Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Teresa Lopes da Silva | -- | 5440 | 2023-12-21 12:24:41 | | | |
| 2 | Peter Tang | Meta information modification | 5440 | 2023-12-22 02:18:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lopes Da Silva, T.; Fontes, A.; Reis, A.; Siva, C.; Gírio, F. Oleaginous Yeast Biorefinery. Encyclopedia. Available online: https://encyclopedia.pub/entry/53031 (accessed on 07 February 2026).
Lopes Da Silva T, Fontes A, Reis A, Siva C, Gírio F. Oleaginous Yeast Biorefinery. Encyclopedia. Available at: https://encyclopedia.pub/entry/53031. Accessed February 07, 2026.
Lopes Da Silva, Teresa, Afonso Fontes, Alberto Reis, Carla Siva, Francisco Gírio. "Oleaginous Yeast Biorefinery" Encyclopedia, https://encyclopedia.pub/entry/53031 (accessed February 07, 2026).
Lopes Da Silva, T., Fontes, A., Reis, A., Siva, C., & Gírio, F. (2023, December 21). Oleaginous Yeast Biorefinery. In Encyclopedia. https://encyclopedia.pub/entry/53031
Lopes Da Silva, Teresa, et al. "Oleaginous Yeast Biorefinery." Encyclopedia. Web. 21 December, 2023.
Copy Citation
Oleaginous yeasts are a potential renewable source of biofuels. However, the yeast-derived biofuels cost is still non-competitive with the fossil fuel prices. To improve the sustainability of yeast-derived biofuels, it is necessary to valorize all yeast biomass fractions, an approach based on the biorefinery concept.
oleaginous yeast
biorefinery
lipids
biofuels
bioproducts
1. Introduction
The increase in the world population has increased the energy demand required to respond to the population’s needs. Fossil fuels currently supply about 80% of the world’s energy. However, this energy source is non-renewable, and the reserves are diminishing. In addition, fossil fuel combustion increases greenhouse gases emissions and the emission of other pollutants, negatively affecting the climate and human health. Also, the geopolitical contexts concerning the main fossil fuel producers generate instability and uncertainty around the world.
Replacing fossil fuels with clean and renewable forms of energy is vital to ensure the sustainability, safety and health of future generations.
Microorganisms have been used as a source of biofuels and bioproducts that are useful for humanity. However, it is well known that the use of microorganisms as source of biofuels is still not economically sustainable as its price remains higher than the price of fossil fuels.
Biorefineries are described as “the sustainable processing of biomass into a spectrum of marketable products (food, feed, materials, chemicals) and energy (fuels, power, heat)” [1].
Therefore, a holistic view of biofuels and bio-compounds production from microbes, based on the biorefinery concept, is urgently needed in order to achieve sustainable biofuels and bioproducts by taking advantage of all microbial biomass fractions and products synthesized by the microorganisms. This may boost the value and profit obtained from the process while also achieving a desired minimum environmental impact. In this way, the economics of the process is enhanced.
In recent years, autotrophic microalgae biorefineries have been intensively studied [2][3][4]. These microorganisms produce various macromolecules that have many applications in industry, including proteins, carbohydrates, pigments, polyunsaturated fatty acids, peptides, exo-polysaccharides (EPS), etc.; these macromolecules may be co-extracted during processing [5]. Autotrophic microalgae need light as an energy source and carbon dioxide (CO2) as carbon source to grow. Due to their capacity to fix carbon dioxide (CO2), these microorganisms contribute to the reduction of greenhouse gas (GHG) emissions. However, the light-dependency of the microalgal cultivations requires expensive and specific equipment design, increasing the process costs at large-scale. In addition, when using low-cost feedstock, such as industrial effluents and waste streams, as substrates for microalgal growth, with these materials usually containing particles and dust, light penetration is hampered due to the shading effect, resulting in low biomass production, leading to low amounts of intracellular products. Moreover, autotrophic microalgae cultivation technology, being light and temperature dependent, is not suitable in areas of high latitude, where most seasons are marked by low temperatures and low insolation (such as in Northern Europe and North America).
Unlike autotrophic microalgae, heterotrophic microorganisms need organic compounds such as carbon as an energy source to grow, and do not require light as an energy source; this reduces the equipment requirements costs. In addition, they grow in conventional bioreactors that are easily scaled-up, and are operated under strictly controlled conditions, which reduces the chance of contamination. Importantly, heterotrophic cultures can attain high cell densities and product productivities and are more efficient in consuming organic carbon, nitrogen and phosphorous compounds than autotrophic cultures [6].
Yeasts are single-cell fungi that grow heterotrophically; they are widely distributed in soil, light water, marine environments and the surface and bodies of various organisms. These microorganisms preferentially metabolize sugars as carbon sources, but they can also utilize a wide range of carbon sources, including amino and organic acids, polyols, alcohols, fatty acids and other compounds, depending on the species. They are resistant to acidic environments, high osmotic pressure and temperature and show high metabolic efficiency. As a result, they can adapt to a variety of adverse environments, making them versatile microorganisms.
Oleaginous microorganisms, also called single cell oils (SCO), can accumulate, intracellularly, more than 20% of their dry weight; as such, they are considered promising microbial platforms for sustainable bio-compounds and biofuels. Compared to other oleaginous microorganisms, such as filamentous fungus and microalgae, yeast show more desirable characteristics because yeast cells are unicellular and may display high growth rates and high cellular lipid content [7]. Unlike autotrophic microalgae, yeast cultivation does not require land use change and does not compete with any agricultural activity. They can utilize low-cost substrates such as industrial effluents, wastes and residues to produce triglycerides (TAGs) [8][9], which are chemically equivalent to oils produced from edible crops, making them alternative edible oils for food industry, as well as substrates used in synthesis of the oleochemicals such as fuels, soaps, plastics, paints, detergents, textiles, rubber, surfactants, lubricants, additives for the food and cosmetic industry and many other chemicals [9]. Like oleaginous microalgae, oleaginous yeast can grow on low-cost substrates, such as industrial effluents and byproducts, producing intracellular products including carbohydrates, proteins, lipids and pigments with commercial interest. There are several studies describing lipid production from oleaginous yeasts for biofuels [10][11][12].
