Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christos Dordas | -- | 2947 | 2023-12-18 20:17:30 | | | |
| 2 | Peter Tang | Meta information modification | 2947 | 2023-12-19 03:46:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arampatzis, A.S.; Pampori, A.; Droutsa, E.; Laskari, M.; Karakostas, P.; Tsalikis, L.; Barmpalexis, P.; Dordas, C.; Assimopoulou, A.N. Luteolin in Prevention and Treatment of Periodontal Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/52892 (accessed on 07 February 2026).
Arampatzis AS, Pampori A, Droutsa E, Laskari M, Karakostas P, Tsalikis L, et al. Luteolin in Prevention and Treatment of Periodontal Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/52892. Accessed February 07, 2026.
Arampatzis, Athanasios S., Aspasia Pampori, Eleftheria Droutsa, Maria Laskari, Panagiotis Karakostas, Lazaros Tsalikis, Panagiotis Barmpalexis, Christos Dordas, Andreana N. Assimopoulou. "Luteolin in Prevention and Treatment of Periodontal Disease" Encyclopedia, https://encyclopedia.pub/entry/52892 (accessed February 07, 2026).
Arampatzis, A.S., Pampori, A., Droutsa, E., Laskari, M., Karakostas, P., Tsalikis, L., Barmpalexis, P., Dordas, C., & Assimopoulou, A.N. (2023, December 18). Luteolin in Prevention and Treatment of Periodontal Disease. In Encyclopedia. https://encyclopedia.pub/entry/52892
Arampatzis, Athanasios S., et al. "Luteolin in Prevention and Treatment of Periodontal Disease." Encyclopedia. Web. 18 December, 2023.
Copy Citation
Polyphenols, a class of flavonoids, are secondary metabolites that play a crucial role in plant adaptation to both biotic and abiotic environments, including UV radiation, high light intensity, low/high temperatures, and attacks from pathogens, among others.
extraction
aromatic
medicinal plants
secondary metabolites
pharmaceutical use
1. Introduction
Higher plants produce, through the photosynthesis process, all the necessary substances for their growth and also for all the other life forms of nature. These are called ‘primary metabolites’. In addition, plants have the ability to biosynthesize a large number of substances that have specific functions and are known as secondary metabolites. Polyphenols are one of the most common groups of secondary metabolites widely distributed in all plant species [1]. The content of polyphenols increases in response to various factors, such as ultraviolet (UV) radiation, high light intensity, low/high temperature, salinity, drought, etc. These conditions cause the creation of free oxygen and nitrogen radicals due to the stress they cause to the plants. One of the functions of polyphenols is the reduction of the effect caused by the presence of free radicals [2]. A class of polyphenols are flavonoids, which are the largest group of phenolic compounds, accounting for more than 5000 different compounds present in plant species [3]. In the last decade, flavonoids have been studied systematically due to experimental and clinical research as they are used as anti-cancer, antioxidant, anti-inflammatory, and antiviral compounds. They also act as cardioprotective, neuroprotective and chemoprotective agents [4].
Flavonoids are polyhydroxy–phenolic compounds produced through the phenylpropanoid biosynthetic pathway in plants [5]. They have 15 carbon atoms (phenolic compounds of the type C6–C3–C6) with the structure of two benzene rings joined by a heterocyclic oxygen-centered ring. Flavonoids are divided into seven major subclasses: flavan-3-ols, flavones, flavonols, flavanones, anthocyanins, chalcones and isoflavonoids (Figure 1). Flavonoids include, in particular, flavones, such as luteolin and tetramethoxyluteolin. Flavones are structurally characterized by a double bond and an oxygen atom in the heterocyclic ring C of the flavonoid skeleton. Flavones, such as apigenin and luteolin, can be found in plants showing a wide range of substitutions, including methylations, hydroxylations, acylations, and glycosylations leading mainly to O- or C-glycosides [6].
