Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekaterina Mikhailova | -- | 7522 | 2023-12-21 22:29:52 | | | |
| 2 | Lindsay Dong | Meta information modification | 7522 | 2023-12-22 03:51:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mikhailova, E.O. Green Synthesis and Biomedical Application of Selenium Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/53049 (accessed on 09 March 2026).
Mikhailova EO. Green Synthesis and Biomedical Application of Selenium Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/53049. Accessed March 09, 2026.
Mikhailova, Ekaterina O.. "Green Synthesis and Biomedical Application of Selenium Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/53049 (accessed March 09, 2026).
Mikhailova, E.O. (2023, December 21). Green Synthesis and Biomedical Application of Selenium Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/53049
Mikhailova, Ekaterina O.. "Green Synthesis and Biomedical Application of Selenium Nanoparticles." Encyclopedia. Web. 21 December, 2023.
Copy Citation
Selenium nanoparticles (SeNPs) are extremely popular objects in nanotechnology. “Green” synthesis has special advantages due to the growing necessity for environmentally friendly, non-toxic, and low-cost methods.
selenium nanoparticles
green synthesis
capping agents
antibacterial activity
anticancer activity
1. Introduction
Green technologies are a relatively young trend developed in recent decades with interest growing like a snowball. This is not surprising, because the goal pursued in their development is to protect nature, eliminate the damage caused to the environment in the past, and preserve the Earth’s natural resources. Therefore, searching for ways to synthesize various compounds using eco-friendly technologies is the most important task. Nanoparticles have become one such topic deserving close attention. Their huge variety (inorganic nanoparticles, organic nanoparticles, ceramic nanoparticles, and carbon-based nanoparticles [1][2][3][4][5][6]), biosynthesis simplicity, and remarkable physicochemical, biological, and catalytic properties can literally open a new era for the creation of modern drugs solving different biomedical tasks. The biological synthesis of nanoparticles by living organisms—bacteria, fungi, and plants [7]—is an inexhaustible source of medicines that enable the solving of large-scale problems in the treatment of various diseases—bacterial and viral infections, oncological diseases, parasitic invasions, inflammatory processes, diabetes, and also in biosensing, bioimaging, drug delivery, diagnostics, etc. [8][9][10].
Metal nanoparticles are one of the most popular objects in green synthesis, and the number of articles on this subject is steadily growing from year to year. Biosynthesized silver nanoparticles with a wide range of useful biological properties are the most popular among them [11][12]. However, other nanoparticles are of particular interest. Among those are selenium nanoparticles. The selenium (Se) element has the atomic number 34 and is located in group 16 and period 4 of the D.I. Mendeleev chemical element periodic system. Selenium is a non-metal, and it is classified as a chalcogen element. It was discovered by J.J. Berzelius in 1817 during his search for the method of sulfuric acid production [13]. Selenium received its name (from Greek σελήνη—the Moon) due to the fact that in nature it is a satellite of chemically similar tellurium (named after the Latin word telluris—Earth). For a long time, until the middle of the 20th century, selenium was considered poisonous, but in 1957, an important discovery inverted this idea upside down—it turned out to be able to prevent severe myodystrophy and cirrhosis of the animal liver [14]. Moreover, in the 1970s, it was found that selenium is part of proteins containing amino acids such as selenmethionine, selencysteine, and methylselenysteinine [15][16]. Selenium’s vitality for humans and animals was shown in further studies. Selenium as a trace element plays an important role in various physiological process: it is necessary for normal immune system functioning, participates in redox process regulation and iodine metabolism, and also performs anti-inflammatory and antiviral functions [17][18][19]. Selenium also acts as an antioxidant enzyme cofactor (glutathione peroxidase and thioredoxin reductase) protecting the human body from reactive oxygen species (ROS) [20]. Recently, the potential role of selenium in reproductive function in women and fertility in men was revealed [21][22]. There is a lot of evidence in favor of Se as an inducer of cancer cell apoptosis with minimal side effects on normal cells [23]. In 2009, the WHO even provided data on the daily intake of selenium, which should be 50–55 µg per day in the human diet, depending on individual body weight [24]. Selenium deficiency can lead to serious disorders in the body—liver necrosis, muscular dystrophy, and thyroid dysfunction [25]—and can also cause disorders in the heart, bones, muscles, etc. [26], while normal Se consumption contributes to growth and fertility improvement (shown in domestic chickens) [27]. Unfortunately, the line between selenium deficiency and toxicity is very thin, so it is extremely important to take this into account when creating drugs based on it. Selenium compounds exist in nature in four oxidation degrees: selenate (Se6+), selenite (Se4+), selenide (Se2−), and elementary selenium (Se0) [28]. The first three forms are toxic even at low concentrations, and only the latter is insoluble in water and, in essence, is non-toxic [29]. To achieve the desired therapeutic effect, selenium-based nanoparticles can be created, which can alleviate toxicity problems. In this regard, SeNP synthesis mediated by living organisms (bacteria, fungi, algae, and higher plants) can solve several important problems at once: to be an environmentally friendly alternative to expensive, energy-intensive, and potentially toxic chemical and physical methods for producing nanoparticles and to create nanoparticles with diverse and unique biological properties by the virtue of “green” bioreactors. Biocompounds can not only take part in nanoparticle synthesis but also act as capping agents capable of preventing agglomeration, provide functional groups for the drug attachment [30], stabilize NPs and, due to their own biological potential, have a targeted effect.
2. The Proposed Mechanism of SeNPs
The creation of nanoparticles using biological methods has provoked a real boom in this nanotechnology field over the past decades. Environmentally friendly green synthesis, which does not require high energy costs or the use of hazardous and toxic substances, has other undeniable advantages compared to physical and chemical methods for producing nanoparticles. The application of biological substrates (bacteria, fungi, algae, and plants) as a matrix for the NP synthesis and packaging allows for the production of an immeasurable number of variants, each having their own, sometimes unique properties, provided by nature itself and the organism where the process takes place. Low toxicity, biodegradability, and biocompatibility are the key parameters of the bionanoparticle’s positive reputation. Metal nanoparticles synthesized biologically are extremely popular objects [31][32][33]. Among non-metallic NPs, special attention is being focused on selenium nanoparticles. The interest in selenium compounds is not an accident as they directly participate in the most important biological processes in human and animal cells. Selenium compounds in the Se0 form are the least toxic, which makes them a potential aid in the fight against various diseases.
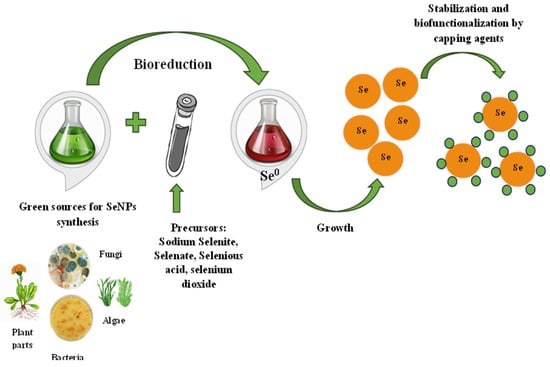
SeNP biosynthesis is a simple, one-step process that does not require toxic chemicals, high temperatures, or complex equipment. It is possible to realize green synthesis in two ways—directly in living organisms or with the help of bioreagents extracted from them. Sodium selenite/selenate, selenous acid, and selenium dioxide can be used as precursors for the synthesis of SeNPs added to a “bioreactor”—bacteria or fungi culture fluid, plant extract, etc. [34][35][36]. The precursors’ addition to the bioextract leads to the appearance of a red, red–orange, or orange solution color, indicating the reduction of selenium compounds and the formation of colloidal SeNPs. Further, the processes of the stabilization and capping of nanoparticles take place [37]. The proposed synthesis mechanism is presented in Figure 1. The selenium salt reduction to Se0 occurs due to different biopolymers capable of performing this role. For example, it is assumed that the enzymes thioredoxin reductase, nitrite reductase, or other membrane reductases are responsible for SeNP synthesis in bacteria [38]. Bacterial reductases have the ability to reduce the water-soluble oxyanion SeO42− to SeO32− and then to insoluble elemental selenium Se0 in a two-stage reaction. A variety of proteins, terpenoids, vitamins, flavonoids, tannins, polysaccharides, and other biologically active substances may be responsible for both the reduction process and SeNP stabilization and capping, preventing their agglomeration in aqueous solution during plant synthesis [39]. Capping agents, as important nanoparticle components, are very attractive to study owing to their own therapeutic significance.

Figure 1. The proposed mechanism of selenium nanoparticles synthesis.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM), used to estimate the size and shape of SeNPs, can be distinguished as widely used methods for assessing the biosynthesized selenium nanoparticle properties: transmission electron microscopy (TEM), UV/Vis spectrophotometry, and dynamic light scattering (DLS) to evaluate the nanoparticle physical properties; scanning electron microscopy (SEM) to analyze the morphology of the nanoparticles; X-ray diffraction measurements to study the nanoparticle structure; as well as FTIR analysis (Fourier transform infrared spectroscopy), providing the opportunity to characterize biomolecules involved in SeNP synthesis and stabilization [40][41][42].
2.1. By Bacteria
Biologically active substance synthesis by microorganisms is a very popular method that has become increasingly important in recent years. This is a simple, cost-effective, environmentally friendly method widely used in biotechnology. SeNP biosynthesis is no exception, especially due to bacteria being capable of the biotransformation of selenium compounds. Apparently, this is one of the leading reasons for the large-scale microorganism-mediated SeNP production, in contrast to metal nanoparticles, where plant nanoparticles occupy the lion’s share of research [43]. The used microbes are extremely diverse—from bacteria of the genus Bacillus, Pseudomonas, Lactobacillus, and Escherichia coli to more exotic microorganisms.
Literature data indicate that both Gram-positive and Gram-negative bacteria can synthesize SeNPs. The reduction of SeO32− and SeO42− to Se0 is one of the mechanisms for toxic Se oxyanion removal in aerobic and anaerobic conditions [44]. Bacteria are able to implement this process intracellularly (in the periplasmic space) and extracellularly; however, the extracellular variant seems to be more preferable because nanoparticles are easier to extract. The intracellular mechanism consists of several stages: the Se oxyanions transport into the cell mainly due to sulfate permeases; these compounds’ reduction by bacterial enzymes to Se0 proceeds with their subsequent release from the cell; the elementary Se0 assembling into SeNPs by the continuous reduction of Se oxyanions to Se0; and the isolation of SeNPs from bacterial cells using centrifugation and filtration methods.