2. Low-Cost Feedstock for Oleaginous Yeast Production
Oleaginous yeasts are characterized by accumulating more than 20% of their dry cell (DCW) weight as lipids. Among more than 600 known yeasts species, only 30 show this characteristic. Several of the more promising yeasts in terms of lipid production belong to the genera Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus, Trichosporon and Lipomyces; the most studied oleaginous species are Y. lipolytica, L. starkey, R. toruloides, Rhodotorula glutinis, Trichosporon fermentans and Cryptococcus curvatus [13].
The type of substrates used to grow the yeasts contribute to the overall costs and the environmental impact of the process. It is desirable to use low-cost feedstock such as industrial byproducts, wastes or lignocellulosic materials. Different types of feedstocks have been used to grow oleaginous yeast. However, glucose remains the most used substrate to grow these microorganisms despite being an expensive carbon source, which increases the costs of the overall process [14]. Therefore, efforts must be made to find low-cost substrates for yeast lipid production and improve the conversion efficiently of these substrates into intracellular lipids.
2.1. Wastewater
Nowadays, biological wastewater (WW) treatment is well accepted as it is considered to be more environmentally friendly and cost effective than chemical treatments. The use of oleaginous yeasts in biological treatment of wastewater is very attractive when compared to traditional aerobic and anaerobic digestion technologies, which require highly sophisticated and expensive systems such as up-flow anaerobic sludge blanket digestion or expanded granular sludge bed digestion [15].
There are several oleaginous yeast strains that can grow in different types of WW. For instance, Trichosporon cutaneum ACCC 20271 was able to grow on cellulosic ethanol fermentation WW containing glucose, xylose, acetic acid, ethanol and part of the phenolic compounds. When grown in a 3-L bioreactor, COD (chemical oxygen demand) was reduced by 55.1%, and the yeast cells produced 13.3% (w/w) of lipid content, corresponding to 2.16 g/L of lipids and 16.7 mg/Lh of lipid productivity [16].
Other WW types have also been used to grow oleaginous yeasts. WW resulted from butanol fermentation with high COD content, containing acetic and butyric acids and residual sugars as xylose and arabinose. This was used to produce intracellular lipids by the oleaginous Trichosporon dermatis [17]. After five days of cultivation, the COD removal rate was notably high (68%), while the yeast biomass and lipid concentration attained 7.4 g/L and 13.5% (w/w), respectively.
2.2. Agri-Food Industry Wastes
The agri-food industry produces large amounts of waste and residues. Its sustainability depends on the efficient management of these residues, aiming towards their valorization. This strategy represents a method for converting low-value feedstock into high value products, addressing one of the main goals of the circular economy: reduction of waste by recycling.
Sugarcane is used for sugar production. Molasses is the main byproduct from the sugar industry, and primarily contains sucrose, with smaller amounts of other sugars, proteins, minerals, vitamins, amino acids and antioxidants [18][19]. It has been successfully used as carbon source in media formulations for oleaginous yeasts growth in several studies. Lakshmidevi et al. [19] studied the growth of two yeast strains, Rhodosporodium toruloides and Rhodotorula glutinis, grown on glucose yeast extract mineral medium (GYM) and molasses medium for comparison purposes. While the lipid content was higher when the two yeasts were grown on the molasses medium, the carotenoid content was higher when the yeasts were grown on GYM.
Waste from the food industry has also been used as feedstock for oleaginous yeast growth. The yeast Rhododporidium azoricus DBVPG 4620 was cultivated on pumpkin peel wastes hydrolysate without the addition of nutrients. To enhance the lipid accumulation, a two-stage process was performed in a 2L-bioreactor, using, in a sequential way, an addition food waste, a syrup derived from candied fruits manufacture, rich in available sugars, without any pre-treatment. The yeast culture achieved 0.45 g/L biomass with 55% of lipids, and a lipid concentration and productivity of 24 g/L and 0.26 g/Lh, respectively [20].
Whey permeate was also used to produce microbial lipids by the yeast Apiotrichum curvatum (synonym Cryptococcus curvatus), reporting a lipid content and productivity of 50% (w/w) and 2 g/Lh, respectively, in a partial recycling culture [21].
Half of the total global biomass on the planet is composed of lignocellulosic biomass. This has been considered as a possible feedstock for biofuels production from microorganisms since it is abundant and not food competitive. Cellulose, hemicellulose and lignin, at various proportions, are the main polymers that compose the lignocellulosic biomass. The first two are composed of sugar polymers, which may be converted to sugar monomers such as glucose and xylose after a hydrolysis reaction step.
2.3. Crude Glycerol
The biodiesel industry generates raw glycerol as byproduct. Its discharge in the environment is a serious threat [22]. In addition, crude glycerol recovery and purification from the industrial biodiesel process is expensive. Previously, only pure glycerol was used as the carbon source in media formulations, since the impurities present in crude glycerol (methanol, ethanol, salts, metals and soaps) can inhibit the growth of some microorganisms [23][24]. However, purification of crude glycerol is a difficult task; hence, its utilization without any treatment is a value-added approach. In addition, the biological conversion of glycerol impurities is a viable way to enhance the economics of the overall process. Indeed, the utilization of crude glycerol has many advantages in microbial fermentations, without the requirement for any purification step. Low-cost, greater degree of reduction, higher availability and less CO2 emitted during the fermentations are advantages of the crude glycerol when used as carbon source for microbial growth when compared to sugars. In addition, glycerol shows a higher NADH generation rate and degree of reduction [25]. Strains from the genera Trichosporonoides, Rhodosporidium, Candida, Rhodotorula, Lipomyces, Schizosaccharomyces, Yarrowia and Cryptococcus can grow on glycerol [25][26] but other genera have been used. Kumar et al. [27] used the yeast Pichia guilliermondii to grow on a medium containing crude glycerol, CSL and mineral salt. Polburee et al. [28] studied 23 oleaginous yeast strains grown on a complex medium containing crude glycerol in shaking flasks. The ascomycetous species Pichia manshurica, Kodamaea ohmeri, Candida silvae and Meyerozyma caribbica, and the basidiomycetous species Rhodotorula taiwanensis, Sporidiobolus ruineniae, Cryptococcus laurentii, Cryptococcus cf. podzolicus and Rhodosporidium fluviale, displayed at least 20% (w/w) lipid content. The yeast strain that achieved the highest lipid content was Rhodosporidium fluviale DMKU-RK253, with 65.2% w/w, corresponding to a lipid concentration of 3.9 g/L.