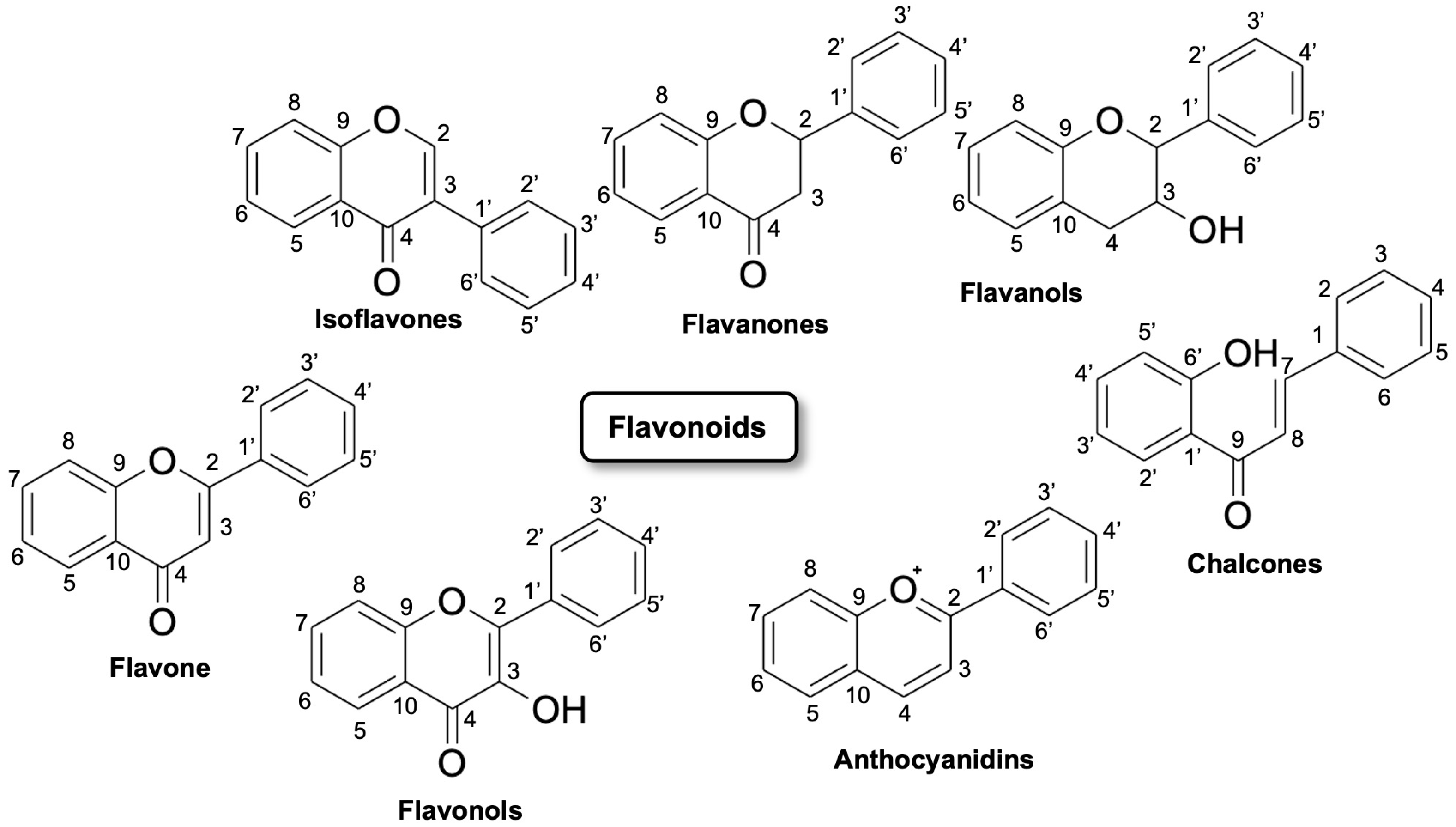
Figure 1. Flavonoids classification and their possible chemical structure [7].
Luteolin (3′,4′,5,7-tetrahydroxyflavone) belongs to a group of naturally occurring compounds called flavonoids, which are naturally found in several plant species. Because of their abundance in foods, e.g., vegetables, fruits, and medicinal plants, flavonoids are common compounds that act as antioxidants, estrogenic regulators, and antimicrobial agents [8]. Chemically, luteolin has a C6–C3–C6 structure that contains two benzene rings and one oxygen-containing ring with a C2–C3 carbon double bond (Figure 2). Structure-activity relationship studies have shown that the presence of hydroxyl moieties at carbons 5, 7, 3′ and 4′ positions of the luteolin structure and the presence of the 2−3 double bond are responsible for its multiple pharmacological effects [9]. The hydroxyl moieties and the 2–3 double bonds are important structural features in luteolin that are associated with its biochemical and biological activities (anti-cancer, antioxidant, anti-inflammatory, neuroprotective, etc.) [10]. As in other flavonoids, luteolin is often glycosylated in plants, and the glycoside is hydrolyzed to free luteolin during absorption [11].
