface areas, high porosities, and ease of functionalization, similar to metal–organic frameworks (MOFs) and zeolitic imidazolate frameworks (ZIFs). The highly ordered π-π conjugated system in COFs and their independently accessible regular pores provide high levels of electronic conductivity. It is for this reason that these materials are often used as excellent photocatalysts, for gas adsorption and separation, electrochemical sensing, and energy storage applications [53][54]. The following subsections provide an overview of the different chemical structures of COFs.
2.1.1. The B-O Structure of COFs
In 2005, Yaghi et al. synthesized COF-1 and COF-5, typical examples of the B-O structure [47]. One method for the synthesis of COF-1 is based on the self-condensation of phenylboronic acid, a process in which the boronic acid molecules in phenylboronic acid undergo dehydration in order to form a two-dimensional B3O3 ring (boroxine ring). The boronic acid molecules in 1,4-phenyldiboronic acid have the same capability of undergoing condensation during dehydration to form a layered hexagonal framework (COF-1). It is possible to obtain an extendable layered structure (COF-5) by dehydrating and condensing 1,4-phenyldiboronic acid with 2,3,6,7,10,11-hexahydroxytriphenylene. However, it should be noted that these types of COFs have a poor water stability due to the reversible reaction of boronic acid ester formation, which causes their hydrolysis when exposed to acid, alkali, or atmospheric water vapor, thus impairing the quality of their framework [55].
2.1.2. The Imine Structure of COFs
COFs are typically connected by imine bonds when amines and aldehydes undergo the Schiff base reaction, which produce C=N bonds [56]. Yaghi et al., in 2009, reported the first example of this type of COF. During the experimental process, COF-300 was found to be structurally stable at 490 °C and insoluble in both water and common organic solvents [57]. The application prospects of COFs connected by imine bonds are greater than those of COFs linked by B-O bonds. In addition, COFs with oxime bonds can be considered as another type of imine-based COFs, which are formed by reacting hydrazine compounds with aldehydes or ketones, such as COF-42 and COF-43 [58].
The Banerjee group introduced functional groups -OH into their structures in order to improve their stability and crystallinity. As a result of the reaction between 2,5-dimethoxybenzaldehyde (Dma) and 2,5-dihydroxybenzaldehyde (Dha) with 5,10,15,20-tetra(4-aminophenyl)-21H,23H-porphine (Tph), the COFs of interest were synthesized. The results indicated that, compared to DmaTph, the O-H···N=C interaction in the DhaTph structure partially protected the COFs from hydrolysis under aqueous and acidic conditions, thereby improving their crystallinity and porosity [59].
2.1.3. The C=C Structure of COFs
During the Knoevenagel condensation reaction, the active methylene group in a compound is dehydrated and condensed with an aldehyde or ketone under the catalysis of a base, leading to the formation of a thermally stable compound. In spite of this, due to the limitations of the reaction conditions, it has been difficult to apply this principle to the synthesis of COFs for a long time. The first time this principle was applied was in 2016, when Zhang and Feng synthesized two-dimensional conjugated COFs (2DPPV) with C=C connectivity [60].
2.1.4. The Triazine Structure of COFs
Covalent triazine-based frameworks (CTFs), which are triazine-based COFs, were first synthesized in 2008 by Kuhn et al. The self-polymerization of dinitrile occurs under the catalysis of zinc chloride at 400 °C, followed by the polymerization into polymers based on the triazine structure [61]. It is important to note that these COFs have excellent thermal stability as well as chemical stability, but because of the high temperatures and strong acid catalysis required for the reaction process, their subsequent applications are limited. There are also many research groups that are dedicated to developing milder preparation methods.
2.1.5. The Other Structure of COFs
Furthermore, there are many other ways of connecting COFs besides those mentioned above, such as C-C [62], aminal [63], imide [64], ester [65], and quinoline [66]. Their chemical stabilities, thermal stabilities, large surface areas, and designable pore sizes make them highly promising in a wide range of applications.