2.4. Hydrophobic Wastes
Hydrophobic wastes, such as volatile fatty acids (VFAs), are obtained during anaerobic fermentation (AF), a simple method that transforms organic wastes into a digestate containing organic acid compounds of carbon length C3–C5, which can be used as carbon source for yeast lipid production [25][29]. The use of these organic acids as carbon source for the yeast growth may be a sustainable strategy for the concomitant waste treatment and yeast lipid production because it might improve the overall process from the economic and environmental point of view. In addition, economic studies demonstrated that VFAs obtained from the AF of food wastes cost 27.6 EUR/ton, less than 10% of the price of 1 ton of glucose [30]. Furthermore, when compared to hexoses carbon sources metabolism, VFAs show higher theoretical conversion efficiencies and shorter metabolic pathways to lipid production [31]. Therefore, VFAs are considered to be a promising alternative carbon source for microbial lipids production. Some oleaginous yeast strains such as Yarrowia lipolytica, Cryptococcus curvatus and Cryptococcus albidus can grow on VFAs [30]. Acidic conditions are commonly adopted to grow oleaginous yeast on VFAs because they are favorable to yeast cultivation. However, under an acidic pH environment, VFAs are largely in the undissociated form, which is toxic for microbial cells [32]. To overcome this limitation, Gao et al. [31] used alkaline conditions to grow Yarrowia lipolytica on food wastes and fruit and vegetables wastes after AF at pH = 6.0, 7.0 and 8.0, in order to alleviate the severe inhibition resulting from the presence of high-content VFAs. The highest biomass and lipid production was achieved on FVW fermentate, at pH 8 (11.84 g/L and 3.08 g/L, respectively), with a lipid content of 26.02% (w/w). In addition, Llamas et al. [28] studied the growth of five yeast strains (Cutaneotrichosporon curvatum NRRL-Y-1511, Lipomyces lipofer NRRL-Y-11555, Rhodotorula (Rhodosporidium) toruloides NRRL-Y-27012, Cyberlindnera (Williopsis) saturnus NRRL-Y-17396 and Yarrowia lipolytica ACA DC 5010) in VFAs-rich digestate of Chlorella vulgaris biomass at three different VFAs concentrations. At pH 6.5., C. curvatum showed the highest lipid production (36.9% w/w), with a lipid yield of 0.11 g/g of VFAs, similar to the yield obtained with sugar-based media.
3. Lipid Production by Oleaginous Yeasts
Lipids produced by yeasts are complex molecules such as free fatty acids (FFA), sterols, polyprenols, phospholipids, glycolipids, sphingolipids, mono-, di-, tri-acylglycerols and carotenoids. However, not all of these molecules are present in all oleaginous yeast species. The major lipid compounds present in yeasts are triacylglycerols, while mono-, di-acylglycerols, FFA, steryl esters and carotenoids are present in lower proportions [33]. The de novo TAGs synthesis occurs whenever the yeast cells are exposed to carbon excess conditions, as well as to the depletion of a specific nutrient (usually nitrogen). Under these circumstances, cells canalize the excess carbon towards lipid synthesis instead of cell division. In addition, under nitrogen limiting conditions, the concentration of adenosine monophosphate (AMP) is decreased due to its cleavage by AMP-deaminase and NAD+-isocitrate dehydrogenase (NAD+-ICDH) inhibition, resulting in the accumulation of citrate in the mitochondria. Citrate is then transferred to the cytoplasm where it is hydrolyzed by the enzyme ATP-citrate lyase (ACL), considered the key for the lipid synthesis in oleaginous microorganisms [34]. In the fatty acid synthase complex (FAS), the acetyl-CoA is used for de novo fatty acids synthesis; the resulting products (palmitoyl-CoA, and stearoyl-CoA) are transferred to the endoplasmic reticulum, in which they are converted into triacylglycerols (TAGs) or, through NADPH-dependent desaturation and or/an elongation, before being used for TAGs synthesis through the Kennedy pathway, to produce lysophosphatidic acid (LPA), phosphatidic acid (PA) and diacylglycerol (DAG). Lastly, the TAGs are intracellularly stored as lipid droplets. According to Ratledge [35], the NADPH requested for lipid production in oleaginous microbes is provided in several ways, such as NADP+, the oxidative pentose phosphate pathway and the malic enzyme.
When fats or other hydrophobic compounds are used as the sole carbon and energy source, ex novo lipid synthesis occurs. Typically, these substrates are fatty acids (FA) and TAG, which may be used as an energy source or modified by enzymes [36]. The free fatty acids (FFA) available as a substrate or resulting from a lipase-catalyzed hydrolysis of the TAGs are transported, by active transport, into the cells, wherein they are assimilated for growth or are accumulated as lipid droplets [37]. It should be noted that, when the yeast cells synthesize ex novo lipids, lower quantities of TAGs are obtained when compared with the de novo yeast lipid synthesis based on sugar-based substrates [38].