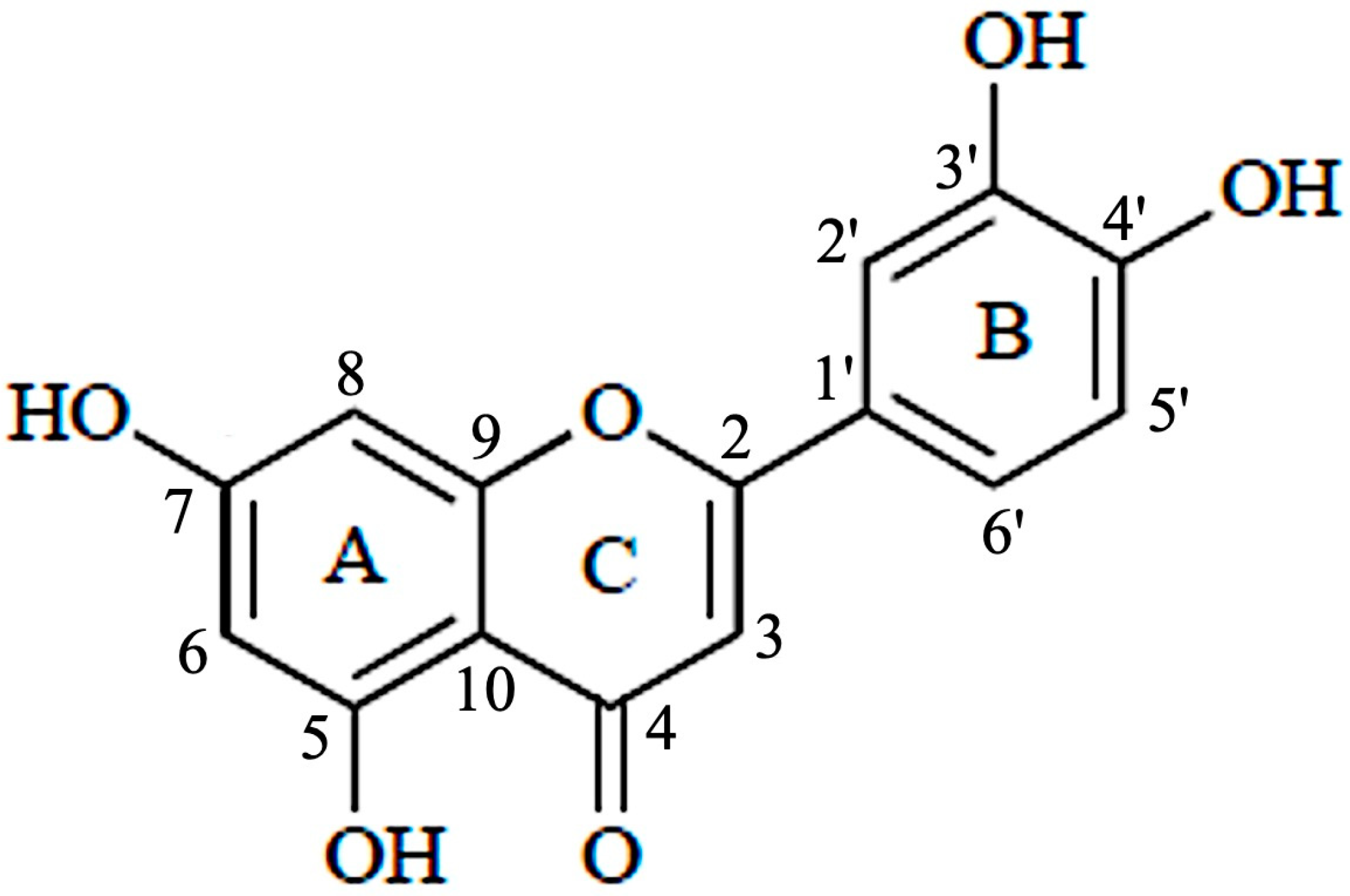
Figure 2. Structure of luteolin.
2. Determination of Luteolin Content in Plants of the Greek Flora
Areas that have favorable climate conditions for plant species [12], such as Greece, are ideal for the cultivation of aromatic and medicinal plants [13]. Aromatic and medicinal plants are important for the protection of the environment. In particular, since ancient times, they have been used as fresh, frozen or dry essential oils, originally for the food, pharmaceutical and cosmetic industries [12]. It is estimated that 50,000–70,000 species of higher plants may be used in traditional and modern medicinal systems throughout the world, and about 3000 belong to the group of medicinal and aromatic plants [14]. Aromatic plants contain chemical substances, such as essential oils, polyphenols, glycosides, quinones, flavonols/flavonoids, terpenes, alkaloids, polypeptides or their oxygen-substituted derivatives [15][16]. Some bioactive compounds have therapeutic value, such as antioxidant and antiseptic activities and may reduce the risk of cancer or cardiovascular diseases and treatment of respiratory diseases, stomach or inflammatory disorders [17][18]. Luteolin is a common flavonoid that belongs to the subclass of flavones and exists in many types of plants, including fruits, vegetables, and medicinal herbs [9]. Luteolin, in medicinal plants, is most commonly found in leaves, having been isolated from many plants [19]. The major natural sources of luteolin, based on the literature, are celery, thyme, dandelion, clover flower, chamomile, carrots, peppers, olive oil, peppermint, thyme, rosemary, oregano, and parsley [20][21]. Luteolin is a powerful antioxidant with anti-inflammatory properties that can be used to treat diseases such as periodontitis [22][23].