An interesting object for intracellular selenium nanoparticle production are the Gram-negative bacteria Delftia sp., known for their unique metabolic abilities to transform various pollutants; in this case, the reduction of selenite to SeNPs did not have harmful effects on the cell structure [45]. Moreover, selenium nanoparticles synthesized intracellularly using Lactobacillus pentosus were agglomerated in the surrounding medium [46]. Khoei et al. showed that after the synthesis by Burkholderia fungorum, SeNPs were present both intracellularly and extracellularly. It is assumed that the SeNP formation mechanism can be conditionally attributed to cytoplasmic enzymatic activation mediated by electron donors. Then, biogenic nanoparticles are probably released from bacterial cells as a result of cell secretion or lysis [47]. The following hypotheses of SeNP export from the cell can be found in the literature: (1) the bubbles’ formation on the outer membrane (membrane vesicles), occurring in response to stress by encapsulating various harmful substances in the bubbles of a lipid bilayer membrane for removal outside the cell; (2) secretion due to the specialized proteins (for example, the SefA protein in Thauera elenatis) [47][48]. Extracellular SeNP synthesis was shown for Pseudomonas alcaligenes [49], Enterococcus faecalis [50], Bacillus subtilis [51], on the membrane surface of bacteria isolated from wastewater from glass factories [52], etc.
Despite the mechanism of bacteria-mediated biosynthesis not being fully understood, the most important role is assigned to enzymes. It was found that NADH-dependent reductases are responsible for the biomimetic reduction of SeO32− to Se0 nanospheres in Pseudomonas aeruginosa [40]. Nitrate reductases are capable of reducing in E. coli [53] and arsenate reductase in Bacillus selenitireducens [54]. It is proposed that membrane-bound reductases are able to produce reduced Se0 through enzymatic electron transfer [55]. Thus, in B. fungorum strains synthesizing SeNPs, cytoplasmic reductase accepts electrons from NADH and NADPH. Free thiols and thiol groups of proteins may participate in SeO32− reduction in both strains as well [47].
However, there is evidence of another mechanism of SeNP bacterial synthesis. In the filamentous bacterium Streptomyces sp., the intracellular Se(IV) reduction was usually driven by reduced thiols such as glutathione (GSH) in the cytoplasm via the Painter reaction, and further, SeNPs were released through cell lysis or fragmentation [56]. The mechanism suggests that selenite is converted by glutathione or glutathione reductase as the main electron donors for hydrogen selenide (H2Se) via selenodiglutathione (GS-Se-SG) and glutathionylselenol intermediates. Initially unstable selenodiglutathione formed, and then reacts with residual GSH to form diglutathione (GSSG) and elemental selenium. The electron source for GSH regeneration is mainly NADPH. This variant of SeNP synthesis involving GSH was discovered for Idiomarina sp. [57]. Glutathione reductase and selenocysteine lyase can participate in the selenium reduction to SeNPs in lactobacilli [58].
2.2. By Fungi
Unfortunately, there are few data on the mechanism of SeNP synthesis by fungi, although research is underway. Fungi-mediated SeNP synthesis is possible both intracellularly and extracellularly. Fungi as nanoparticle manufacturers attract attention owing to their ability to secrete many extracellular metabolites that can increase the nanoparticle yield and their stability. Thus, extracellular selenium nanoparticle synthesis was presented in Penicillium crustosum as a producer of various enzymes (amylase, cellulase, gelatinase, and xylanase) [59]. At the same time, SeNPs in Mariannaea sp. were biosynthesized intracellularly, released from cells, and subsequently increased in size outside the fungal cell [60]. It is supposed that the key role in the synthesis process belongs to enzymes working as reducing and stabilizing agents. Moreover, a lot of proteins are adsorbed on the surface of nanoparticles, forming a stable selenium bioconjugate system, and lipid and amide groups might play an important role during the formation process [60]. The intracellular synthesis mechanism is characteristic of Saccharomyces cerevisiae [61]. There are suggestions that membrane-bound oxidoreductases and quinones were engaged in metal NP synthesis using yeast strains. The possible involvement of sulfite reductase in nanoparticle formation in Saccharomyces boulardii was shown [62]. As the pH value increased in the yeast’s internal environment, reductase-reducing metal ions with the simultaneous production of NPs were activated. Quinones also have strong nucleophilic and redox properties, making them more applicable for metal ion reduction and their conversion into NPs.
High pH values of the medium had a positive effect on selenium nanoparticle synthesis [59][60][63][64]. Also, the alkaline medium prevented NP agglomeration and stabilized the coating agents on the NP surfaces by interacting with protein amino groups [60]. As in the case of bacteria, the microorganism’s growth phase is important. So, the stationary growth phase for maximum Trichoderma sp. in SeNP synthesis was demonstrated [64].
Capping agents are striking parameter-stabilizing nanoparticles and are of particular interest for their further biomedical use. As such, proteins and enzymes can bind to the formed SeNPs in several ways—by cysteine residues, free amino groups, and the electrostatic attraction of negatively charged carboxylate groups [59], as well as lipids [60], alkenes, alkanes, alcohols [64], polysaccharides [65], phenols, primary amines [66], carbohydrates, and amino acids [67].
2.3. By Algae
Another original “bionanofactory” for the production of selenium nanoparticles is algae. With many valuable biologically active compounds capable of selenium reduction and participating in nanoparticle coating, algae can be an effective tool for creating SeNPs. Such synthesis can be mediated by a variety of compounds, such as amines, amides, alkaloids, terpenoids, antioxidants (polyphenols and tocopherols), polysaccharides, proteins, pigments (carotenoids), and phycobilins contained in algae extracts and involved in selenium reduction and nanoparticle stabilization [68]. However, this mechanism is practically unstudied. Thus, synthesis in cyanobacteria (blue–green algae) was found both extracellularly, or on the surface of the cell wall, and inside the cell [69]. Selenate reductases and selenite reductases are responsible for intracellular synthesis, while extracellular synthesis occurs under the action of various phytocompounds with reducing potential [69].
2.4. By Plants
The huge diversity of plant selenium nanoparticles is so great and difficult to describe. The rich flora of our planet and the knowledge accumulated about medicinal plants (for example, traditional Chinese medicine or Ayurveda) and their disease treatment application, along with well-known plants consumed in food, allow using different herb parts as a “biofactory”—roots from Blumea axillaris [70], peel from Solanum melongena [71], wheat seedlings from Triticum aestivum [72], leaves from Azadirachta indica, Petroselinum crispum [73][74], and others, stem from banana [75], flowers from Calendula officinalis [76], and many others. However, since the vast majority of research is conducted in order to obtain a targeted effect (antibacterial, anticancer, antioxidant, anti-inflammatory, antiparasitic, etc.), the mechanism of SeNPs plant synthesis is not given such significant attention.
The smaller size and high ratio of surface area to volume of NPs give them the possibility to interact closely with the microorganism cell membrane, which facilitates interaction and intracellular diffusion, causing significant damage to the membranes and having a toxic effect on DNA or cell proliferation inhibition by processes mediated by reactive oxygen species [76]. For example, in onion and ginger extracts, selenium is reduced by phenolic compounds (quercetin and gingerols), where the enol group of the extract’s phenolic compounds breaks the O-H bond, which releases an electron to reduce Se [77]. Polyphenolic compounds contain different functional groups capable of forming nanoparticles. It is assumed that the tautomeric conversion of a flavonoid from an enol to a keto form can release a chemically active hydrogen atom that can reduce metal ions to form NPs during synthesis using aquatic extracts of black and green tea [78].
It is supposed that they participate not only at the reduction stage but also at the initiation stage of NP formation and their further aggregation [78]. In Crocus sativus extract-mediated synthesis, reduction can occur due to catechin and gallic acid. As a rule, the ortho-hydroxyl groups of these compounds are involved in SeNP synthesis. Se4+ is reduced to Se0 when two electrons are released in the flavonoid ring. The catechin ring is oxidized to a 3,4-quinone ring as a stable final product.
The pH of the medium for SeNP synthesis is also significant, as it affects the bioreduction of precursors. For example, in Muntigia calabura-mediated synthesis, it was found that Se nanoparticles retained a spherical shape in the pH range of 5–6, while a neutral medium ensured the joint presence of spherical and nanorod nanoparticles, i.e., the pH level directly affected the formation of nanomaterials, especially during crystal formation [79]. Due to the presence of various compounds in M. calabura extract, the surfactant layer can be formed and effectively maintain the spherical nanoparticle shape without any agglomeration.
Since nanoparticles tend to agglomerate, their stabilization is necessary to suppress their excessive growth by coating them with a polymer or surfactant layer, decreasing the interaction between nanoparticles. Biocompounds can act as phyto-capping agents, including those contributing to the reduction of selenium salts to SeNPs. The selenium nanoparticle’s stability is usually assessed by measuring the zeta potential. SeNPs synthesized by plant extracts are covered with a bio-organic layer, consisting of proteins, polysaccharides, and lipids, with a significant proportion of ionized carboxylic groups. These groups, specific to both the side chains of some amino acid residues and carboxylated polysaccharides, are responsible for the negative values of the SeNP zeta potentials; the higher the negative values of the zeta potentials, the more stable the nanoparticles [80].
2.5. Other Biosynthesis
Green selenium nanoparticle synthesis is not limited to different “biofactories”. Separate biomolecules having biological activity are also used, providing anticancer, antioxidant, anti-inflammatory and other impacts. Such examples include SeNP synthesis using berberine, a plant alkaloid with anticancer and antisclerotic effects [81]; apigenin is one of the most common aglycones of flavones, a natural antioxidant with anti-inflammatory and anticarcinogenic properties [82]; bee propolis, known for many centuries for its antimicrobial, antioxidant, anti-inflammatory, immunomodulatory, and cardioprotective properties, can synthesize nanoparticles due to its broad range of biomolecules like phenols, flavonoids, alkaloids, steroids, terpenoids, etc. [83]; by bioactive compounds of honey [84]; by Carvacrol—a member of the monoterpenoid phenols, possessing antimutagenic, analgesic, antimicrobial, antitumor, antiparasitic, and antioxidant activity [85]; by enzyme synthesis using bovine serum albumin as a reducing agent [86]; with naringenin and baicalin participation as capping agents—flavonoids with antimicrobial and anti-inflammatory effects [87]—and zein (a class of prolamine proteins contained in corn) [88]. Such an abundance of approaches to biosynthesis and applied biological objects represent an inexhaustible storehouse of possibilities both for SeNP production and for further biomedical application.
3. Green SeNP Application
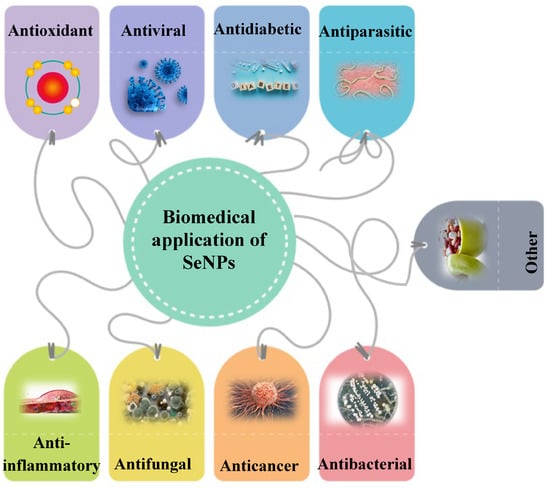
SeNPs have great potential for biomedical application according to their remarkable properties (Figure 2). They have many advantages and useful therapeutic properties, which will be discussed below.