4. Oleaginous Yeast Biorefinery
A microbial biorefinery starts with the biomass production by fermentation. The yeast biomass will be further processed to obtain a wide range of bioproducts (Figure 1):
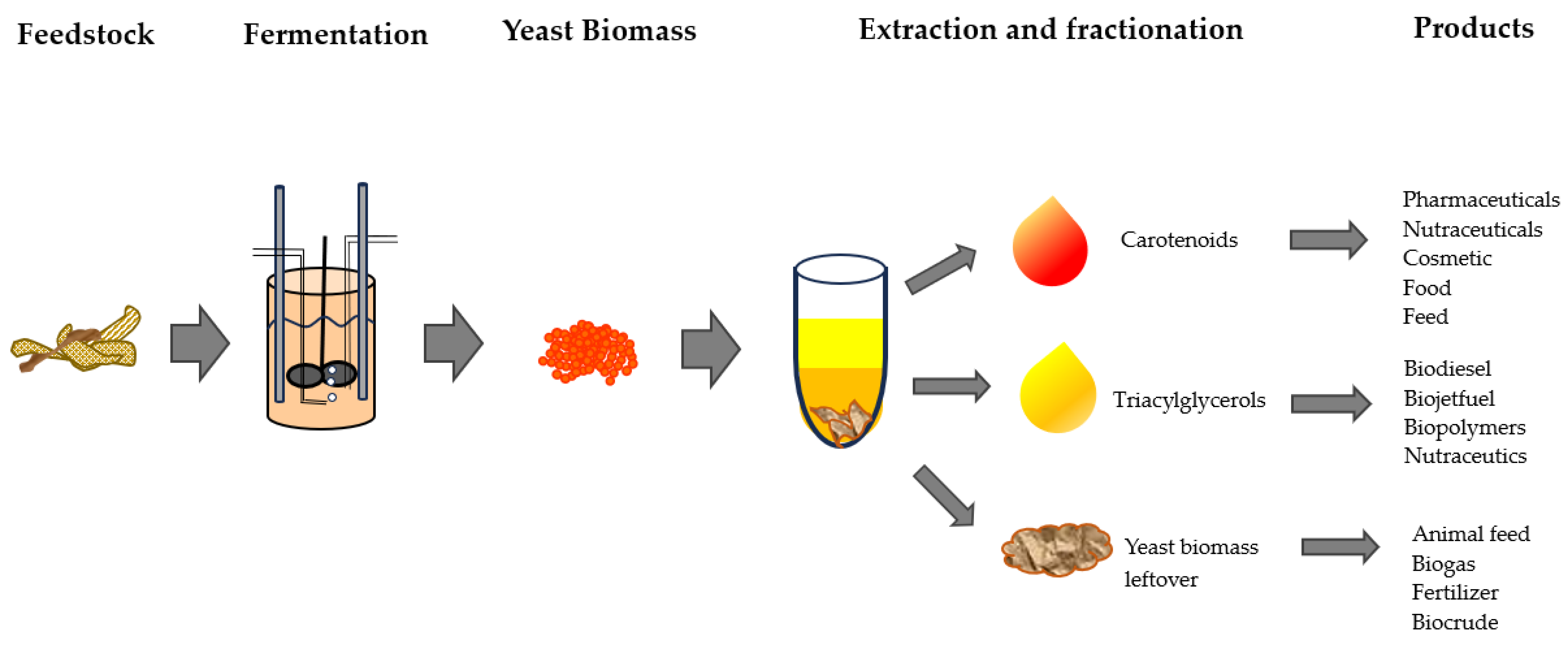
Figure 1. Oleaginous yeast biorefinery.
4.1. Yeast Cultivation Modes
There are several different modes to produce lipids from yeasts. The review of Karamerou and Webb [39] presented a complete description of all the cultivation modes that can be used to grow oleaginous yeasts, explaining the advantages and drawbacks of each one, i.e., the batch mode, fed-batch cultivation, fed-batch cultivation with pulse medium addition, fed-batch cultivation with continuous medium supply, continuous cultivation, repeated batch, two-stage batch cultivation and two-stage cultivation with feed supply. The authors conclude that, among all these cultivation modes, the two-stage cultivation is the most efficient in terms of lipid production since it allows higher cell and lipid yields to be obtained and is an easy technology to scale-up. Poontawee et al. [14] also reported that the most effective way to produce lipids from yeasts is the two-stage cultivation, in which two sequential phases are established. In the first phase, the cells experience nutrient excess conditions to promote cell growth; in the second phase, the cells are exposed to carbon excess and nitrogen limiting conditions so that they will use the carbon for the purposes of lipid storage synthesis instead of cell division, since the lack of nitrogen for de novo protein and nucleotide synthesis halts the cell growth [40]. Some authors have used different conditions in the two stages to improve the lipid production. Qian et al. [41] used different carbon sources in the two stages: glucose was used in the first stage, and a VFA mixture in the second stage. In addition, the optimal conditions for cell growth may not be the same for lipid synthesis. Based on this, Polburee et al. [42] and Polburee and Limting [43] used a lower temperature (optimal for lipid synthesis) during the second stage, resulting in an improved lipid concentration. A two-stage cultivation strategy for lipid and carotenoid by the strain R. glutinis CGMCC No. 2258 was described by Zhang et al. [44]. The first grow stage was carried out under irradiation/high temperature and the second stage was conducted under dark/low temperature conditions, to improve lipid production. Dias et al. [45] found that the optimal pH for R. toruloides NCYC 921 biomass and fatty acids production was 4.0, and for carotenoid production was 5.0. Based on this observation, the authors used pH 4.0 for the first stage and pH 5.0 for the second stage; this resulted in an increase in the carotenoid content by 51% when compared to the yeast growth conducted at a constant medium pH.
When growing oleaginous yeast cells on lignocellulosic feedstock, two different strategies are possible: separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). Grubisic et al. [46] studied both approaches. They added a recycle cellulase step in the SHF and carried out the SSF in batch and fed-batch mode. The authors also added Tween 80 to the lignocellulosic slurry to improve the hydrolysis rate, having observed that the Tween 80 presence improved the lipid yield without affecting the yeast growth. The authors concluded that the fed-batch SFF is the most efficient and economical strategy. However, particular attention must be paid to SSF cultures since the lignocellulosic slurry particles will be mixed with the yeast cells, which may misestimate the biomass and products quantification.
Other strategies have been used to improve the lipid production by oleaginous yeasts. Several authors have reported the use of oleaginous yeast and microalgae mixed cultures to boost lipid production. In fact, the complementary metabolisms of yeast and microalgae (heterotrophic/autotrophic, respectively) improve the yeast growth and lipid production. Microalgal cells require CO2 and sunlight to grow, producing intracellular lipids while producing oxygen (O2) throughout the process of photosynthesis [47]. On the contrary, oleaginous yeasts require organic carbon and O2 to grow. Therefore, when both microorganisms are grown together in mixed cultures, yeast will have an additional supply of O2 produced by microalgae, while the latter will have an additional supply of CO2 produced by the yeast cells when compared to the O2/CO2 availabilities existing in yeast/microalgae pure cultures carried out under the same conditions. In addition, in mixed cultures, the medium pH usually increases due to the microalgae metabolism, which may mitigate the acidic environment that the yeast cells are usually exposed to when grown in pure cultures. In addition, due to the medium pH auto-adjustment that occurs during the evolution of yeast-microalgae mixed cultures, no chemicals or pH control equipment are needed to control the mixed culture medium pH, thereby reducing the process costs. Dias et al. [48] reported an increase of 36% in R. toruloides NCYC 921 lipid content (26.3% w/w) when the yeast was grown together with the microalga T. obliquus on primary brewery effluent supplemented with 100 g/L of sugarcane molasses and 2 g/L of urea when compared to the respective yeast pure culture (19.4% w/w).