The olive tree (Olea europaea, Oleaceae), along with its fruit (olive) and leaves, is considered to be very important, exhibiting high nutritional and medicinal value. Besides the main bioactive compound oleuropein, olive drupes and leaves have been found to contain luteolin and its derivatives, among other compounds. Blekas and co-workers (2002) studied different samples of table olives obtained from the retail market that were representative of the main Greek cultivars Conservolea (Amfissa), Nychati (Kalamata) and Chalkidiki. The analyses—using a high-performance liquid chromatography system coupled with diode array detector (HPLC-DAD) and reference compounds—showed that luteolin was among the most abundant phenols in all samples, yet Kalamata olives contained the highest quantity [24]. Luteolin was also detected in olive oils that were extracted from various Greek cultivars Koroneiki, Tsounati, Adramitini, Throubolia, Native from Zakynthos, Lianolia, Asprolia, and Thiaki [25][26][27]. The identification of luteolin was performed either using NMR [25][26] or HPLC-UV and LC-DAD-mass spectrometry (MS) [27]. Moreover, luteolin and luteolin 7-O-glucoside were determined in fresh olive fruits, as well as in Greek-style and Spanish-style processed olives from the Kalamon cultivar. Luteolin 7-O-glucoside was identified and quantified by means of HPLC-UV [28], as well as HPLC-DAD and LC-(ESI)-MS/MS [29]. In another study, olive leaves from the Greek cultivars Koroneiki, Megaritiki and Kalamon were examined and found to contain luteolin 7-O-glucoside. LC-(ESI)-MS/MS method was employed to determine glycoside content in the olive leaves [30]. 4-O-glucoside and 7-O-glucoside of luteolin were present in the leaves and drupes of samples collected from several major Greek olive varieties, such as Koroneiki, Lianolia Kerkyras, Mastoidis, Adramytini, Megaritiki, Gaidourelia, Kalamata, Konservolia, and Chalkidiki. The two metabolites were identified and quantified by using reference compounds and HPLC-UV analysis [31].
The Lamiaceae family constitutes one of the most significant botanical families, including a large number of aromatic and medicinal plants, such as Origanum vulgare, Rosmarinus officinalis, Salvia officinalis and Thymus vulgaris, among others. Luteolin and/or its derivatives have been identified in various plant species of the Lamiaceae family. As mentioned above, luteolin was detected in samples collected from different sites in Greece regarding the following species: O. vulgare [32][33], R. officinalis [34], S. officinalis [34], S. fruticosa [33], and Thymus vulgaris [34]. The above analyses were performed by means of HPLC-DAD and using reference compounds. Additionally, luteolin was present in Mentha spicata [32], Nepeta cataria and N. sibthorpii [35][36], Teucrium chamaedrys [34] and T. polium [37]. Other studies have reported that various luteolin derivatives have also been detected in species belonging to the Lamiaceae family. Specifically, 6-hydroxy luteolin 7-O-glucoside, luteolin 7-O-glucuronide, luteolin 7-O-rutinoside, luteolin diglucuronide, 6-methoxyluteolin 7-O-glucoside, and 6-methoxyluteolin derivative were tentatively identified via HPLC-DAD and LC-(ESI)-MS/MS methods in Salvia fruticosa and S. pomifera [38][39]. Choulitoudi et al. reported that Satureja thymbra was found to contain luteolin 7,4′-di-O-glucuronide, 6-OH luteolin 7,3′-dimethyl ether and 6-OH luteolin 7,3′,4′-trimethyl ether. The above components were identified through HPLC-DAD-ESI-MS/MS analysis [40].
Members of the Asteraceae family were analyzed through NMR and MS methods for their flavonoid content, and it was reported that luteolin was identified in Crepis incana [41] and Cynara species [42]. Moreover, the derivatives luteolin 7-O-rutinoside, luteolin 7-O-glucoside, luteolin 7-O-malonylglycoside, luteolin 7-O-β-d-glucoside, luteolin 7-O-β-d-rutinoside, and luteolin 7-O-β-gentiobioside, were also present in Cynara cardunculus, Cynara humilis and Cynara cornigera [42][43].