Figure 2. Biomedical application of SeNPs.
3.1. Antibacterial Activity
The search for new antibacterial drugs is a very urgent problem due to the increasing resistance of pathogenic microorganisms to well-known and widely used antibiotics. Human pathogens are the most popular for selenium nanoparticle antimicrobial activity studies. These include Staphylococcus aureus, which, upon entering the bloodstream, can cause endocarditis, pneumonia, osteomyelitis, and other infections. In addition, hospital strains, such as methicillin-resistant S. aureus (MRSA), are extremely resistant to antibiotics, making the fight against them difficult. E. coli, normally inhabiting the intestines, can, however, cause pathology ingested into other human body organs or cavities. P. aeruginosa can be stable to many classes of antimicrobial and invoke infections, especially in patients with weakened immunity [89]. Despite the antimicrobial mechanism of SeNP action not having been fully investigated, there are a number of well-established assumptions. The SeNPs’ biocidal properties depend on their size and shape: spherical nanoparticles of smaller size—50–100 nm—are more effective than larger nanoparticles because they can easily penetrate into the bacterial cell/membrane and affect the bacterial biological activity [89][90]. SeNPs are active against both Gram-positive and Gram-negative microorganisms [91]. The more negative membrane charge of the Gram-negative bacteria formed from lipopolysaccharides, as well as the cytoplasmic membrane and the outer cell membrane containing a thin peptidoglycan layer between them with a periplasmic compartment, allows selenium nanoparticles to bind effectively to bacterial cells [89][92].
Apparently, more complex mechanisms are involved in the process: interaction with vital cellular components (RNA, DNA and ribosomes) in order to change and deactivate their intracellular processes; ATP depletion and a change in the membrane’s polarity; and the ROS generation and free radical formation (hydroxyl radicals, superoxide anions, and hydrogen peroxide) after penetration through bacterial membranes, which can damage DNA and proteins through oxidative stress, to enhance the peroxidation of membrane lipids and subsequently increase the cytoplasmic contents’ leakage, suppressing metabolism and leading to cell death [91]. Thus, in experiments with P. aeruginosa, E. coli, Vibrio parahemolyticus, S. aureus, B. cereus, and B. subtilis enhanced protein leakage was demonstrated, moreover, bio-SeNPs may accelerate proteins and polysaccharide leakage from bacterial cytoplasm [93]. It is supposed that the negative charges discovered on the protein content in bacterial walls might cause interaction with ionic species generated by the existence of SeNPs [94].
Undoubtedly, capping agents are of great value in the SeNPs antibacterial activity. For example, anacardiac acids from Amphipterygium glaucum, inhibitors of bacterial histidine protein kinase in two-component regulatory systems, can act as capping agents. These proteins participated in the expression regulation of the virulence factor through quorum sensing (a mechanism of communication between unicellular organisms) [95]. It is assumed that the biologically active substances (BAS) from C. officinalis extract present on the SeNPs surface are capable of damaging the signaling receptors necessary to carry out quorum sensing [96]. The SeNPs coating of compounds such as flavonoids, terpenoids, alkaloids and other BAS from plant extracts is a possible reason for their antimicrobial potential because they can inhibit enzymes responsible for DNA replication and gene expression necessary for the microbe’s survival.
It is believed that more than 80% of all chronic human infectious diseases are associated with biofilms [97]. In this regard, SeNPs, having antibacterial properties, can become potential agents against biofilm formation. Thus, SeNPs (with an average particle size of 28 nm) produced by P. vermicola have an antibiofilm effect on S. aureus, B. cereus, E. coli and Salmonella enteritidis, which lose their ability to form a biofilm 95% higher [98]. SeNPs were able to inhibit biofilm formation as well as disaggregate mature glycocalyx (glycoprotein and glycolipid cover), ultimately led to the bacterial cell death in P. aeruginosa [99]. The biofilm degradation of E. coli, S. aureus and P. aeruginosa [100], Salmonella typhi [101], P. mirabilis [102], Enterococcus faecalis, Salmonella typhimurium, and S. enteritidis [103], Klebsiella pneumoniae [104], Acinetobacter sp. [18] was shown.
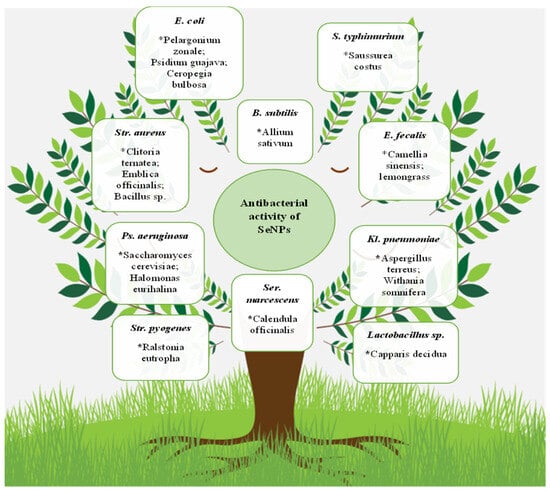
It should be noted that, in general, the antibacterial properties’ multiformity of selenium nanoparticles are huge. SeNPs overwhelming effect on microorganisms, including the Gram-positive bacteria S. aureus [50][105][106][107][108][109][110][111][112][113][114][115][116][117], B. subtilis [36][91][113][118], Streptococcus mutans [114][116], E. faecalis [75], Mycobacterium tuberculosis [119][120], and Micrococcus luteus [121], and the Gram-negative E. coli [107][118][122][123][124], S. typhimurium [90][125][126][127], S. typhi [128], P. aeruginosa [91][100][129], Klebsiella pneumoniae [130][131], P. mirabilis [77][132], Vibrio harveyi and Vibrio parahaemolyticus [133], Shigella sp. [70], and Serratia marcescens [96], was demonstrated. Some of these microorganisms and nanoparticle bioproducers are presented in Figure 3.

Figure 3. Antibacterial SeNP activity. The biosources of selenium nanoparticles are indicated by stars. * Pelargonium zonale; Psidium guajava; Ceropegia bulbosa; Allium sativum; Saussurea costus; Clitoria ternatea; Emblica officinalis; Bacillus sp.; Camellia sinensis; lemongrass; Saccharomyces cerevisiae; Halomonas eurihalina; Ralstonia eutropha; Calendula officinalis; Aspergillus terreus; Withania somnifera; Capparis decidua.
3.2. Antifungal Activity
Despite the development and introduction of new antifungal drugs, the problem of increasing microorganism resistance to them still needs to be solved. Fungi such as Candida yeast and filamentous fungi like Aspergillus are widespread and bring huge costs to health systems in different countries. They exist as opportunistic human microorganisms with a suppressed and defective immune system, and some strains have high resistance to antifungal drugs. The nanoparticle application is one of the new ways to tackle this problem. The proposed mechanism of antifungal action is similar to those of bacteria: the smaller size and high ratio of surface area to volume allow them to interact closely with the cell membranes of microorganisms (reaction with a thiol (-SH) protein group affects membrane permeability), which facilitates interaction and intracellular diffusion, causing significant membrane damage and having a toxic effect on DNA, disrupting the mitochondrial membrane, changing gene expression, or inhibiting cell proliferation by ROS [76].
The biofilm formation of C. albicans is a separate medical problem. It was found that bio-SeNPs from Paenibacillus terreus are able to suppress the gene expression involved in morphogenesis and biofilm formation in C. albicans [134]. Adhesion was the first step for the initiation of biofilm formation in C. albicans and was mediated by the expression of Als genes that encoded the adhesion proteins that helped the cells adhere to the hydrophobic surfaces or attach themselves to the endothelial and epithelial cells. Als3, 4, and 6 genes were downregulated in the presence of SeNPs, and the Hwp1 gene that was involved in hyphal growth and biofilm formation was also downregulated. The suppression of the Efg1 gene probably led to the suppression of the hyphae cell wall gene expression, such as Hwp1 and Als3. It was observed that the expression of the Phr1 gene inhibited the transition to hyphae, and suppression of the Phr1 gene regulation probably inhibits morphogenesis in C. albicans in the presence of selenium nanoparticles [134].
3.3. Antiviral
Viruses are non-cellular infectious agents that pose one of the greatest threats to humanity, and the COVID-19 pandemic became direct proof of that. Newly available antiviral drugs with a minimal toxic effect, especially broad-acting, could be the key to solving a lot of medical problems. Unfortunately, there is not much information about biosynthesized SeNPs yet, but they also inspire serious hope in the struggle against various human viruses. Dengue fever, a viral infection transmitted by humans through infected mosquito bites, became a global problem after World War II and spread to more than 110 countries, mainly in Asia and South America. Thus, it was shown that selenium nanoparticles synthesized by the actinobacterium Streptomyces minutiscleroticus had antiviral activity against type 1 dengue virus [105]. The antiviral activity tended to increase with an increase in dose, and concurrently a reduction in viral growth was shown [105]. It is especially necessary to note the antiviral effect of Portulaca oleracea-based SeNPs on the inhibition of the hepatitis A virus (HAV) and Coxsackie B virus (Cox-B4), which are widespread everywhere and can trigger severe complications [135].
The exact antiviral mechanism of selenium nanoparticles is still to be explored; however, based on data on chemically produced SeNPs (most often with vitamin C participation), it is assumed that antiviral activity is associated not only with direct virus destruction but also with its role in regulating selenoprotein function, making SeNPs ideal candidates for antiviral drugs with a wide spectrum of antiviral activity [136]. Apparently, nanoparticles are able to penetrate directly into the host cell, besides direct interaction with the virus surface glycoproteins, and exhibit their antiviral activity by binding to viral factors and host cellular factors, thereby blocking the virus replication mechanism [137]. SeNPs probably resist the proliferation of viruses in Vero cells and inhibit the apoptotic protein activation by viruses inside host cells [135]. It was shown that SeNPs can effectively inhibit the production of ROS by the H1N1 virus [138]. H1N1 induced intracellular apoptosis in MDCK (Madin Darby Canine Kidney) cells, and SeNPs inhibited the generation of caspase-3-mediated apoptosis by increasing the Gpx1 (glutathione peroxidase 1) level by regulating the apoptosis-signaling pathway [138]. SeNPs could prevent H1N1 from infecting MDCK cells and causing cell apoptosis by blocking chromatin condensation and DNA fragmentation, along with the inhibition of ROS generation and activation of p53 phosphorylation [139]. It was found that SeNPs loaded with oseltamivir prevented the host cell apoptosis induced by EV71 (Enterovirus 71) infection through the mitochondrial pathway and reduced the generation of reactive oxygen species [140].
3.4. Anticancer
Cancer has become a real scourge of humanity over the past hundred years. The progressive number of cancer cases all over the world is of serious concern and requires a speedy way out of this situation. The new strategies for cancer treatment imply improving drug targeting as well as reducing their toxicity. The nanotechnological approach seems to be one of the most promising in this context. Successful results were shown in SeNP studies to induce cytotoxicity in cancer cells. Although the molecular mechanism of the SeNPs’ antitumor effect has not been fully revealed, research is in an active phase.