4.2. Yeast Cultures Monitoring Techniques
During any bioprocess development, it is essential to monitor several culture parameters, such as biomass concentration and substrate consumption, to evaluate process performance and, if possible, to adjust the process control strategy, aiming at improving the product yields.
The most used analytical methods for microbial growth monitoring are dry cell weight (DCW) measurement and colony forming units (cfu) determination. However, these results are only available a considerable time after the sample harvesting, often after the process conclusion. Optical density (OD) is also used as a fast technique for cell concentration quantification. However, it gives limited information since it does not provide knowledge on the cell status or metabolism. In addition, it assumes a linear correlation between the OD and DCW, which is not possible to ensure throughout the cultivation, particularly when the cells attained the stationary phase as they become smaller [49].
In addition, stress conditions such as nutrient limitation, inefficient aeration and mixing and changes in the oxygen tension and pH often occur during yeast cultivations, from laboratory to large scale production, resulting environmental heterogeneities that will affect the cell physiological status, thereby reducing product quantity and quality. Furthermore, these stress conditions may damage or kill the yeast cells, which may lead to a large proportion of dead or dormant cells (which are partially or completely metabolically inactive) in the broth, thereby affecting culture performance.
Flow cytometry (FC) is a powerful technique for at-line monitoring cell status and growth during microbial cultures, giving information at the single-cell level. FC allows cell counting and physiological cell status detection at near real time (at-line). This information, available soon after the sample harvesting, is crucial since it allows for us to understand the cell stress response to the environment; it also allows the process control strategy to be changed during the cultivation course (by changing the feed strategy, speed/aeration rate, medium composition, etc.) in order to reduce the proportion of stressed/dead cells and improve process efficiency.
Yeasts are particularly suitable for FC analysis since the cells are large enough to be discriminated from the background (cell debris and undesirable particles) in contrast to bacteria, which are barely discriminated. In addition, a few yeast strains produce intracellular carotenoids, thereby allowing the yeast cells to be easily identified. This is similar to autotrophic microalgae, which produce chlorophyll, that are also easily identified.
FC has also been used to monitor yeast and microalgae mixed cultures. As yeast and microalgae cells have different shapes and sizes, FC allows yeast to be differentiated from microalgae cells, giving information on each microbial population in the mixed culture [47]. Beyond cell counting and status detection, FC can provide at-line quantification of a few intracellular products such as lipids [48][50] and carotenoids [51], providing the results immediately after sample collection. This allows for sample collection at the optimal cultivation time. Ami et al. [52] studied a method based on Fourier transform infrared spectroscopy (FTIR) as quick way to quantify lipid accumulation in oleaginous yeasts.
Substrates consumption is also an important parameter to monitor throughout the yeast cultivations in order to calculate consumption rates and process yields. This is usually conducted by HPLC, using specific columns. In addition, several kits for the fast quantification of sugars, organic acids and glycerol concentration are available on the market.
Dissolved oxygen (DO) tension is another important parameter that must be monitored throughout yeast cultivation since oleaginous yeasts are obligatory aerobic microorganisms and the production of yeast intracellular products strongly depends on the oxygen availability. Therefore, it is important to ensure adequate aeration and mixing rates so that the cells can have oxygen excess conditions. In bioreactors, DO is usually monitored through oxygen probes. In shake flask cultivations, readers for O2, biomass and pH measurements may be integrated into the shaking incubator [53].
5. Downstream Processing Techniques
5.1. Biomass Separation
The downstream processing of the yeast biomass includes harvesting, de-watering, biomass concentration or drying, cell disruption, product extraction, recovery and, if needed, fractionation and purification of desired products [54].
Several separation techniques can be used to separate microbial cells from the aqueous broth, which include centrifugation, sedimentation, flocculation or microfiltration and ultrafiltration. Centrifugation is the most used technique to separate yeast cells from aqueous media during lab-scale cultivations. However, this technique is not suitable for large-scale fermentations since oleaginous yeast biomass has a similar density to that of the aqueous media [33]. Filtration can also be used for this purpose, but, if the cells produce surfactants or polysaccharides that remain stuck to cells walls, this technique should be avoided, since these compounds make the filtration of the aqueous phase through the membrane difficult. Flocculation involves the addition of a flocculant compound to the broth that will promote cell aggregation, facilitating the separation of the cell aggregates from the aqueous broth. However, for further lipid extraction, attention must be paid to the solvents used in the extraction, since most of the organic solvents used in the lipid extraction can also extract the flocculant, thereby contaminating the yeast oil.
The yeast products extraction step can be performed on wet biomass (obtained after centrifugation) or dry biomass (which can be obtained by oven heat, spray drying or freeze drying) before any treatment. In fact, the use of wet biomass decreases the process energetic costs. However, the water presence may reduce the products yield. For example, when using organic solvents to extract the intracellular lipids from wet biomass, the extraction yield is usually low, since the water molecules hinder the organic solvent penetration and diffusion through the cells, thereby reducing lipid recovery. Lipid extraction from dry biomass is usually considered to be more efficient despite the drying step being energetically unfavorable.