Due to their high functionality, as well as their highly ordered π-π conjugated systems, independent open pores, and high specific surface area, COF materials facilitate rapid electron transfer and energy storage. Moreover, COFs possess electrodes with high specific surface areas and a dense exposure of catalytic active sites, and the interconnected pores facilitate diffusion and contact between the analytes and the active sites. Therefore, it has been found that electrodes constructed using COFs directly or with electrochemically active molecules are ideal electrodes for electrochemical sensing analysis [22][67]. It has been reported that various types of electrochemically active COFs have been developed as a result of COFs’ ability to be easily controlled by functional groups. In Wang’s research team, an electrochemically active two-dimensional COFThi-TFPB was synthesized by introducing sulfur as an electroactive monomer, which was then grown on carbon nanotube surfaces functionalized with amines. It was applied to the construction of ascorbic acid (AA) and pH sensors [68]. In Lu’s research laboratory, a topological skeleton COF-LZU1 based on Fe3+ coordination was prepared, as well as Fe3O4/N composites for enzyme-free plasma component detection [69]. According to Wang’s research group, the self-redox-active COFDHTA-TTA was used as an electroactive material in the construction of electrochemical sensors for H2O2, pH, glucose, etc., which demonstrated excellent stability and performance in the detection of these targets [70]. Zhang’s research group utilized COF nanocomposites doped with Au NPs as signal probes for catechin testing [71]. It is important to recognize that the output of electrical signals is a critical component in the design of electrochemical sensors. It follows that the crucial issue in the application of COFs to electrochemical sensing is the development of more versatile electrode materials, the design of electroactive COFs, or the development of COFs that are capable of performing more than one function. Consequently, COF materials may be developed and applied to electrochemical analysis with some potential and feasibility.
2.2. Principles of Electrochemical Detection and COF-Based Electrochemical Sensors
The electrochemical sensor detects and quantifies chemical components in a sample using electrochemical principles. The selection of electrode materials is essential for the construction of the electrochemical sensing interface, and COFs have gained considerable attention as highly promising electrode materials. It is well known that COFs possess a variety of porous structures, low toxicity, and excellent biocompatibility, which make them ideal for the construction of sensing interfaces. The application of COFs to electrochemical sensors is therefore becoming increasingly popular. There have been more than 100 publications in this field (from WOS) over the last five years.
It is also possible to modify COFs with different functional groups or metal ions to develop a number of highly specific and targeted sensors [72]. In addition to their outstanding stability, they are widely used in electrochemical sensors due to their high durability [73]. With electrochemical sensors based on COFs, the real-time monitoring of analytes is possible with minimal sample preparation and rapid analysis. Over the last several years, COFs have attracted an increasing amount of attention owing to their excellent performance and their ability to be used in the development of new electrochemical sensors. There has been a steady increase in the number of articles related to COF-based electrochemical sensors since Wang and colleagues detected Pb2+ using COF-based electrochemical sensors in 2018 [74]. It is primarily in the food industry that electrochemical sensors based on COF are used for the detection of hazards associated with food.
References
- Singh, B.K.; Tiwari, S.; Dubey, N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: A review. J. Sci. Food Agric. 2021, 101, 4879–4890.
- Chen, J.; Yu, X.; Qiu, L.; Deng, M.; Dong, R. Study on Vulnerability and Coordination of Water-Energy-Food System in Northwest China. Sustainability 2018, 10, 3712.
- Ma, T.; Wang, H.; Wei, M.; Lan, T.; Wang, J.; Bao, S.; Ge, Q.; Fang, Y.; Sun, X. Application of smart-phone use in rapid food detection, food traceability systems, and personalized diet guidance, making our diet more health. Food Res. Int. 2022, 152, 110918.
- Zhao, F.; He, J.; Li, X.; Bai, Y.; Ying, Y.; Ping, J. Smart plant-wearable biosensor for in-situ pesticide analysis. Biosens. Bioelectron. 2020, 170, 112636.
- Sarker, A.; Kim, J.E.; Islam, A.; Bilal, M.; Rakib, M.R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy metals contamination and associated health risks in food webs-a review focuses on food safety and environmental sustainability in Bangladesh. Environ. Sci. Pollut. Res. Int. 2022, 29, 3230–3245.
- Su, Z.; Li, T.; Wu, D.; Wu, Y.; Li, G. Recent Progress on Single-Molecule Detection Technologies for Food Safety. J. Agric. Food Chem. 2022, 70, 458–469.
- Filho, W.L.; Setti, A.F.F.; Azeiteiro, U.M.; Lokupitiya, E.; Donkor, F.K.; Etim, N.N.; Matandirotya, N.; Olooto, F.M.; Sharifi, A.; Nagy, G.J.; et al. An overview of the interactions between food production and climate change. Sci. Total Environ. 2022, 838, 156438.