Among the SeNPs’ mechanisms of antitumor action in vitro, the greatest attention is paid to apoptosis, characterized by nuclear chromatin condensation, cytoplasm compression, and membrane dysfunction. It was found that oxidative stress and ROS generation are the main cytotoxicity mechanisms induced by SeNPs, where ROS modulates apoptosis by regulating the enzymatic activity involved in cell death pathways. The cancer cells exhibit an acidic pH and an imbalanced redox state. These conditions in cancer cells initiate the pro-oxidant conversion of SeNPs and trigger the development of free radicals in malignant cells. This leads, on the one hand, to mitochondrial membrane destruction, causing mitochondrial protein leakage, and, on the other hand, to stress in the endoplasmic reticulum (ER). The mitochondrial membrane destruction results in the outflow of various proteins and triggers apoptosis through caspase activation (a family of protease enzymes playing an important role in programmed cell death) [141]. Selenium nanoparticles can stimulate p53 expression in cancer cells, leading to caspase-9 activation, mitochondrial membrane potential depletion, and the induction of apoptosis. In addition, in cellular processes, DNA structure is damaged, causing the cell cycle to stop and, ultimately, cell death.
ROS are highly reactive molecules, including oxygen ions, free radicals, and peroxides. ROS are produced as a natural byproduct of normal cellular metabolism and play a significant role in cell signaling. ROS can chemically bind to nucleic acids and proteins; therefore, ROS generation is a substantial cellular event induced by Se compounds and results in cell apoptosis and/or cell cycle arrest [142]. It was found that SeNPs fabricated in Undaria pinnatifida polysaccharide caused a dose-dependent increase in the intracellular level of ROS, the most important mediator in the induction of apoptosis in vitro for A375 human melanoma cells, as well as DNA fragmentation, an important biochemical sign of cellular apoptosis [143].
The mitochondrial respiratory chain is a potential source of ROS, such as superoxide and hydrogen peroxide. The loss of mitochondrial membrane potential is associated with caspase activation and the initiation of the apoptotic cascade. When mitochondrial membrane integrity is violated, cytochrome C moves from the mitochondria to the cytosol, triggering a signaling caspase cascade that damages DNA.
The main role of caspase-9 is in the internal transmission of the apoptosis signal, while caspase-8 is involved in the external apoptosis signal transmission, and caspase-3 is a decisive factor in apoptosis implementation. Thus, an increase in the activity of caspase-3, the most important signaling module of the apoptosis cascade that plays a vital role in protein breakdown, provokes the programmed destruction of lung cancer cells, as was demonstrated for E. coli-mediated SeNPs [144]. Treatment with Carvacrol-mediated SeNPs directly targeted Bcl-2, Bax, and caspase-3, leading to the mitochondrial leakage of cytochrome C and subsequent activation of the apoptotic cascade in MCF-7 cells [85]. SeNPs dose-dependently increased caspase-3 levels in cancer cells; moreover, in normal cells, the SeNP concentration necessary to induce an increase in the caspase-3 level was found to be significantly higher compared to RAW 264.7, Caco-2, MCF-7, and IMR-32 cancer cells [145].
The significant induction of oxidative stress markers, such as ROS, 8-OHDG, LPO, and NO, was accompanied by a decrease in antioxidant marker levels (CAT, SOD, GPx activity and GSH levels) in MCF-7 cells exposed to green SeNPs, in contrast to control cells [85]. Possessing antioxidant properties, GSH not only protects the cell from toxic free radicals, but also generally determines the redox characteristics of the intracellular environment. It was found that ROS generation converts GSH to GSSG (glutathione disulfide) through the oxidation process. Oxidized glutathione is reduced by the enzyme glutathione reductase induced by oxidative stress. The ratio of reduced and oxidized glutathione formed in the cell is one of the most important parameters showing the oxidative stress level [142]. SeNPs increased oxidative stress and reduced the level of endogenous antioxidants such as GSH, superoxide dismutase, catalase and GSH peroxidase in MCF-7 cells [146]. Spermacoce hispida SeNP treatment decreased the levels of endogenous antioxidant such as GSH, superoxide dismutase, catalase, and GSH peroxidase [147] in the hepatocarcinoma cell line (HepG2), and also induced cell cycle arrest at the sub-G1 phase and subsequently led to apoptosis [148]. SeNPs from Carica papaya effectively influenced the viability of cancer cells through lactate dehydrogenase (LDH) leakage, meaning damage to the cell membrane.
The proliferating cell nuclear antigen (PCNA) is called the “ringmaster of the genome” because it regulates the cell cycle and participates in DNA synthesis, suggesting its active role in oncogenesis. Therefore, PCNA is widely used as a cell proliferation marker in both healthy and malignant tissues since it usually reflects cell proliferation activity [85]. Interestingly, SeNPs reduced the PCNA expression level in MCF-7 cells, showing their role in suppressing oncogenesis and proliferation in breast cancer by inhibiting PCNA gene expression [85].
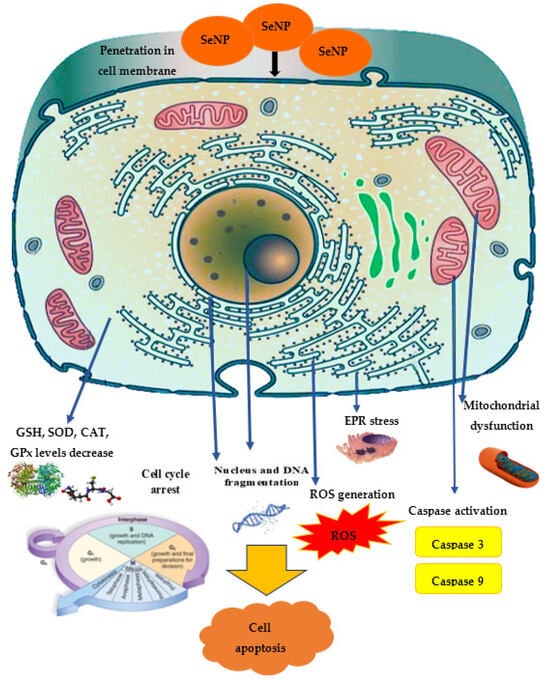
In general, the spectrum of SeNPs’ anticancer activity demonstrated in vitro is quite wide: in leukemia cells [149], MCF-7 [132][150], AGS (human gastric adenocarcinoma cell line) [151], HeLa [57][105], HepG2 [122][152], 4T1 [153], SW480 (human colorectal adenocarcinoma cell lines) [154], MDA-MB-231 (mammary gland adenocarcinoma cell lines) [155], and A2780 cells (human ovarian cancer cell line) [156]. The proposed mechanism of anticancer effect is demonstrated in Figure 4.

Figure 4. The proposed mechanism of SeNPs’ anticancer activity.
3.5. Antioxidant Activity
Hydrophilic/lipophilic, enzymatic/non-enzymatic compounds act as antioxidants, inhibiting oxidation by neutralizing the oxidative action of free radicals and other substances. Antioxidant activity was found for many nanoparticles, including SeNPs [157]. Green SeNPs’ ability to neutralize these free radicals can be explained by the nanoparticle dispersibility in the medium owing to their tiny particle size, in addition to high chemical activity, as well as the antioxidant activity of the covering biomolecules. The antioxidant capacity analysis in vitro is usually realized with the help of such molecules as 1,1-diphenyl-2-picryl-hydrazyl (DPPH), where the free electron of nitrogen in DPPH is reduced by hydrogen present in antioxidants, ABTS (2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) for checking both hydrophilic and lipophilic antioxidants, and the FRAP assay. The ferric reduction antioxidant power (FRAP) assay is based on electron transfer rather than hydrogen transfer, unlike DPPH and ABTS [158].
An excess of ROS can provoke irreversible oxidative damage to proteins, DNA, and cellular lipids in the human body. ROS, most often represented by superoxide anionic radicals—(O2−), hydroxyl radicals (·OH), and hydrogen peroxide (H2O2), can play a role in the initiation of diseases such as diabetes mellitus, Alzheimer’s disease, coronary heart disease, cancer, arteriosclerosis, and other health disorders associated with aging. Antioxidants protect the human body from oxidative damage and are able to slow down the progression of these processes to a certain degree by reacting directly with free radicals or improving antioxidant enzyme activity. Literature data indicate that SeNP treatment significantly inhibited intracellular ROS production in IPEC-J2 cells exposed to H2O2 and reduced the MMP loss (mitochondrial membrane potential) caused by oxidative stress induced by H2O2 [159]. Such an effective mitigation of the H2O2-induced oxidative damage effects of IPEC-J2 may be associated with an increase in the antioxidant enzyme levels, including GPx and SOD [159]. Selenium nanoparticles from B. paralicheniformis can protect IPEC-J2 cells from oxidative stress by inhibiting ROS production [160]. SeNPs from Lycium barbarum, having positive antioxidant activity, protected PC12 from toxicity induced by H2O2 [157]. The antioxidant activity of selenium nanoparticles from Pantoea agglomerans was demonstrated using primary cultures of umbilical vein endothelial cells (HUVECs) [55].
3.6. Antidiabetic Activity
Diabetes mellitus is a group of endocrine diseases conjugated with impaired glucose uptake owing to absolute or relative (violation of interaction with target cells) insufficiency of the hormone insulin. As a result, hyperglycemia develops—a persistent increase in blood glucose. The increase in disease cases on the planet causes serious concerns in medicine practice and requires the search for new, high-quality drugs with minimal side effects. The malady is associated with oxidative stress, reactive nitrogen species production (RNS) and ROS generation, accompanied by inflammation, β-cell dysfunction, hyperlipidemia, insulin resistance, and impaired glucose tolerance, leading to diabetic complications. Therefore, biocompounds with antioxidant activity seem to be the most suitable way to solve this problem. Literature data indicate that SeNPs produced by biochemical methods (most often by the reduction of sodium selenite with glutathione in the presence of bovine serum albumin (BSA)) raise the insulin concentration in the blood serum in mice with diabetes mellitus [161]. SeNPs could significantly decrease hepatic (serum ALT, AST, and ALP) and renal (serum uric acid, urea, and creatinine) function markers, total lipid, total cholesterol, triglyceride and low-density lipoprotein cholesterol levels, and glucose-6-phosphatase activity. At the same time, SeNPs increased malic enzyme, hexokinase, and glucose-6-phosphate dehydrogenase activity, liver and kidney glycogen contents, and high-density lipoprotein cholesterol levels. In addition, SeNPs were able to prevent histological injury in the hepatic and renal tissues of rats [161]. As for green SeNPs, very promising results were discovered, too. In in vitro experiments, selenium nanoparticles produced using Acacia catechu had an inhibitory effect on alpha-amylase, regulating blood glucose levels [162]. SeNPs functionalized with a novel polysaccharide (RTFP-3) extracted from Rosa roxburghii fruit in INS-1 cells effectively blocked intracellular ROS overproduction, mitochondrial damage, the activation of caspases-3, -8, and -9 in INS-1 cells, indicating that SeNPs functioned by reducing oxidative stress and down-regulating the uncoupling protein-2 expression (UCP-2 is an uncoupling protein, functioning to disperse the proton gradient generated through the inner membrane of mitochondria, and is thus associated with a decrease in the membrane potential of mitochondria) [163]. SeNPs from Hibiscus sabdariffa were able to enhance the decrease in testosterone levels in the blood serum in streptozotocin (STZ)-induced diabetes rats. SeNPs can significantly reduce oxidative stress indicators in testicular tissues, such as nitric oxide and MDA, restore impaired activity of antioxidant enzymes (CAT, GR, SOD and GPx), increase glutathione activity in testicular tissues, and are also able to prevent histological damage in rat testicles [164].