5.2. Cell Disruption
It is well known that the yeast cell wall is composed of chitin microfibrils, and that these microfibrils are responsible for the rigidness of the wall [54]. Therefore, to release and valorize the various yeast biomass fractions, it is necessary to efficiently break the yeast wall before any extraction from the yeast biomass occurs. Several approaches can be used to break the yeast cells walls, such as mechanical action which, by force, using energy transfer by waves or heat, breaks the cells, thereby disintegrating their structure [55]. Bead milling (BM), high pressure homogenization (HPH), microwave irradiation and ultrasonication are example of mechanical disruption methods. Chemical (alkali and acid, cationic detergents) and biological techniques (using enzymes) may also be used to disrupt yeast cells. However, depending on the intracellular products of interest, attention must be paid to the chosen disruption method. Mechanical techniques promote total cell disruption, resulting in the release of the total cellular internal content in small fragments, which will make the purification of a target product difficult. Non-mechanical treatments (chemicals and biologicals) are milder and more selective but are less efficient in terms of intracellular product recovery [56]. In any case, when selecting a cell disruption method, attention must be paid to the advantages and drawbacks, bearing in mind the characteristics of the desired final bioproduct.
5.3. Lipid Extraction
The first fraction to be extracted from the oleaginous yeast biomass is usually the lipids. Several lipid extraction methods are known for extracting the intracellular lipids from yeast cells. The Blight and Dyer and Folch methods [57] are considered standard procedures for the extraction and separation of lipids from microorganisms and biological tissues at the lab-scale. These methods use methanol, chloroform and water, which are added to the sample in a two-step extraction, then, after phase separation, lipids are quantified in the chloroform phase by gravimetry after the solvent evaporation. Due to the use of organic solvents non-GRAS, these methods are not suitable for processes related to food and feed applications and are also difficult for large scale lipid extractions. As a result, Gorte et al. [58], based on the method described by Cheng and Rosentrater (2017) [59], used an extraction method using ethanol and hexane to extract intracellular lipids from oleaginous yeasts; this can be used for food purposes due to the lower toxicity of these solvents when compared to those traditionally used (methanol and chloroform).
However, there are some limitations when using these methods at large-scale, even SCO2, due to the high costs involved. Kumar and Banerjee [60] used the ultrasonic assisted extraction method (UAE) coupled with chloroform/methanol solvent system to extract the intracellular lipids from Trichosporum sp., reporting a 95–97% of conversion efficiency. The authors claimed that the UAE method is a potential green extraction technique, is easy to scale-up, and one that reduced time, energy, and solvent consumption when compared to the traditional Soxhlet technique. However, the authors did not discuss the toxicity of the chloroform and methanol used in the UAE method.
It must be highlighted that there are no downstream processes procedures that can be applicable to all yeast species. Therefore, the lipid extraction procedure must be optimized for each specific strain. Gorte et al. [58] studied the effect of different cell disruption and lipid extraction methods on the lipid content of two oleaginous yeasts (Saytozyma podzolica and Apiotrichum porosum). They found that BM and HPH methods were the best for S. podzolica lipid extraction, while direct transesterification was the most appropriate for A. porosum after the BM step.
5.4. Other Oleaginous Yeast Bioproducts
A strategy to improve the value derived from the yeast lipid extraction process consists of co-extracting other valuable compounds, although only a few studies referring to this approach have been published.
For instance, oleaginous red yeasts, classified in the subphylum Pucciniomycotina, show orange, red or pink colors due to the presence of carotenoids [61]. These microorganisms produce not only TAG, but also valuable carotenoids. The TAG (saponifiable) fraction has a wide range of applications, including biodiesel (converting the yeast fatty acids into methyl esters by transesterification) and biojetfuel (converting the yeast fatty acids into hydrocarbons suitable for aviation by hydroprocessing of esters and fatty acids). As the yeast fatty acids profile is strongly dependent on the growth conditions, tailoring them to obtain higher proportions of essential fatty acids for nutrition can be another application for the yeast oil. Nowadays, polymers are obtained from petrochemical industry; this has raised environmental concerns. Many polymers can be produced from vegetable oils, including polyuretane, polyolefin, polyester and polyether, all of which have several industrial applications (foams, vehicles and house coatings, building insulation, surfboards and skateboards, etc.). Since the oil composition of oleaginous yeast is like that of vegetable oils, the former can be a cleaner and environmentally sustainable alternative for the production of biopolymers. Importantly, vegetable oil or yeast oil-based biopolymers can be used for medical purposes (prosthesis), being preferable to petrochemical based-polymers, since the former are biocompatible, representing less danger of rejection [62].
Thermochemical methods can be used to obtain a variety of products from oleaginous yeast strains. Bi et al. [63] produced Cryptococcus curvatus biomass from sweet sorghum bagasse hydrolysates. A direct transesterification was applied to the wet yeast biomass in order to convert the intracellular lipids into biodiesel. The residual yeast biomass, obtained after the transmethylation, and the sorghum bagasse residues, obtained after the pretreatment, were used to produce “biocrude” through hydrothermal liquefaction (HTL); this is a process that converts whole wet biomass into the energy-dense liquid fuel precursor, called ‘biocrude’, and is a promising alternative to conventional lipid extraction methods as it does not require a dry feedstock or additional steps for lipid extraction. The HTL that led to the highest biocrude yield (68.9%, with a high heating value (HHV) of 38.2 MJ/kg) used the catalyst K2CO3 at 1.00 mol/L, conducted at 350 °C. This study proposed a method for producing biocrude and biodiesel from yeast biomass generated from fermenting lignocellulosic sugars.
The sustainable and efficient oleaginous yeast biorefinery aims to produce a wide range of bioproducts such as polyunsaturated fatty acids and carotenoids with food, nutraceutical, cosmetic and pharmaceutical applications, and biofuels (biodiesel, biojetfuel and biogas). The leftover yeast biomass contains proteins and carbohydrates that may be further used as animal feed or converted into biocrude by HTL for further conversion into renewable gasoline and diesel. Recently, the whole oleaginous yeast red biomass was considered a promising material with many appealing biological functions that can be used in the food industry, as a pharmaceutical material, or in the feed industry [64]. The efficient oleaginous yeast biomass biorefinery uses all the biomass fractions with minimal environmental impact and with zero waste.
References
- IEA Bioenergy Task42. Available online: https://www.ieabioenergy.com/wp-content/uploads/2014/09/IEA-Bioenergy-Task42-Biorefining-Brochure-SEP2014_LR.pdf (accessed on 9 September 2023).
- Jacob-Lopes, E.; Queiroz, M.; Zepka, L. Microalgal biorefineries. In Biomass Production and Uses; Jacob-Lopes, E., Zepka, L., Eds.; IntechOpen: London, UK, 2015; Chapter 5.