- Khan, M.R.; Alammari, A.M.; Aqel, A.; Azam, M. Trace analysis of environmental endocrine disrupting contaminant bisphenol A in canned, glass and polyethylene terephthalate plastic carbonated beverages of diverse flavors and origin. Food Sci. Technol. 2021, 41, 210–217.
- Xiang, Q.; Huangfu, L.; Dong, S.; Ma, Y.; Li, K.; Niu, L.; Bai, Y. Feasibility of atmospheric cold plasma for the elimination of food hazards: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2023, 63, 4431–4449.
- Snyder, F. No country is an island in regulating food safety: How the WTO monitors Chinese food safety laws through the Trade Policy Review Mechanism (TPRM). J. Integr. Agric. 2015, 14, 2142–2156.
- Walorczyk, S.; Kopeć, I.; Szpyrka, E. Pesticide Residue Determination by Gas Chromatography-Tandem Mass Spectrometry as Applied to Food Safety Assessment on the Example of Some Fruiting Vegetables. Food Anal. Methods 2015, 9, 1155–1172.
- Nicolich, R.S.; Werneck-Barroso, E.; Marques, M.A.S. Food safety evaluation: Detection and confirmation of chloramphenicol in milk by high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2006, 565, 97–102.
- Pico, Y.; Font, G.; Ruiz, M.J.; Fernandez, M. Control of pesticide residues by liquid chromatography-mass spectrometry to ensure food safety. Mass. Spectrom. Rev. 2006, 25, 917–960.
- Hingmire, S.; Oulkar, D.P.; Utture, S.C.; Ahammed Shabeer, T.P.; Banerjee, K. Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem. 2015, 176, 145–151.
- Jara, M.D.L.; Alvarez, L.A.C.; Guimaraes, M.C.C.; Antunes, P.W.P.; de Oliveira, J.P. Lateral flow assay applied to pesticides detection: Recent trends and progress. Environ. Sci. Pollut. Res. Int. 2022, 29, 46487–46508.
- Du, H.; Xie, Y.; Wang, J. Nanomaterial-sensors for herbicides detection using electrochemical techniques and prospect applications. TrAC Trends Anal. Chem. 2021, 135, 116178.
- Xue, R.; Kang, T.-F.; Lu, L.-P.; Cheng, S.-Y. Electrochemical Sensor Based on the Graphene-Nafion Matrix for Sensitive Determination of Organophosphorus Pesticides. Anal. Lett. 2013, 46, 131–141.
- Zheng, Y.; Mao, S.; Zhu, J.; Fu, L.; Moghadam, M. A scientometric study on application of electrochemical sensors for detection of pesticide using graphene-based electrode modifiers. Chemosphere 2022, 307, 136069.
- Singh, V.V. Recent Advances in Electrochemical Sensors for Detecting Weapons of Mass Destruction. A Review. Electroanalysis 2016, 28, 920–935.
- Xue, R.; Liu, Y.S.; Huang, S.L.; Yang, G.Y. Recent Progress of Covalent Organic Frameworks Applied in Electrochemical Sensors. ACS Sens. 2023, 8, 2124–2148.
- Fukatsu, A.; Kondo, M.; Masaoka, S. Electrochemical measurements of molecular compounds in homogeneous solution under photoirradiation. Coord. Chem. Rev. 2018, 374, 416–429.
- Ma, X.; Pang, C.; Li, S.; Xiong, Y.; Li, J.; Luo, J.; Yang, Y. Synthesis of Zr-coordinated amide porphyrin-based two-dimensional covalent organic framework at liquid-liquid interface for electrochemical sensing of tetracycline. Biosens. Bioelectron. 2019, 146, 111734.
- Wang, M.; Hu, M.; Liu, J.; Guo, C.; Peng, D.; Jia, Q.; He, L.; Zhang, Z.; Du, M. Covalent organic framework-based electrochemical aptasensors for the ultrasensitive detection of antibiotics. Biosens. Bioelectron. 2019, 132, 8–16.
- Yan, X.; Song, Y.; Liu, J.; Zhou, N.; Zhang, C.; He, L.; Zhang, Z.; Liu, Z. Two-dimensional porphyrin-based covalent organic framework: A novel platform for sensitive epidermal growth factor receptor and living cancer cell detection. Biosens. Bioelectron. 2019, 126, 734–742.
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230.
- Ling, P.; Lei, J.; Zhang, L.; Ju, H. Porphyrin-encapsulated metal-organic frameworks as mimetic catalysts for electrochemical DNA sensing via allosteric switch of hairpin DNA. Anal. Chem. 2015, 87, 3957–3963.