3.7. Anti-Inflammatory Activity
Inflammation is one of the most significant initial stages in the development of many pathological conditions. In the light of ongoing research, selenium nanoparticles appear to be a possible anti-inflammatory agent. Cytokines such as IL-6, IL-8, TNF-α. and IFN-γ can act together to initiate and regulate the inflammation process. IL-6, as an essential cytokine, is involved in various pathologies that can be improved by IL-6 inhibition. The expression of cytokines is regulated by various mechanisms. The activation of toll-like receptors (TLRs) induces pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β via nuclear factor-kappa B (NF-κB). In in vitro experiments, SeNPs biosynthesized by L. lactis capped with polysaccharides significantly attenuated the increase in IL-6, IL-8, IFN-γ, and TNF-α in IPEC-J2 cells [159]. The potential application of SeNPs using Syzygium aromaticum against epileptic seizures and damage to the cerebral cortex was shown in pentylenetetrazol-induced epilepsy in rats [165]. Green SeNPs reduced the levels of pro-inflammatory cytokines and suppressed the activity of the glial fibrillar acidic protein, showing their inhibitory effect on epilepsy-associated inflammation, and leading to their anti-inflammatory activity against PTZ-induced neuroinflammation. The authors attribute this protective effect not only to the SeNP activity itself but also to the plant extract flavonoids, phenols, and GSH components as capping agents that can prevent free radical accumulation and reduce oxidative stress, subsequent inflammation, and apoptosis [165]. The anti-inflammatory effect of selenium nanoparticles may have the potential to reduce acute colitis symptoms. Thus, the levels of TNF-α and IL-6 in the colon in mice with DSS (dextran sulfate sodium)-induced colitis were markedly reduced by SeNPs decorated with Ulva lactuca polysaccharides [166]. SeNPs inhibited the activation of macrophages by suppressing the nuclear translocation of NF-κB, which drives the transcription of these pro-inflammatory cytokines [166].
4. Toxicology
Toxicity is one of the most important areas of nanoparticle evaluation. Despite the wide range of useful biomedical properties, such an assessment is strictly mandatory before implementation. Most studies confirm that SeNPs are less toxic than sodium selenite. In addition, they are more bioavailable due to their small size [167]. The data presented in [167] suggest that the SeNPs’ toxicity depends on several interrelated parameters, such as the nanoparticle size, chemical composition, dose, and exposure time, affected the organism’s biological response. The toxicological studies showed that the main targets of SeNP toxicity are not only their pro-oxidant properties but also their interaction with metabolic pathways and molecular signaling pathways, including apoptosis, and the ability of small nanoparticles to penetrate various tissues. Interestingly, however, despite affecting cancer cells and causing their death, SeNPs do not harm normal cells, improve the bioenergetic phenotype of normal cells, or cause any genetic or chromatin damage [24]. Undoubtedly, the synthesis method is also important. Thus, biosynthesized SeNPs showed low toxicity in mice compared to SeNPs obtained by chemical methods, which demonstrated the important role of Bacillus sp. MSh-1 in the conversion of a highly toxic Se compound to the less toxic SeNPs [168]. Biosynthesized selenium nanoparticles with A. sativum showed interesting biocompatible features and limited cytotoxicity when compared with chemically synthesized selenium nanoparticles [169].
Little or no toxic effect is often shown toward normal cells. Thus, human dendritic cells and fibroblasts exposed to bacterial-mediated SeNPs did not show any cell viability loss, any increase in ROS release, or any significant increase in the pro-inflammatory and immunostimulating cytokine secretion (IL-12 stimulates natural killer cells and T-lymphocytes; IL-8 causes chemotaxis and activation of leukocytes; IL-6 and TNF-α cause inflammation and a systemic acute phase reaction characterized by fever). Moreover, the authors believe that inorganic selenium is unable to stimulate human DCs alone and that organic molecules on the surface of biogenic SeNPs must be responsible for the observed effect [170]. The Kaempferia parviflora-mediated SeNPs showed significant cytotoxicity in human gastric adenocarcinoma cells (AGS cells) but not in normal cells [171]. The selective ability to kill only cancer cells was shown for selenium nanoparticles from Crocus sativus, significantly reducing the growth rate of breast cancer cells (4T1 and MCF7 cells) without adversely affecting normal cells [172]. Similar data were obtained for normal cells (Vero and WI38) with SeNPs from Penicillium verhagenii [64]. No hazardous effect against normal Vero cells was observed for Polycladia myrica SeNPs [173] and CHO cells lines for Psidium guajava nanoparticles [122], HBL100 cell line for Carica papaya SeNPs [164], or THLE2 normal liver cells for Spirulina platensis SeNPs [125]. Allium sativum SeNPs did not cause damage to vertebrate erythrocytes and macrophages [169]. A lower cytotoxic effect and such reduced cytotoxicity might be due to the phytochemicals of M. koenigii berry extract incorporated with the SeNPs [174].
5. Conclusions
Thus, strengthening SeNPs’ properties with biocompounds possessing medical potential can become an important milestone on the way to producing environmentally friendly and safe medicines for many diseases [175][176]. Each of the biosynthesis options has its own undeniable advantages. The cultivation of bacteria and fungi to produce SeNPs allows for quickly scaling the process; at the same time, synthesis with plants opens up the widest possibilities for using their own therapeutic functions, coupled with toxicity reduction. The crucial role of capping agents has been shown in much research. These molecules mainly modulate the chemical surface composition, morphology, and size distribution of nanoparticles, preventing agglomeration and enhancing the kinetics of nanoparticle reduction by forming complex structures with metal ions in precursor salts. They are capable of performing a stabilizing function. For example, it was shown that the SeNP surface was strongly adsorbed and passivated by sulfated Ganoderma lucidum polysaccharide molecules, leading to the formation of a homogeneous and spherical morphology. Polysaccharide molecules can be closely absorbed around the Se particles, according to the surface absorption effect of the nanoparticles, and stability may be related to the electrostatic repulsion of negatively charged sulfate groups of the polysaccharide [177]. Polysaccharide–protein complexes isolated from Asian clam (Corbicula fluminea) and Pu-Erh tea crude polysaccharides were stabilizers of SeNPs [178][179]. In addition, there is evidence regarding bacterial SeNPs that some biomolecules are bound more strongly than others to the core metalloid matrix, so that the diverse capping layer components differentially contribute to the overall structural characteristics of the nanoparticles [180]. Moreover, Anoectochilus burmannicus extract can acts as a cryoprotectant and/or lyoprotectant during the freeze-drying process of the SeNPs, resulting in the complete resuspension of the particles with the preservation of both physical and biological properties [181]. There are many such examples, not to mention the fact that most researchers approve the cell extract biomolecules’ participation as enhancers of the selenium nanoparticles biological functions. Biological molecules coating the nanoparticles not only reduce their side effects but also improve their characteristics like long-term stability and biocompatibility (speed up the uptake and retention of nanoparticles), and enhance their antimicrobial and anticancer activity [182].
The SeNPs’ antibacterial activity against well-known pathogenic microorganisms is intensively studied. The results of the antibiofilm formation are particularly important in this regard. For example, selenium nanoparticles inhibit S. aureus adherence and microcolony formation on polystyrene, glass, and catheter surfaces [183]. This useful property can be applied in the fight against hospital infections. Selenium nanoparticles may be considered antibacterial drug carriers.
References
- Sun, M.; Wang, T.; Li, L.; Li, X.; Zhai, Y.; Zhang, J.; Li, W. The Application of Inorganic Nanoparticles in Molecular Targeted Cancer Therapy: EGFR Targeting. Front. Pharmacol. 2021, 12, 702445.
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245.
- Zadeh Mehrizi, T.; Shafiee Ardestani, M. Application of non-metal nanoparticles, as a novel approach, for improving the stability of blood products: 2011–2021. Prog. Biomater. 2022, 11, 137–161.
- Qasim, M.; Clarkson, A.N.; Hinkley, S.F.R. Green Synthesis of Carbon Nanoparticles (CNPs) from Biomass for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 1023.
- Mondal, R.; Yilmaz, M.; Mandal, A. Green synthesis of carbon nanoparticles: Characterization and their biocidal properties. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Kharisov, B., Kharissova, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 277–306.
- Thomas, S.C.; Harshita; Mishra, P.K.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188.
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370.
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.M.; Islam, F.; Emran, T.B.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742.
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902.
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2023, 1–27.
- Jabbar, A.; Abbas, A.; Assad, N.; Naeem-ul-Hassan, M.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; Hanbashif, A.; Amin, H.M.A. A highly selective Hg2+ colorimetric sensor and antimicrobial agent based on green synthesized silver nanoparticles using Equisetum diffusum extract. RSC Adv. 2023, 13, 28666.
- Ullah, S.; Khalid, R.; Rehman, M.F.; Irfan, M.I.; Abbas, A.; Alhoshani, A.; Anwar, F.; Amin, H.M.A. Biosynthesis of phytofunctionalized silver nanoparticles using olive fruit extract and evaluation of their antibacterial and antioxidant properties. Front. Chem. 2023, 11, 1202252.
- Trofast, J. Berzelius’ discovery of selenium. Chem. Int. 2011, 33, 16–19.
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 Against Dietary Necrotic Liver Degeneration. 1951. Nutrition 1999, 15, 255.
- Flohe, L. The labour pains of biochemical selenology: The history of selenoprotein biosynthesis. Biochim. Biophys. Acta 2009, 1790, 1389–1403.
- Chaudhary, S.; Umar, A.; Mehta, S.K. Progress Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. Prog. Mater. Sci. 2016, 83, 270–329.
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128.
- Esmaeili, S.; Fazelifard, R.S.; Ahmadzadeh, S.; Shokouhi, M. The importance of selenium to human health. Lancet 2000, 356, 233–241.
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium nanoparticles: Synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mat. Res. Express 2019, 6, 0850d8.
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119.
- Qazi, I.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053.
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Zhou, G. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268.
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812.
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074.
- Saha, U.K.; Fayiga, A.O.; Hancock, D.W.; Sonon, L.S. Selenium in Animal Nutrition: Deficiencies in Soils and Forages, Requirements, Supplementation and Toxicity. Int. J. Appl. Agric. Sci. 2016, 2, 112.