3. Effect of Crop Management on Luteolin Content
Luteolin’s concentration, as well as the quantitative and qualitative composition of the products of secondary metabolites, depends on the genotype of the plant, the crop management (fertilization, irrigation), the growth stage of the plant, soil and climate conditions such as ultraviolet (UV) radiation, strong light, low/high temperature, drought, etc. [44]. These conditions can cause the creation of free radicals due to the stress they cause to the plants, and one of the functions of flavonoids in plants is the reduction of the effect caused by the presence of reactive oxygen species (ROS) [2]. It is possible that luteolin can protect plants from abiotic and biotic stresses. Also, it was proposed that flavonoids, where luteolin belongs, can function as signal molecules, phytoalexins, allopathic compounds, detoxifying agents, antimicrobial defensive compounds and UV filters [45]. They also protect plants from drought, heat and freezing [46][47].
Fertilization is a factor that can affect the concentration of flavonoids. For example, luteolin-7-O-glucoside was found to be higher at the first growth stage and also at the 150 kg N ha−1 treatment [44]. In addition, crop management, such as organic and conventional, can affect luteolin content. Particularly, luteolin concentration was higher by 51% in the organic system compared with the conventional one [48]. In the same study, luteolin content was increased by 39% with the application of 20 kg N ha−1 (1.741 mg 100 g−1 dry weight) compared to 0 kg N ha−1 (1.383 mg 100 g−1 dry weight). Regarding the effect of N fertilization on the organic management system, it was reported that there were no significant changes [48]. Moreover, in another work, the effect of genotype, the ripening stage and their interactions in different growing systems (organic and conventional) were studied in different species of Capsicum. It was observed that the ripening stage significantly affected the concentration of luteolin; specifically, luteolin content appeared higher in ripening fruits, as well as in the organic system [49].
In a field experiment carried out in Pisa, Italy, different amounts of nitrogen fertilizer were applied to Stevia rebaudiana: N0 (without N fertilization), N50 (50 kg N ha−1), N150 (150 kg N ha−1), N300 (300 kg N ha−1) and Norg (150 kg N ha−1, as organic nitrogen from Nutex N7 based on wool wastes, poultry manure, blood, and pomace with 7% organic N and 35% organic carbon). In addition, leaf samplings were accomplished at three vegetative stages (H1, H2, and H3), where H1 and H2 were during the vegetative growth and H3 at the flowering stage. The experiment showed that luteolin-7-O-glucoside was significantly affected by the amount of nitrogen fertilization (N), harvest time (H), and NxH interaction. Luteolin-7-O-glucoside was higher in the H1 compared to the other harvests with 150 kg N ha−1. In H2, a decrease in Luteolin-7-O-glucoside was recorded compared to H1, while in H3, the amount of this compound increased in each N treatment. Values similar in H1 were recorded in the N0 and Norg treatments [44].
Abiotic stress can affect the concentration of luteolin as it was found that water stress was among the main factors that increase the content of secondary metabolites, especially polyphenols/flavonoids [50][51][52]. Water stress increased the concentration of luteolin in Chrysanthemum plants [52]. In addition, another study conducted with Lactuca sativa plants found that drought stress and UV-B affected the flavonoid content, such as quercetin, luteolin and anthocyanin, as there was an increase in luteolin content under water stress [53].
One of the most common abiotic stresses is salinity, as it affects many agricultural areas worldwide (Vafadar et al., 2020). Also, salinity stress affects the secondary metabolism, and it was found that the concentration of phenolic and flavonoid compounds increased [54][55][56]. In addition, it was found that the concentration of luteolin was increased by up to 75 mM NaCl in Dracocephalum kotschyi [57].
Agati et al. [58] reported that salinity and UV radiation significantly enhanced the biosynthesis of luteolin 7-O-glycosides. Other researchers studied the effect of salt stress using Solanum nigrum seedlings, and the results of the experiment showed increased flavonoid accumulation and decreased root and leaf dry biomass in the treatment with the highest salt concentration [59]. Similar results were reached by other researchers [60][61].
The growth stage of the plants can affect the flavonoid content and especially the content of luteolin, as it was found by several studies in Origanum majorana. Specifically, the highest content is found at the bud stage, as well as in the early vegetative stages and at full bloom [62][63][64].
4. Methods of Luteolin Extraction from Different Plant Species
There are various extraction techniques that are reported in the literature and that have been used by many research groups in order to efficiently acquire luteolin and/or luteolin-enriched extracts. Both conventional and modern methods have been applied for extracting luteolin from different plant sources. From maceration and Soxhlet extraction to ultrasound-assisted and microwave-assisted extraction, each technique offers several advantages while exhibiting certain limitations. The choice of the extraction method is highly dependent on the quantity and characteristics of the plant material, as well as the properties of the bioactive compound(s) to be extracted.