- Ramajayam, K.; Ganesan, S.; Ramesh, P.; Beena, M.; Kokulnathan, T.; Palaniappan, A. Molecularly Imprinted Polymer-Based Biomimetic Systems for Sensing Environmental Contaminants, Biomarkers, and Bioimaging Applications. Biomimetics 2023, 8, 245.
- Zhou, B.; Sheng, X.; Xie, H.; Zhou, S.; Huang, L.; Zhang, Z.; Zhu, Y.; Zhong, M. Molecularly Imprinted Electrochemistry Sensor Based on AuNPs/RGO Modification for Highly Sensitive and Selective Detection of Nitrofurazone. Food Anal. Methods 2023, 16, 709–720.
- Svalova, T.S.; Saigushkina, A.A.; Verbitskiy, E.V.; Chistyakov, K.A.; Varaksin, M.V.; Rusinov, G.L.; Charushin, V.N.; Kozitsina, A.N. Rapid and sensitive determination of nitrobenzene in solutions and commercial honey samples using a screen-printed electrode modified by 1,3-/1,4-diazines. Food Chem. 2022, 372, 131279.
- Van Dersarl, J.J.; Mercanzini, A.; Renaud, P. Integration of 2D and 3D Thin Film Glassy Carbon Electrode Arrays for Electrochemical Dopamine Sensing in Flexible Neuroelectronic Implants. Adv. Funct. Mater. 2014, 25, 78–84.
- Wu, T.; Alharbi, A.; Kiani, R.; Shahrjerdi, D. Quantitative Principles for Precise Engineering of Sensitivity in Graphene Electrochemical Sensors. Adv. Mater. 2018, 31, 1805752.
- Zhang, M.; Guo, W. Simultaneous electrochemical detection of multiple heavy metal ions in milk based on silica-modified magnetic nanoparticles. Food Chem. 2023, 406, 135034.
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214.
- Jain, U.; Saxena, K.; Hooda, V.; Balayan, S.; Singh, A.P.; Tikadar, M.; Chauhan, N. Emerging vistas on pesticides detection based on electrochemical biosensors—An update. Food Chem. 2022, 371, 131126.
- Yue, X.; Luo, X.; Zhou, Z.; Bai, Y. Selective electrochemical determination of tertiary butylhydroquinone in edible oils based on an in-situ assembly molecularly imprinted polymer sensor. Food Chem. 2019, 289, 84–94.
- Yu, L.; Sun, L.; Zhang, Q.; Zhou, Y.; Zhang, J.; Yang, B.; Xu, B.; Xu, Q. Nanomaterials-Based Ion-Imprinted Electrochemical Sensors for Heavy Metal Ions Detection: A Review. Biosensors 2022, 12, 1096.
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nanomicro Lett. 2020, 12, 45.
- Park, J.; Hwang, J.C.; Kim, G.G.; Park, J.U. Flexible electronics based on one-dimensional and two-dimensional hybrid nanomaterials. InfoMat 2019, 2, 33–56.
- Sebastian, V.; Arruebo, M.; Santamaria, J. Reaction engineering strategies for the production of inorganic nanomaterials. Small 2014, 10, 835–853.
- Liu, T.; Chu, Z.; Jin, W. Electrochemical mercury biosensors based on advanced nanomaterials. J. Mater. Chem. B 2019, 7, 3620–3632.
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage. Adv. Mater. 2017, 29, 1602300.
- Ma, Y.; Li, B.; Yang, S. Ultrathin two-dimensional metallic nanomaterials. Mater. Chem. Front. 2018, 2, 456–467.
- Liu, Y.; Pang, H.; Wang, X.; Yu, S.; Chen, Z.; Zhang, P.; Chen, L.; Song, G.; Saleh Alharbi, N.; Omar Rabah, S.; et al. Zeolitic imidazolate framework-based nanomaterials for the capture of heavy metal ions and radionuclides: A review. Chem. Eng. J. 2021, 406, 127139.
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal-Organic Framework Nanoparticles. Adv. Mater. 2018, 30, e1800202.
- Zhang, X.; Hou, L.; Samori, P. Coupling carbon nanomaterials with photochromic molecules for the generation of optically responsive materials. Nat. Commun. 2016, 7, 11118.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170.
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272.
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568.