- Agarwal, R.; Behari, J.R. Effect of selenium pretreatment in chronic mercury intoxication in rats. Bull. Environ. Contam. Toxicol. 2007, 79, 306–310.
- Nabi, F.; Arain, M.A.; Hassan, F.; Umar, M.; Rajput, N.; Alagawany, M.; Syed, S.F.; Soomro, J.; Somroo, F.; Liu, J. Nutraceutical role of selenium nanoparticles in poultry nutrition: A review. Poult. Sci. J. 2020, 76, 459–471.
- Rasche, B.; Amin, H.M.A.; Clarke, S.J.; Compton, R.G. Polyselenides on the route to electrodeposited selenium. J. Electroanal. Chem. 2019, 835, 239–247.
- Malyugina, S.; Skalickova, S.; Skladanka, J.; Slama, P.; Horky, P. Biogenic Selenium Nanoparticles in Animal Nutrition: A Review. Agriculture 2021, 11, 1244.
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172.
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84.
- Ali, S.; Iqbal, M.; Naseer, A.; Yaseen, M.; Bibi, I.; Nazir, A.; Khan, M.I.; Tamam, N.; Alwadai, N.; Rizwan, M.; et al. State of the art of gold (Au) nanoparticles synthesis via green routes and applications: A review. ENMM 2021, 16, 100511.
- Mikhailov, O.V.; Mikhailova, E.O. Elemental silver nanoparticles. Biosynthesis and bio application. Materials 2019, 12, 3177.
- Ho, C.T.; Nguyen, T.H.; Lam, T.-T.; Le, D.-Q.; Nguyen, C.X.; Lee, J.-H.; Hur, H.-G. Biogenic synthesis of selenium nanoparticles by Shewanella sp. HN-41 using a modified bioelectrochemical system. EJB 2021, 54, 1–7.
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748.
- Vyas, J.; Rana, S. Synthesis of selenium nanoparticles using Allium sativum extract and analysis of their antimicrobial property against gram positive bacteria. Pharma Innov. 2018, 7, 262–266.
- Vetchinkina, E.; Loshchinina, E.; Kursky, V.; Nikitina, V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J. Microbiol. 2013, 51, 829–835.
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547.
- Abadi, B.; Hosseinalipour, S.; Nikzad, S.; Pourshaikhali, S.; Fathalipour-Rayeni, H.; Shafiei, G.; Adeli-Sardou, M.; Shakibaie, M.; Forootanfar, H. Capping Agents for Selenium Nanoparticles in Biomedical Applications. J. Clust. Sci. 2023, 34, 1669–1690.
- Dwivedi, S.; AlKhedhairy, A.A.; Ahamed, M.; Musarrat, J. Biomimetic Synthesis of Selenium Nanospheres by Bacterial Strain JS-11 and Its Role as a Biosensor for Nanotoxicity Assessment: A Novel Se-Bioassay. PLoS ONE 2013, 8, e57404.
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299.
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28.
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70.
- Tendenedzai, J.T.; Chirwa, E.M.N.; Brink, H.G. Performance Evaluation of Selenite (SeO32−) Reduction by Enterococcus spp. Catalysts 2021, 11, 1024.
- Pescuma, M.; Aparicio, F.; Zysler, R.D.; Lima, E.; Zapata, C.; Marfet, J.A.; Velez, M.L.; Ordonez, O.F. Biogenic selenium nanoparticles with antifungal activity against the wood-rotting fungus Oligoporus pelliculosus. Biotechnol. Rep. 2023, 37, e00787.
- Tayo, A.; Olawunmi, B.; Solomon, Y.; Alao, O.; Christianah, B. Antibacterial activity of intracellular greenly fabricated selenium nanoparticle of Lactobacillus pentosus ADET MW861694 against selected food pathogens. Int. J. Biotechnol. 2021, 10, 39–51.
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjälä, K.; Bernardi, P.; Vallini, G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017, 34, 1–11.
- Debieux, C.M.; Dridge, E.J.; Mueller, C.M.; Splatt, P.; Paszkiewicz, K.; Knight, I. A bacterial process for selenium nanosphere assembly. Proc. Natl. Acad. Sci. USA 2011, 108, 13480–13485.
- Ashengroph, M. Synthesis analysis and antibacterial activity of selenium nanoparticles produced by Pseudomonas alcaligenes. JMW 2019, 12, 252–266.
- Shoeibia, S.; Mashreghi, M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139.
- Huang, S.; Yu, K.; Wen, L.; Long, X.; Sun, J.; Liu, Q.; Zheng, Z.; Zheng, W.; Luo, H.; Liu, J. Development and application of a new biological nano-selenium fermentation broth based on Bacillus subtilis SE201412. Sci. Rep. 2023, 13, 2560.
- Sardarabadi, H.; Zohrab, F.; Tavakolizadeh, M.; Akhlaghi, M.; Salarinia, R. Biosynthesis of selenium nanoparticles using the bacteria isolated from the glass-making factories effluents. Heliyon 2023.
- Avazeri, C.; Turner, R.J.; Pommier, J.; Weiner, J.H.; Giordano, G. Tellurite and selenate reductase activity of nitrate reductases from Escherichia coli: Correlation with tellurite resistance. Microbiology 1997, 143, 1181–1189.
- Afkar, E.; Lisak, J.; Saltikov, C.; Basu, P.; Oremland, R.S. The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiol. Lett. 2003, 226, 107–112.
- Torres, S.K.; Campos, V.L.; Leon, C.G.; Rodrıguez-Llamazares, S.M.; Rojas, S.M.; Gonzalez, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012, 14, 1236.
- Tan, Y.; Yao, R.; Wang, R.; Wang, D.; Wang, G.; Zheng, S. Reduction of selenite to Se(0) nanoparticles by filamentous bacterium Streptomyces sp. ES2-5 isolated from a selenium mining soil. Microb. Cell Fact. 2016, 15, 157.
- Srivastava, P.; Kowshik, M. Anti-neoplastic selenium nanoparticles from Idiomarina sp. PR58-8. Enzyme Microb. Technol. 2016, 95, 192–200.
- Martínez, F.G.; Moreno-Martin, G.; Pescuma, M.; Madrid-Albarrán, Y.; Mozzi, F. Biotransformation of Selenium by Lactic Acid Bacteria: Formation of Seleno-Nanoparticles and Seleno-Amino Acids. Front. Bioeng. Biotechnol. 2020, 8, 506.
- Fouda, A.; Hassan, S.E.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834.
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ A 571 and their characterization. Colloids Surf. A Physicochem. Eng. 2019, 571, 9–16.
- Faramarzi, S.; ·Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—Fabrication and characterization. Arch. Microbiol. 2020, 202, 1203–1209.
- Patel, N.; Kaler, A.; Jain, S.; Banerjee, U.C. Cells of Saccharomyces boulardii and its Evaluation as Anticancer Agent. Curr. Nanosci. 2013, 9, 463–468.
- Tugarova, A.V.; Vetchinkina, E.P.; Loshchinina, E.A.; Burov, A.M.; Nikitina, V.E.; Kamnev, A.A. Reduction of Selenite by Azospirillum brasilense with the Formation of Selenium Nanoparticles. Microb. Ecol. 2014, 68, 495–503.
- Sekyerebea Diko, C.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583.
- Liang, X.; Perez, M.A.M.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G. M Fungal formation of selenium and tellurium nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 7241–7259.
- Hussein, H.G.; El-Sayed, E.-S.R.; Younis, N.A.; Hamdy, A.E.H.A.; Easa, S.M. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Express 2022, 12, 68.
- Nassar, A.-R.A.; Eid, A.M.; Atta, H.M.; El Naghy, W.S.; Fouda, A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci. Rep. 2023, 13, 9054.
- Muthusamy, G.; Thangasamy, S.; Raja, M.; Chinnappan, S.; Kandasamy, S. Biosynthesis of silver nanoparticles from spirulina microalgae and its antibacterial activity. Environ. Sci. Pollut. Res. 2017, 24, 19459–19464.
- Afzal, B.; Fatma, T. Selenium Nanoparticles: Green Synthesis and Exploitation. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 473–484.
- Dash, J.P.; Mani, L.; Nayak, S.K. Antibacterial activity of Blumea axillaris synthesized selenium nanoparticles against multidrug resistant pathogens of aquatic origin. EJBAS 2022, 9, 65–76.
- Ali, B.M.H.; Almashhedy, L.A.M. Green Synthesis Optimization and Characterization of Selenium Nanoparticle Using Aqueous Extract of Peel Solanum melongena L. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 102007.
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Guo, Y. Absorption and Bio-Transformation of Selenium Nanoparticles by Wheat Seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597.
- Hawsah, M.A.; Abdel-Gaber, R.; Al-Quraishy, S.; Aljawdah, H.M.A.; Maodaa, S.N.; Al-Saebi, E. Green synthesis of selenium nanoparticles using Azadirachta indica leaves extract: Evaluation of anthelmintic and biocompatibility potential. Food Sci. Technol. 2023, 43, e13223.
- Fritea, L.; Laslo, V.; Cavalu, S.; Costea, T.; Vicas, S.I. Green biosynthesis of selenium nanoparticles using parsley (Petroselinum crispum) leaves extract. Stud. Univ. Vasile Goldiş 2017, 27, 203–208.
- Khullar, D.; Rajeshkumar, S. Phyto-assisted Synthesis and Assessment of Anti-Inflammatory and Antioxidant Properties of Selenium Nanoparticles Prepared Using Banana Stem Extracts-An Invitro Study. J. Med. Dent. Sci. 2021, 9, 83–85.
- Lazcano-Ramírez, H.G.; Garza-García, J.J.O.; Hernández-Díaz, J.A.; León-Morales, J.M.; Macías-Sandoval, A.S.; García-Morales, S. Antifungal Activity of Selenium Nanoparticles Obtained by Plant-Mediated Synthesis. Antibiotics 2023, 12, 115.
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Perez-Larios, A. Antimicrobial activity of green synthesized Se nanoparticles using ginger and onion extract: A laboratory and in silico analysis. Part. Sci. Technol. 2022, 41, 319–329.
- Sentkowska, A.; Pyrzyńska, K. Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and HerbWater Extracts. Appl. Sci. 2023, 13, 1071.
- Tuyen, N.N.K.; Huy, V.K.H.; Duy, N.H.; An, H. Green synthesis of selenium nanorods using Muntigia calabura leaf extract: Effect of pH on characterization and bioactivities. Waste Biomass Valorization 2023.
- Pyrzynska, K.; ·Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480.
- Othman, M.S.; Obeidat, S.T.; Al-Bagawi, A.H.; Fareid, M.A.; Fehaid, A.; Abdel Moneim, A.E. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J. Integr. Med. 2022, 20, 65–72.