- Chew, K.W.; Yap, J.; Show, P.; Suan, N.; Juan, J.; Ling, T.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Techol. 2017, 229, 53–62.
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal biorefinery concepts’ development for biofuel and bioproducts: Current perspective and bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623.
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146.
- Ruiz, J.; Wijffels, R.; Dominguez, M.; Barbosa, M. Heterotrophic vs autotrophic production of microalgae: Bringing some light into everlasting cost controversy. Algal Res. 2022, 64, 102698.
- Ageitos, J.; Vallejo, J.; Veiga-Crespo, P.; Villa, T. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227.
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50.
- Zymanczyk-Duda, E.; Brzezinka-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a versatile tool in biotechnology. In Yeast; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2017; Chapter 1.
- Liu, X.; Wang, D.; Li, A. Biodiesel production of Rhodosporidium toruloides using different carbon sources of sugar-containing wastewater: Experimental analysis and model verification. J. Clean Prod. 2021, 323, 129112.
- Darvishi, F.; Fathi, Z.; Ariana, M.; Moradi, H. Yarrowia lipolytica as a workhorse for biofuel production. Biochem. Eng. J. 2017, 127, 87–96.
- Santek, M.; Lisicar, J.; Musak, L.; Spolijaric, I.; Beluhan, S.; Santek, B. Lipid production by the yeast Trichosporon oleaginosus on the enzymatic hydrolysate of alkaline pretreated corn cobs for biodiesel production. Energy Fuels 2018, 32, 12501–12513.
- Adrio, J.L. Oleaginous yeasts: Promising platforms for the production of oleochemicals and biofuels. Biotechnol. Bioeng. 2017, 114, 1915–1920.
- Poontawee, R.; Lorliam, W.; Polburee, P.; Limtong, S. Oleaginous yeasts: Biodiversity and cultivation. Fungal Biol. Rev. 2023, 44, 100295.
- Rayaan, M.; Alshayqi, I. A Review on oleaginous microorganisms for biological wastewater treatment: Current and future prospect. J. Environ. Treat. Tech. 2021, 9, 280–288.
- Wang, J.; Hu, M.; Zhang, H.; Bao, J. Converting chemical oxygen demand (COD) of cellulosic ethanol fermentation wastewater into microbial lipid by oleaginous yeast Trichosporon cutaneum. Appl. Biochem. Biotecnhol. 2017, 182, 1121–1130.
- Peng, W.; Huang, C.; Chen, X.; Xiong, L.; Chen, X.; Chen, Y.; MA, L. Microbial conversion of wastewater from butanol fermentation to microbial oil by oleaginous yeast Trichosporon dermatis. Renew. Energy 2013, 55, 31–34.
- Zhang, A.; Wang, J.; Jiang, H. Microbial production of value-added products and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860.
- Lakshmidevi, R.; Ramakrishnan, B.; Ratha, S.; Bahaskar, S.; Chinnasamy, S. Valorization of molasses by oleaginous yeasts for single cell oil (SCO) and carotenoids production. Env. Technol. Innov. 2022, 21, 101281.
- Donzella, S.; Serra, I.; Fumagalli, A.; Pellegrino, L.; Mosconi, G.; Scalzo, R.; Compagno, C. Recycling industrial food wastes for lipid production by oleaginous yeasts Rhodosporidiobolus azoricus and Cutaneotrichosporon oleaginosum. Biotechnol. Biofuels Bioprod. 2022, 15, 51.
- Ykema, A.; Verbree, E.; Kater, M.; Smit, H. Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in wheypermeate. Appl. Microbiol. Biotechnol. 1988, 29, 211–218.
- Brandenburg, J.; Blomqvist, J.; Shapaval, V.; Kohler, A.; Samples, S.; Sandgen, M.; Passoth, V. Oleaginous yeasts respond differently to carbon source present in lignocellulose hydrolysate. Biotechnol. Biofuels 2021, 14, 124.
- Kumar, L.; Yellapu, S.; Tyagi, S.; Tyagi, R.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 122155.
- Kosamia, N.; Samavi, M.; Uprety, B.; Rakshit, S. Valorisation of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Catalysts 2020, 10, 609.
- Qin, L.; Liu, L.; Zeng, A.; Wei, D. From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresour. Technol. 2017, 245, 1507–1519.
- Signori, L.; Ami, D.; Posteri, R.; Giuzzi, A.; Mareghetti, P.; Porro, D.; Branduardi, P. Assessing an affective feeding strategy to optimize crude glycerol utilization as sustainable carbon source for lipid accumulation in oleaginous yeasts. Microbial Cell Fact. 2016, 15, 76.
- Kumar, R.; Dhanarajan, G.; Bhaumik, M.; Chopra, J.; Sen, R. Performance evaluation of a yeast biorefinery as a sustainable model for co-production of biomass, bioemulsifier, lipid, biodiesel and animal-feed components using inexpensive raw materials. Sustain. Energy Fuels 2017, 1, 923.
- Polburee, P.; Yongmanitchai, W.; Lertwattanasakul, N.; Ohashi, T.; Fujiyama, K.; Limtong, S. Characterization of oleaginous yeasts accumulating high levels of lipid when cultivated in glycerol and their potential for lipid production from biodiesel-derived crude glycerol. Fungal Biol. 2015, 119, 1194–1294.
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Rego, E. Screening of oleaginous yeasts for lipid production using fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553.
- Fei, Q.; Nam, C.; Shang, L.; Choi, J. Exproring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol. Bioprocess Eng. 2011, 15, 482–487.
- Gao, R.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3.
- Mira, N.; Teixeira, M.; Sá-Correia, I. Adaptive response and tolerance to weak acids in Saccharomyces cerevisae. A genome-wide view. Omics A J. Int. Biol. 2010, 14, 525–540.
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid recovery from oleaginous yeasts: Perspectives and challenges for industrial applications. Fuel 2020, 259, 116292.
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP:citrate lyase from Mus musculus. J. Biotechnol. 2014, 192 PtA, 78–84.
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochemie 2004, 86, 807–815.
- Robles-Iglesias, R.; Naveira-Pazos, C.; Fernández-Blanco, C.; Veiga, M.; Kennes, C. Factors affecting the optimization and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew Sust. Energ. Rev. 2023, 171, 113043.