- Hu, F.; Xue, Y.; Xu, J.; Lu, B. PEDOT-Based Conducting Polymer Actuators. Front. Robot. AI 2019, 6, 114.
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124.
- Xu, F.; Xu, H.; Chen, X.; Wu, D.; Wu, Y.; Liu, H.; Gu, C.; Fu, R.; Jiang, D. Radical covalent organic frameworks: A general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage. Angew. Chem. Int. Ed. Engl. 2015, 54, 6814–6818.
- Chen, X.; Addicoat, M.; Irle, S.; Nagai, A.; Jiang, D. Control of crystallinity and porosity of covalent organic frameworks by managing interlayer interactions based on self-complementary pi-electronic force. J. Am. Chem. Soc. 2013, 135, 546–549.
- Xiang, Z.; Cao, D.; Huang, L.; Shui, J.; Wang, M.; Dai, L. Nitrogen-Doped Holey Graphitic Carbon from 2D Covalent Organic Polymers for Oxygen Reduction. Adv. Mater. 2014, 26, 3315–3320.
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983.
- Vyas, V.S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.V. Exploiting Noncovalent Interactions in an Imine-Based Covalent Organic Framework for Quercetin Delivery. Adv. Mater. 2016, 28, 8749–8754.
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klock, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571.
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481.
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. Engl. 2013, 52, 13052–13056.
- Zhuang, X.; Zhao, W.; Zhang, F.; Cao, Y.; Liu, F.; Bi, S.; Feng, X. A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton. Polym. Chem. 2016, 7, 4176–4181.
- Kuhn, P.; Thomas, A.; Antonietti, M. Toward Tailorable Porous Organic Polymer Networks: A High-Temperature Dynamic Polymerization Scheme Based on Aromatic Nitriles. Macromolecules 2009, 42, 319–326.
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2007, 2, 687–691.
- Jiang, S.Y.; Gan, S.X.; Zhang, X.; Li, H.; Qi, Q.Y.; Cui, F.Z.; Lu, J.; Zhao, X. Aminal-Linked Covalent Organic Frameworks through Condensation of Secondary Amine with Aldehyde. J. Am. Chem. Soc. 2019, 141, 14981–14986.
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 4503.
- Zhao, C.; Lyu, H.; Ji, Z.; Zhu, C.; Yaghi, O.M. Ester-Linked Crystalline Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 14450–14454.
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized pi clouds. Nat. Commun. 2013, 4, 2736.
- Sun, Y.; Waterhouse, G.I.N.; Xu, L.; Qiao, X.; Xu, Z. Three-dimensional electrochemical sensor with covalent organic framework decorated carbon nanotubes signal amplification for the detection of furazolidone. Sens. Actuators B Chem. 2020, 321, 128501.
- Wang, L.; Xie, Y.; Yang, Y.; Liang, H.; Wang, L.; Song, Y. Electroactive Covalent Organic Frameworks/Carbon Nanotubes Composites for Electrochemical Sensing. ACS Appl. Nano Mater. 2020, 3, 1412–1419.
- Lu, Z.; Shi, Z.; Huang, S.; Zhang, R.; Li, G.; Hu, Y. Covalent organic framework derived Fe(3)O(4)/N co-doped hollow carbon nanospheres modified electrode for simultaneous determination of biomolecules in human serum. Talanta 2020, 214, 120864.
- Xu, M.; Wang, L.; Xie, Y.; Song, Y.; Wang, L. Ratiometric electrochemical sensing and biosensing based on multiple redox-active state COFDHTA-TTA. Sens. Actuators B Chem. 2019, 281, 1009–1015.
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018, 276, 362–369.
- Pan, F.; Tong, C.; Wang, Z.; Han, H.; Liu, P.; Pan, D.; Zhu, R. Nanocomposite based on graphene and intercalated covalent organic frameworks with hydrosulphonyl groups for electrochemical determination of heavy metal ions. Mikrochim. Acta 2021, 188, 295.
- Zhang, T.; Ma, N.; Ali, A.; Wei, Q.; Wu, D.; Ren, X. Electrochemical ultrasensitive detection of cardiac troponin I using covalent organic frameworks for signal amplification. Biosens. Bioelectron. 2018, 119, 176–181.
- Zhang, T.; Gao, C.; Huang, W.; Chen, Y.; Wang, Y.; Wang, J. Covalent organic framework as a novel electrochemical platform for highly sensitive and stable detection of lead. Talanta 2018, 188, 578–583.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
02 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