- Al-Otaibi, A.M.; Al-Gebaly, A.S.; Almeer, R.; Albasher, G.; Al-Qahtani, W.S.; Abdel Moneim, A.E. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 2022, 29, 47539–47548.
- Shubharanim, R.; Mahesh, M.; Murthy, V.N.Y. Biosynthesis and Characterization, Antioxidant and Antimicrobial Activities of Selenium Nanoparticles from Ethanol Extract of Bee Propolis. J. Nanomed. Nanotechnol. 2019, 10, 522.
- Amiri, H.; Hashemy, S.I.; Sabouri, Z.; Javid, H.; Darroudi, M. Green synthesized selenium nanoparticles for ovarian cancer cell apoptosis. Res. Chem. Intermed. 2021, 47, 2539–2556.
- Othman, M.S.; Aboelnaga, S.M.; Habotta, O.A.; Moneim, A.E.A.; Hussein, M.M. The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells. Appl. Sci. 2023, 13, 7039.
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. A novel one-pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2016, 44, 471–477.
- Pérez Gutiérrez, R.M.; Gómez, J.T.; Martínez Jerónimo, F.F.; Paredes-Carrera, S.P.; Sánchez-Ochoa, J.C. Effects of Selenium Nanoparticles Using Potential Natural Compounds Naringenin and Baicalin for Diabetes. Biointerface Res. Appl. Chem. 2023, 13, 597.
- Wang, H.; Li, Z.Z.; Liang, X.Y. A novel zein-selenium complex nanoparticle with controllable size: Quantitative design, physical properties and cytotoxicity in vitro. Food Chem. 2023, 402, 134470.
- dos Santos Souza, L.M.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.T.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. CRGSC 2022, 5, 100303.
- Al-Saggaf, M.S.; Tayel, A.A.; Ghobashy, M.O.I.; Alotaibi, M.A.; Alghuthaymi, M.A.; Moussa, S.H. Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens. Green Process. Synth. 2020, 9, 477–487.
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.G.; Almahri, A.; Ahmed, M.K.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97.
- Ogunleye, G.; Oyinlola, K.A.; Akintade, O.; Fashogbon, R.; Adesina, T. Green synthesis, Characterization and Antimicrobial potential of Selenium Nanoparticles from Ocimum gratissimum. TURJAF 2022, 10, 2903–2912.
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX Environ. Res. 2021, 194, 110630.
- Nastulyavichu, A.; Kudryashov, S.; Smirnov, N.B.; Saraeva, I.; Rudenko, A.E.; Tolordava, E.R.; Ionin, A.; Romanova, Y.; Zayarny, D. Antibacterial coatings of Se and Si nanoparticles. Appl. Surf. Sci. 2019, 469, 220–225.
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; León-Morales, J.M. Selenium nanoparticles based on Amphipterygium glaucum extract with antibacterial, antioxidant, and plant biostimulant properties. J. Nanobiotechnol. 2023, 21, 252.
- Hernández-Díaz, J.A.; Garza-García, J.J.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial Activity of Biosynthesized Selenium Nanoparticles Using Extracts of Calendula officinalis against Potentially Clinical Bacterial Strains. Molecules 2021, 26, 5929.
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm–related infections. Pathogens 2013, 2, 288–356.
- El-Deeb, B.; Al-Talhi, A.; Mostafa, N.; Abouassy, R. Biological Synthesis and Structural Characterization of Selenium Nanoparticles and Assessment of Their Antimicrobial Properties. ASRJETS 2018, 45, 135–170.
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial activity of biogenically produced spherical Se-nanomaterials embedded in organic material against Pseudomonas aeruginosa and Staphylococcus aureus strains on hydroxyapatite-coated surfaces. Microb. Biotechnol. 2017, 10, 804–818.
- Alam, H.; Khatoon, N.; Khan, M.A.; Husain, S.A.; Saravanan, M.; Sardar, M. Synthesis of Selenium Nanoparticles Using Probiotic Bacteria Lactobacillus acidophilus and Their Enhanced Antimicrobial Activity Against Resistant Bacteria. J. Clust. Sci. 2019, 31, 1003–1011.
- Ullah, A.; Mirani, Z.A.; Binbin, S.; Wang, F.; Chan, M.W.H.; Aslam, S.; Yonghong, L.; Hasan, N.; Naveed, M.; Hussain, S.; et al. An elucidative study of the anti-biofilm effect of selenium nanoparticles (SeNPs) on selected biofilm producing pathogenic bacteria: A disintegrating effect of SeNPs on bacteria. Process Biochem. 2023, 126, 98–107.
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241.
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT 2015, 63, 1001–1007.
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893.
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39.
- Barma, M.D.; Doraikanan, S. Synthesis, characterization and antimicrobial activity of selenium nanoparticles with Clitoria ternatea on oral pathogens. Int. J. Health Sci. Res. 2022, 6, 2529–2538.
- Fardsadegh, B.; Vaghari, H.; Mohammad-Jafari, R.; Najian, Y.; Jafarizadeh-Malmiri, H. Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract. Green Process. Synth. 2019, 8, 191–198.
- Pandey, P.; Kumar, A. Antimicrobial and antioxidant activity of synthesized selenium nanoparticles against biofilm forming Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Health Sci. Res. 2022, 6, 9627–9638.
- Han, H.-W.; Patel, K.D.; Kwak, J.-H.; Jun, S.-K.; Jang, T.-S.; Lee, S.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. Selenium Nanoparticles as Candidates for Antibacterial Substitutes and Supplements against Multidrug-Resistant Bacteria. Biomolecules 2021, 11, 1028.
- Singh, N.; Saha, P.; Rajkumar, K.; Abraham, J. Biosynthesis of silver and selenium nanoparticles by Bacillus sp. JAPSK2 and evaluation of antimicrobial activity. Pharm. Lett. 2014, 6, 175–181.
- Pandiyan, I.; Sakthi, D.S.; Indiran, M.A.; Rathinavelu, P.K.; Rajeshkumar, S. Thymus vulgaris Mediated Selenium Nanoparticles, Characterization and its Antimicrobial Activity—An In Vitro Study. Int. J. Dent. Oral Sci. 2021, 8, 3516–3521.
- Wang, Q.; Wang, C.; Kuang, S.; Wang, D.; Shi, Y. Biological Selenite Reduction, Characterization and Bioactivities of Selenium Nanoparticles Biosynthesised by Pediococcus acidilactici DSM20284. Molecules 2023, 28, 3793.
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater. Res. Express 2019, 6, 0850i2.
- Rajagopal, G.; Nivetha, A.; Ilango, S.; Muthudevi, G.P.; Prabha, I.; Arthimanju, R. Phytofabrication of selenium nanoparticles using Azolla pinnata: Evaluation of catalytic properties in oxidation, antioxidant and antimicrobial activities. J. Environ. Chem. Eng. 2021, 9, 105483.
- Katyal, D.; Jain, R.K.; Sreenivasagan, S. Herbal-mediated preparation of nano-sized particles of selenium, its characterization, and evaluation of its antimicrobial activity. J. Adv. Pharm. Technol. Res. 2022, 13, S525–S529.
- Hashem, A.H.; Saied, E.; Ali, O.M. Pomegranate Peel Extract Stabilized Selenium Nanoparticles Synthesis: Promising Antimicrobial Potential, Antioxidant Activity, Biocompatibility, and Hemocompatibility. Appl. Biochem. Biotechnol. 2023, 195, 5753–5776.
- Yang, J.; Wang, J.; Yang, K.; Liu, M.; Qi, Y.; Zhang, T.; Fan, M.; Wei, X. Antibacterial activity of selenium-enriched lactic acid bacteria against common food-borne pathogens in vitro. J. Dairy. Sci. 2018, 101, 1930–1942.
- Puri, A.; Patil, S. Biogenic Synthesis of Selenium Nanoparticles using Diospyros montana Bark Extract: Characterization, Antioxidant, Antibacterial, and Antiproliferative Activity. Biosci. Biotechnol. Res. Asia 2022, 19, 423–441.
- Kazemi, M.; Akbari, A.; Sabouri, Z. Green synthesis of colloidal selenium nanoparticles in starch solutions and investigation of their photocatalytic, antimicrobial, and cytotoxicity effects. Bioprocess Biosyst. Eng. 2021, 44, 1215–1225.
- Adibian, F.; Ghaderi, R.S.; Sabouri, Z. Green synthesis of selenium nanoparticles using Rosmarinus officinalis and investigated their antimicrobial activity. Biometals 2022, 35, 147–158.
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811.
- Alam, H.; Khatoon, N.; Raza, M. Synthesis and Characterization of Nano Selenium Using Plant Biomolecules and Their Potential Applications. BioNanoScience 2019, 9, 96–104.
- Sneka, S.; Santhakumar, P. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. RJPT 2021, 14, 4452–4454.
- Hassan, A.A.; Yousif, M.H.; Abd-Elkhaliq, H.M.M.; Wahba, A.K.A.; El-Hamaky, A.M.a. The Antimicrobial Potential of Selenium Nanoparticles Singly and in Combination with Cinnamon Oil Against Fungal and Bacterial Causes of Diarrhea in Buffaloes. Adv. Anim. Vet. Sci. 2021, 9, 1238–1248.
- El Saied, B.E.F.; Diab, A.M.; Tayel, A.A.; Alghuthaymi, M.A.; Moussa, S.H. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021, 10, 49–60.
- Indhira, D.; Aruna, A.; Manikandan, K.; Albeshr, M.F.; Alrefaei, A.F.; Vinayagam, R.; Kathirvel, A.; Priyan, S.R.; Kumar, G.S.; Srinivasan, R. Antimicrobial and Photocatalytic Activities of Selenium Nanoparticles Synthesized from Elaeagnus indica Leaf Extract. Processes 2023, 11, 1107.
- Hariharan, H.; Al-Dhabi, N.A.; Karuppiah, P.; Rajaram, S.K. Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515.
- Vyasa, J.; Rana, S. Green Synthesis of Selenium Nanoparticles using Allium Sativum Extract and Its Antimicrobial Activity Against Gram Negative Bacteria. IJPBSTM 2019, 9, 350–356.
- Mulla, N.A.; Otari, S.V.; Bohara, R.A.; Yadav, H.M.; Pawar, S.H. Rapid and Size-Controlled Biosynthesis of Cytocompatible Selenium Nanoparticles by Azadirachta indica Leaves Extract for Antibacterial Activity. Mater. Lett. 2020, 264, 127353.
- Alagesan, V.; Venugopa, S. Green Synthesis of Selenium Nanoparticle Using Leaves Extract of Withania somnifera and Its Biological Applications and Photocatalytic Activities. BioNanoScience 2019, 9, 105–116.
- Saied, E.; Mekky, A.E.; Al-Askar, A.A.; Hagag, A.F.; El-bana, A.A.; Ashraf, M.; Walid, A.; Nour, T.; Fawzi, M.M.; Arishi, A.A.; et al. Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals 2023, 13, 450.