5. Pharmacological Activities and Applications of Luteolin
The different pharmacological activities of luteolin have been well described in the literature. Luteolin exhibits a series of biological activities, such as cytotoxic, anti-inflammatory, antioxidant and antibacterial ones. Some of the possible mechanisms through which luteolin exerts its therapeutic effects are the following: preventing DNA alterations in oncogene and tumor-suppressor genes, decreasing vascular endothelial growth factor (VEGF), inhibiting the elevation of reactive oxygen species (ROS), and downregulating inflammatory cytokines (TNF-α) and transcription factors (Nf-κB, STAT3) [21]. Moreover, luteolin, as an anti-inflammatory agent, acts by inhibiting the activation of mast cells and T cells in cases of neuroinflammation and allergic inflammation [65][66][67][68].
In a recent study, luteolin was also found to inhibit in vitro and in vivo (C57BL/6J mice) the activation of NOD-, LRR- and pyrin domain-containing protein 3(NLRP3) inflammasome, therefore supporting luteolin’s role as an anti-inflammatory agent [69]. Moreover, Wang and co-workers demonstrated that luteolin could modify the M1/M2 polarization of macrophages and exert its anti-inflammatory role by decreasing p-STAT3 and increasing p-STAT6 [70]. Alternatively, luteolin reduced the inflammation in an acute gouty arthritis rat model by negatively regulating the TLR/MyD88/NF-κB pathway [71]. Researchers also reported that luteolin protected retinal pigment epithelium cells from increasing interleukin levels –and thereby, inflammation– through inhibition of MAPK and NF-kΒ [72]. Last but not least, luteolin protects ulcerative colitis rats by reducing inflammation and enhancing the composition and diversity of gut microbiota [73]. In a different animal model (TG-AD mice), luteolin was found to exert a protective effect against Alzheimer’s disease through the inhibition of endoplasmic reticulum stress-associated neuroinflammation [74]. A common luteolin derivative, luteolin 7-O-glucoside, showed promising in vitro anti-inflammatory properties by suppressing ROS and STAT3 activation in HUVEC cells [47].
The antioxidant and antibacterial activities of luteolin have been recently reported by different groups, demonstrating the ability of this molecule to reduce ochratoxin-stimulated oxidative stress in vitro through Nrf2 and HIF-1α pathways and suppressing the growth of Trueperella pyogenes by disrupting cell wall and cell membrane integrity, hindering the synthesis of nucleic acids, altering metabolism and modulating protein expression [75][76].
Due to its biological potential, luteolin has been employed as a bioactive molecule in various applications within the food industry and the biomedical field. For instance, luteolin has been incorporated into the active packaging system of different foods as well as an additive [77]. Bi et al. [78] demonstrated that luteolin nanoemulsions displayed potent antioxidant properties, therefore protecting fat-containing foods, such as beef, poultry and fish (Figure 3). Additionally, luteolin acted as a food preservative when added to minced meat by hindering the development of Listeria monocytogenes [79]. Also, a commercially available dietary supplement that contains luteolin, rutin and quercetin was shown to exert an antioxidant effect. Other therapeutic applications of luteolin include the employment of different delivery platforms (micelles, liposomes, nanoemulsions, amorphous solid dispersions) with the aim to increase the active molecule’s bioavailability and its overall therapeutic efficacy [80][81][82][83]. Luteolin and luteolin-containing drug delivery systems have been studied for the treatment of numerous diseases, such as neurological disorders [84], rheumatoid arthritis [85] and different types of cancers [86][87][88] (Figure 3).