- Mota, M.; Múgica, P.; Sá-Correia, I. Exploring yeast diversity to produce lipid-based biofuels from agro-forestry and industrial organic residues. J. Fungi 2022, 8, 687.
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid. Sci. Technol. 2011, 113, 1031–1051.
- Karamerou, E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322.
- Wynn, J.; Behrens, P.; Sundarajan, A.; Hansen, J.; Apt, K. Production of single cell oils by dinoflagellates. In Single Cell Oils; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2005; Chapter 6; pp. 86–98.
- Qian, X.; Zhou, X.; Chen, L.; Zhang, X.; Xin, F.; Dong, W.; Zhang, W.; Ochsenreitherm, K.; Jiang, M. Bioconversion of volatile fatty acids into lipids by oleaginous yeast Apiotrichum porosum DSM27194. Fuel 2021, 290, 119811.
- Polburee, O.; Yongmanitchai, W.; Honda, K.; Ohashi, T.; Yoshida, T.; Fujiyama, K.; Limtong, S. Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Biochem. Eng. J. 2016, 112, 208–2018.
- Polburee, P.; Limtong, S. Economical lipid production from crude glycerol using Rhodosporidiobolus fluvialis DMKU-RK253 in a two-stage cultivation under non-sterile conditions. Biomass Bioenergy 2020, 138, 105597.
- Zhang, Z.; Zhang, X.; Tan, T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour. Technol. 2014, 157, 149–153.
- Dias, C.; Reis, A.; Santos, J.; Lopes da Silva, T. Concomitant wastewater treatment with lipid and carotenoid production by the oleaginous yeast Rhodosporidium toruloides grown on brewery effluent enriched with sugarcane molasses and urea. Process Biochem. 2020, 94, 1–14.
- Grubisic, M.; Mihajlovki, K.; Gruicic, A.M.; Beluham, S.; Santek, B.; Ivancié, S. Strategies for improvement of lipid production by yeast Trichosporon oleaginosus form lignocellulosic biomass. J. Fungi 2021, 7, 934.
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J Biol. Sci. 2012, 19, 257–275.
- Dias, C.; Reis, A.; Santos, J.A.L.; Gouveia, L.; Lopes da Silva, T. Primary brewery wastewater as feedstock for the yeast Rhodosporidium toruloides and the microalga Tetradesmus obliquus mixed cultures with lipid production. Proc. Biochem. 2021, 113, 71–86.
- Hewitt, J.C.; Nebe-Von-Caron, G. An industrial application of multiparameter flow cytometry: Assessment of cell physiological state and its application to the study of microbial fermentations. Cytometry 2001, 59, 554–562.
- Available online: https://eu.sysmex-flowcytometry.com/reagents/yeastcontrol/2935/yeast-control-neutral-lipids# (accessed on 8 November 2023).
- Chen, J.; Wei, D.; Pohnert, G. Rapid estimation of astaxantin and the carotenoid-to-chlorophyll ratio in the green microalga Chromochloris zofingiensis using flow cytometry. Mar. Drugs 2017, 15, 231.
- Ami, D.; Posteri, R.; Mereghetti, P.; Porro, D.; Doglia, S.; Branduardi, P. Fourier transform infrared spectroscopy as a method to study lipid accumulation in oleaginous yeasts. Biotechnol. Biofuels 2014, 7, 12.
- Shanmugam, M.; Sriman, S.; Gummadi, N. Online measurements of dissolved oxygen in shake flask to elucidate its role on caffeine degradation by Pseudomonas sp. Indian Chem. Eng. 2022, 64, 162–170.
- Chopra, J.; Rangarajan, V.; Sen, R. Recent developments in oleaginous yeast feedstock based biorefinery for production and life cycle assessment of biofuels and value-added products. Sustain. Energy Technol. Assess. 2022, 53, 102621.
- Zainuddin, M.; Fai, C.; Ariff, A.; Rios-Solis, L.; Halim, M. Current pretreatment/Cell disruption and extraction methods used to improve intracellular lipid recovery from oleaginous yeasts. Microorganisms 2021, 9, 251.
- Liu, D.; Ding, L.; Sun, J.; Bousseta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Inn. Food Sci. Emerg. Technol. 2016, 36, 181–192.
- Breil, C.; Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. Bligh and Dyer and Folch methods for solid-liquid-liquid extractions of lipids from microorganisms. Comprehension of solvatation mechanisms and toward substitution with alternative solvents. Int. J. Mol. Sci. 2017, 18, 708.
- Gorte, O.; Hollenbach, R.; Papachristou, I.; Steinweg, C.; Silve, A.; Frey, W.; Syldatk, C.; Ochsenteither, K. Evaluation of downstream processing, extraction and quantification strategies for single cell oil produced by the oleaginous yeast Saitozyma podzolica DSM 27192 and Apiotrichum porosum DSM 27194. Front. Bioeng. Biotechol. 2020, 8, 355.
- Cheng, M.; Rosentrater, K. Economic feasibility analysis of soybean oil production by hexane extraction. Ind. Crops Prod. 2017, 108, 775–785.
- Kumar, S.P.; Banerjee, R. Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: A greener and scalable process. Ultrasonics Sonochem. 2019, 52, 25–32.
- Fakankun, I.; Levin, D. Oleaginous red yeasts: Concomitant producers of triacylglycerols and carotenoids. Encyclopedia 2023, 3, 490–500.
- Vasconcelos, N.; Teixeira, J.; Dragone, G.; Teixeira, J. Optimization of lipid extraction from the oleaginous yeasts Rhodotorula glutinis and Lipomyces kenonenkoae. AMB Express 2018, 8, 126.
- Bi, Z.; Zhang, J.; Zhu, Z.; Liang, Z.; Wiltowski, T. Generating biocrude form partially defatted Cryptococcus curvatus yeast residues through catalytic liquefaction. Appl. Energy 2018, 209, 435–444.
- Vysoka, M.; Szotkowski, M.; Slaninova, E.; Dzuricka, L.; Strecanska, P.; Blazkova, J.; Marova, I. Oleaginous yeast extracts and their possible effects on human health. Microorganisms 2023, 11, 492.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
973
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