- Ebrahimzadeh, M.A.; Moradsomarein, M.; Lalerdi, F.S.; Alizadeh, S.R. Biogenic synthesis of selenium nanoparticles using Hibiscus esculentus L. extract: Catalytic degradation of organic dye and its anticancer, antibacterial and antifungal activities. Eur. J. Chem. 2023, 14, 144–154.
- Rajashree, R.; Sathyamoorthy, G.; Gobalakrishnan, M. Marine biomolecule mediated synthesis of selenium nanoparticles and their antimicrobial efficiency against fish and crustacean pathogens. Res. Sq. 2022.
- Nile, S.H.; Thombre, D.; Shelar, A.; Gosavi, K.; Sangshetti, J.; Zhang, W.; Sieniawska, E.; Patil, R.; Kai, G. Antifungal Properties of Biogenic Selenium Nanoparticles Functionalized with Nystatin for the Inhibition of Candida albicans Biofilm Formation. Molecules 2023, 28, 1836.
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, Antiviral, and In-Vitro Cytotoxicity and Mosquitocidal Activities of Portulaca oleracea-Based Green Synthesis of Selenium Nanoparticles. J. Funct. Biomater. 2022, 13, 157.
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B. Using Nano-Selenium to Combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037.
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456.
- Liu, X.; Chen, D.; Su, J.; Zheng, R.; Ning, Z.; Zhao, M.; Zhu, B.; Li, Y. Selenium nanoparticles inhibited H1N1 influenza virus-induced apoptosis by ROS-mediated signaling pathways. RSC Adv. 2022, 12, 3862–3870.
- Zhong, J.; Xia, Y.; Hua, L. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3485–3491.
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C. Inhibition of H1N1 Influenza Virus-Induced Apoptosis by Functionalized Selenium Nanoparticles with Amantadine through ROS-Mediated AKT Signaling Pathways. Int. J. Nanomed. 2018, 13, 2005–2016.
- Ullah, A.; Mu, J.; Wang, F.; Chan, M.W.H.; Yin, X.; Liao, Y.; Mirani, Z.A.; Sebt-e-Hassan, S.; Aslam, S.; Naveed, M.; et al. Biogenic Selenium Nanoparticles and Their Anticancer Effects Pertaining to Probiotic Bacteria—A Review. Antioxidants 2022, 11, 1916.
- Menon, S.; Devi Ks, S.; Santhiya, R.; Rajeshkumar, S.; Kumar, S.V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292.
- Chen, T.; Wong, Y.S.; Zheng, W.; Bai, Y.; Huang, L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf. B Biointerfaces 2008, 67, 26–31.
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B 2019, 191, 123–127.
- Vundela, S.R.; Kalagatur, N.K.; Nagaraj, A.; Kadirvelu, K.; Chandranayaka, S.; Kondapalli, K.; Hashem, A.; Abd Allah, E.F.; Poda, S. Multi-Biofunctional Properties of Phytofabricated Selenium Nanoparticles from Carica papaya Fruit Extract: Antioxidant, Antimicrobial, Antimycotoxin, Anticancer, and Biocompatibility. Front. Microbiol. 2022, 12, 769891.
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer Activity of Biogenic Selenium Nanoparticles: Apoptotic and Immunogenic Cell Death Markers in Colon Cancer Cells. Cancers 2021, 13, 5335.
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835.
- Krishnan, V.; Loganathan, C.; Thayumanavan, P. Green synthesized selenium nanoparticle as carrier and potent delivering agent of s-allyl glutathione: Anticancer effect against hepatocarcinoma cell line (HepG2) through induction of cell cycle arrest and apoptosis. Drug Deliv. Sci. Technol. 2019, 53, 101207.
- Anu, K.; Devanesan, S.; Prasanth, R.; AlSalhi, M.S.; Ajithkumar, S.; Singaravelu, G. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud Univ. Sci. 2020, 32, 2520–2526.
- Forootanfar, H.; Adeli-Sardou, M.; Nikkhoo, M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014, 28, 75–79.
- Alizadeh, S.R.; Abbastabar, M.; Nosratabadi, M.; Ebrahimzadeh, M.A. High antimicrobial, cytotoxicity, and catalytic activities of biosynthesized selenium nanoparticles using Crocus caspius extract. Arab. J. Chem. 2023, 16, 104705.
- Srivatchava, S.; Roy, A.; Kumar, S. Biosynthesis of Selenium Nanoparticles Using Cassia Oleoresin and Its Anticancer Activity Against Liver Cancer Cell Lines. J. Complement. Med. Res. 2021, 12, 48.
- Gharbavi, M.; Mousavi, M.; Pour-Karim, M.; Tavakolizadeh, M.; Sharafi, A. Biogenic and facile synthesis of selenium nanoparticles using Vaccinium arctostaphylos L. fruit extract and anticancer activity against in vitro model of breast cancer. Cell Biol. Int. 2022, 46, 1612–1624.
- Afzal, B.; Naaz, H.; Ahmedi, S.; Zeya, B.; Imtiyaz, K.; Yasin, D.; Sami, N.; Gogry, F.A.; Rizvi, M.A.; Manzoor, N.; et al. Biosynthesis, characterization and biomedical potential of Arthrospira indica SOSA-4 mediated SeNPs. Bioorg. Chem. 2022, 129, 106218.
- Rajasekar, S.; Kuppusamy, S. Eco-Friendly Formulation of Selenium Nanoparticles and Its Functional Characterization against Breast Cancer and Normal Cells. J. Clust. Sci. 2021, 32, 907–915.
- Toubhans, B.; Gazze, S.A.; Bissardon, C.; Bohic, S.; Gourlan, A.T.; Gonzalez, D.; Charlet, L.; Conlan, R.S.; Francis, L.W. Selenium nanoparticles trigger alterations in ovarian cancer cell biomechanics. Nanomedicine 2020, 29, 102258.
- Alhawiti, A.S. Citric acid-mediated green synthesis of selenium nanoparticles: Antioxidant, antimicrobial, and anticoagulant potential applications. Biomass Convers. Biorefin. 2022.
- Bapte, P.; Pansambal, S.; Borgave, S. Biosynthesized Selenium Nanoparticles: An Excellent Bait for Antioxidant Therapy. J. Nanostruct. 2022, 12, 178–193.
- Xu, C.; Qiao, L.; Ma, L.; Yan, S.; Guo, Y.; Dou, X.; Zhang, B.; Roman, A. Biosynthesis of Polysaccharides-Capped Selenium Nanoparticles Using Lactococcus lactis NZ9000 and Their Antioxidant and Anti-inflammatory Activities. Front. Microbiol. 2019, 10, 1632.
- Cheng, Y.; Xiao, X.; Li, X.; Song, D.; Lu, Z.; Wang, F.; Wang, Y. Characterization, antioxidant property and cytoprotection of exopolysaccharide-capped elemental selenium particles synthesized by Bacillus paralicheniformis SR14. Carbohydr. Polym. 2017, 15, 18–26.
- Al-Quraishy, S.; Dkhil, M.A.; Abdel Moneim, A.E. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756.
- Anuse, S.S.; Sumathi, V.; Uma, C.; Sangeetha, D.; Sivagurunathan, P.; Kumar, D.J.M. Antidiabetic effect of Acacia catechu mediated selenium nanoparticles. Uttar Pradesh Zool. Soc. 2022, 43, 115–120.
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticle with a polysaccharide from Rosa roxburghii fruit and its protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553.
- Fan, D.; Li, L.; Li, Z. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514.
- Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Al-Bagawi, A.H.; Fareid, M.A.; Hameed, R.A.; Mohamed, K.M.; Abdelfattah, M.S.; Fehaid, A.; Hussein, M.M.; et al. Green Synthetized Selenium Nanoparticles Using Syzygium aromaticum (Clove) Extract Reduce Pentylenetetrazol-Induced Epilepsy and Associated Cortical Damage in Rats. Appl. Sci. 2023, 13, 1050.
- Zhu, C.; Zhang, S.; Song, C. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20.
- Bano, I.; Skalickova, S.; Arbab, S.; Urbankova, L.; Bano, P.H. Toxicological effects of nanoselenium in animals. J. Anim. Sci. Biotechnol. 2022, 13, 72.
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63.
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-Synthesis of Selenium Nanoparticles Using Garlic Cloves (Allium sativum): Biophysical Characterization and Cytotoxicity on Vero Cells. J. Clust. Sci. 2017, 28, 551–563.
- Cremonini, E.; Zonaro, E.; Donini, M. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771.
- Wang, R.; Ha, K.Y.; Dhandapani, S.; Kim, Y.J. Biologically synthesized black ginger-selenium nanoparticle induces apoptosis and autophagy of AGS gastric cancer cells by suppressing the PI3K/Akt/mTOR signaling pathway. J. Nanobiotechnol. 2022, 20, 441.
- Naemi, S.; Meshkini, A. Phytosynthesis of graphene oxide encapsulated selenium nanoparticles using Crocus sativus petals’ extract and evaluation of their bioactivity. Drug Deliv. Sci. Technol. 2023, 81, 104286.
- Touliabah, H.E.; El-Sheekh, M.M.; Makhlof, M.E.M. Evaluation of Polycladia myrica mediated selenium nanoparticles (PoSeNPS) cytotoxicity against PC-3 cells and antiviral activity against HAV HM175 (Hepatitis A), HSV-2 (Herpes simplex II), and Adenovirus strain 2. Front. Mar. Sci. 2022, 9, 1092343.
- Yazhiniprabha, M.; Vaseeharan, B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater. Sci. Eng. C 2019, 103, 109763.
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598.
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415.
- Wang, J.; Zhang, Y.; Yuan, Y.; Yue, T. Immunomodulatory of Selenium Nano-Particles Decorated by Sulfated Ganoderma Lucidum Polysaccharides. Food Chem. Toxicol. 2014, 68, 183–189.
- Wang, Y.-Y.; Qiu, W.-Y.; Sun, L.; Ding, Z.-C.; Yan, J.-K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide–protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184.
- Lin, X.; Mu, J.; Chen, Z.; Zhang, Y.; Ye, X.; Gao, X.; Chen, W.; Luo, Y.; Li, B. Stabilization and functionalization of selenium nanoparticles mediated by green tea and Pu-Erh tea polysaccharides. Ind. Crops Prod. 2023, 194, 116312.
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620.
- Buacheen, P.; Chaipuang, A.; Karinchai, J.; Nuchuchua, O.; Imsumran, A.; Wongnoppavich, A.; Pimpha, N.; Pitchakarn, P. Stabilization of Antioxidant and Anti-Inflammatory Activities of Nano-Selenium Using Anoectochilus burmannicus Extract as a Potential Novel Functional Ingredient. Nutrients 2023, 15, 1018.
- Bulgarini, A.; Lampis, S.; Turner, R.J.; Vallini, G. Biomolecular composition of capping layer and stability of biogenic selenium nanoparticles synthesized by five bacterial species. Microb. Biotechnol. 2021, 14, 198–212.
- Sonkusre, P.; Singh Cameotra, S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces 2015, 136, 1051–1057.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