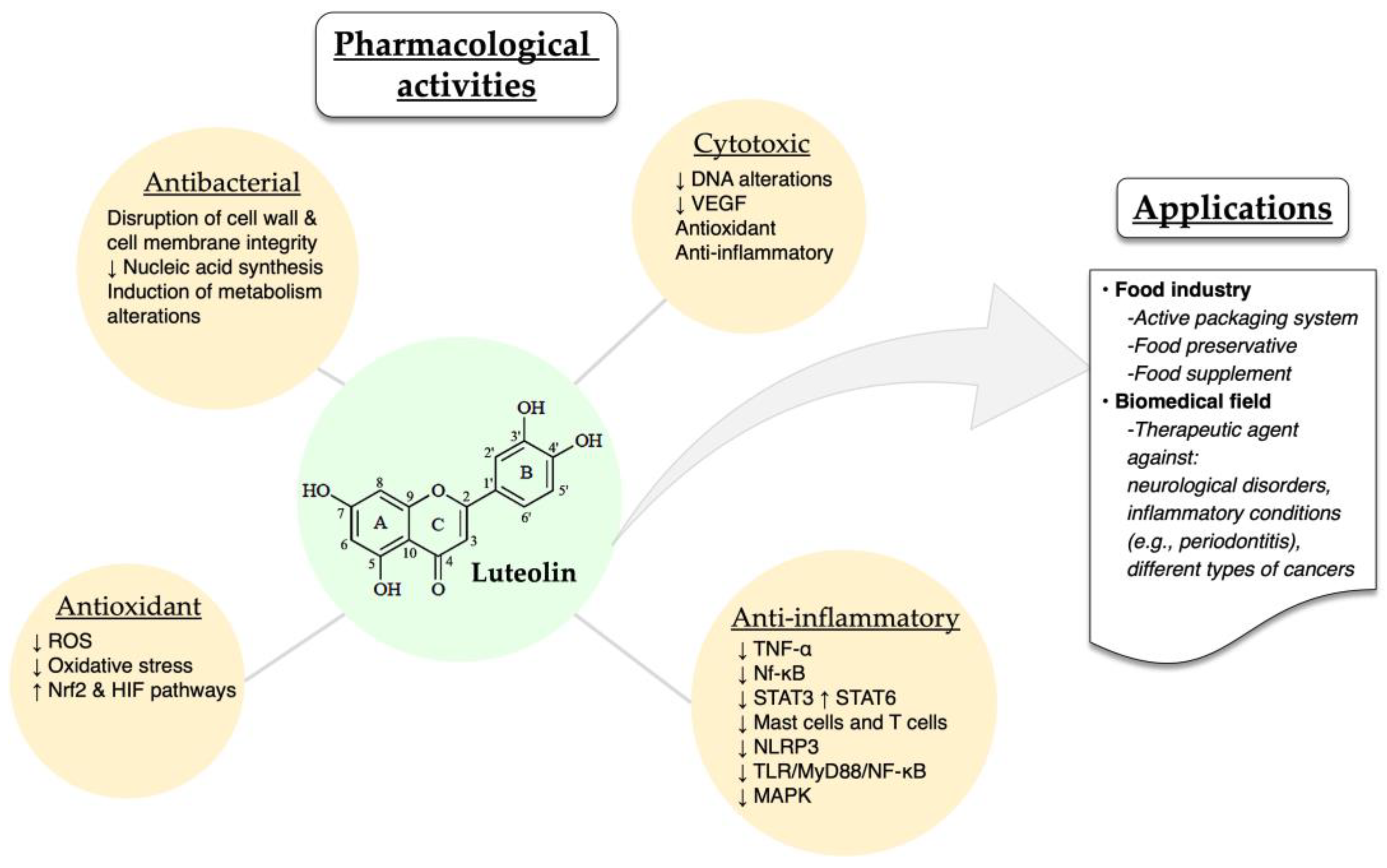
Figure 3. Overview of the pharmacological activities and applications of luteolin. The up and down arrows indicate increases and decreases, respectively.
6. The Effect of Luteolin in the Prevention and Treatment of Periodontal Disease
Among the various available options for managing periodontitis, dental care products containing herbal compounds have been in the spotlight owing to the beneficial pharmacological properties of the bioactive ingredients [89]. In this context, the anti-inflammatory activity of luteolin has been harnessed in order to combat periodontal disease and promote the restoration of damaged bone tissue. Gutiérrez-Venegas and co-workers demonstrated that luteolin could hinder the inflammation in lipopolysaccharide-stimulated human gingival fibroblasts by downregulating a series of mitogen-activated protein kinase family members [90]. In a different study, Wistar rats were used as a model for assessing the use of luteolin in periodontitis prevention. The results suggested that luteolin succeeded in reducing the inflammation and potentially induced osteoblast differentiation [91]. Moreover, it was found that luteolin could facilitate osteogenic differentiation in human periodontal ligament cells through the activation of the Wnt/β–catenin signaling pathway [92]. Casili et al. confirmed that luteolin could alleviate periodontitis symptoms in Sprague-Dawley rats by reducing inflammation through the reduction of TNF-α and IL-6 expression [23]. In relation to the anti-inflammatory activity of luteolin, which is relevant in periodontitis treatment, both in vitro and in vivo studies have shown that luteolin inhibits many pro-inflammatory cytokines (like TNF-α), as well as modulate NF-κB pathway [93].
Luteolin’s antimicrobial properties have been well documented. In some studies, it was attempted to isolate the bioactive compounds of several flavonoids while examining their antimicrobial activities against oral bacteria related to the establishment of periodontitis. Luteolin appeared to exhibit great antimicrobial activity against the oral microbes tested (including Porphyromonas gingivalis) due to the presence of hydroxyl group at the third position [94]. More specifically, it inhibited bacterial growth, leading to a reduction in the total count of bacteria. Taken together, the collected data suggest that luteolin has the potential, as a useful adjunct agent, to regulate both prevention and treatment of periodontal diseases.
References
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural Diversity of Polyphenols and Distribution in Foods. Diet. Polyphen. Their Metab. Health Eff. 2020, 1–29.
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591.
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246.
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243.
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202.
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435.
- Gurung, R.B.; Pandey, R.P.; Sohng, J.K. Apigenin and Naringenin: Natural Sources, Pharmacology and Role in Cancer Prevention. Chapter: Role of Apigenin in Cancer Prevention. Nova Sci. 2015.
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177.
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646.
- Chan, T.S.; Galati, G.; Pannala, A.S.; Rice-Evans, C.; O’Brien, P.J. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidase system. Free. Radic. Res. 2003, 37, 787–794.
- Hempel, J.; Pforte, H.; Raab, B.; Engst, W.; Bohm, H.; Jacobasch, G. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung 1999, 43, 201–204.
- Bogers, R.J.; Craker, L.E.; Lange, D. Medicinal and Aromatic Plants: Agricultural, Commercial, Ecological, Legal, Pharmacological and Social Aspects (Wageningen UR Frontis Series); Springer: Berlin, Germany, 2006; Volume 17, 309p.
- Giannoulis, K.; Evangelopoulos, V.; Gougoulias, N.; Wogiatzi, E. Could bio-stimulators affect flower, essential oil yield, and its composition in organic lavender (Lavandula angustifolia) cultivation? Ind. Crops Prod. 2020, 154, 112611.
- Leaman, D.J. The International Standard for Sustainable Wild Collection of Medicinal and Aromatic Plants (ISSC-MAP): Elements of ISSC-MAP Resource Assessment Guidance Relevant to Cites NDF; International Expert Workshop on CITES Non-Detriment Findings Perennial Plant Working Group (Ornamentals, Medicinal and Aromatic Plants Cancun, Mexico): Cancun, Mexico, 2008.
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17.
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688.
- Kadri, A.; Zarai, Z.; Chobba, I.B.; Bekir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical constituents and antioxidant properties of Rosmarinus officinalis L. essential oil cultivated from South-Western Tunisia. J. Med. Plants Res. 2011, 5, 5999–6004.
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243.
- Nur, A.; Mi-Yeon, K.; Jae, Y.C. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358.
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224.
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59.
- Kostić, Μ.; Kitić, D.; Petrović, M.B.; Jevtović-Stoimenov, T.; Jović, M.; Petrović, A.; Živanović, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59.
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442.
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692.
- Agiomyrgianaki, A.; Petrakis, P.V.; Dais, P. Influence of harvest year, cultivar and geographical origin on Greek extra virgin olive oils composition: A study by NMR spectroscopy and biometric analysis. Food Chem. 2012, 135, 2561–2568.
- Christophoridou, S.; Dais, P. Detection and quantification of phenolic compounds in olive oil by high resolution 1H nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2009, 633, 283–292.
- Kotsiou, K.; Tasioula-Margari, M. Monitoring the phenolic compounds of Greek extra-virgin olive oils during storage. Food Chem. 2016, 200, 255–262.
- Tsantili, E. Quality attributes and their relations in fresh black ripe “Kalamon” olives (Olea europaea L.) for table use—Phenolic compounds and total antioxidant capacity. Int. J. Food Sci. Technol. 2014, 49, 657–665.
- Salis, C.; Papadakis, I.E.; Hagidimitriou, M. Identification and Quantification of Phenolic Compounds in Fresh and Processed Table Olives of Cv. ‘kalamata’. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 1–13.
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 369–376.
- Mitsopoulos, G.; Papageorgiou, V.; Komaitis, M.; Hagidimitriou, M. Phenolic Profile of leaves and drupes in major Greek olive varieties. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 162–166.
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373.
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299.
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195.
- Miceli, N.; Taviano, M.F.; Giuffrida, D.; Trovato, A.; Tzakou, O.; Galati, E.M. Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham. J. Ethnopharmacol. 2005, 97, 261–266.
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52.
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671.
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Hydroglycerolic solvent and ultrasonication pretreatment: A green blend for high-efficiency extraction of Salvia fruticosa polyphenols. Sustainability 2020, 12, 4840.
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45.
- Choulitoudi, E.; Xristou, M.; Tsimogiannis, D.; Oreopoulou, V. The effect of temperature on the phenolic content and oxidative stability of o/w emulsions enriched with natural extracts from Satureja thymbra. Food Chem. 2021, 349, 129206.
- Barda, C.; Ciric, A.; Soković, M.; Tsoukalas, M.; Skaltsa, H. Phytochemical investigation of Crepis incana Sm. (Asteraceae) endemic to southern Greece. Biochem. Syst. Ecol. 2018, 80, 59–62.
- Chinou, I.; Harvala, C. Polyphenolic Constituents from the Leaves of two Cynara Species Growing in Greece. Planta Medica 1997, 63, 469–470.
- Petropoulos, S.A.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017, 8, 2022–2029.
- Tavarini, S.; Sgherri, C.; Ranieri, A.M.; Angelini, L.G. Effect of nitrogen fertilization and harvest time on steviol glycosides, flavonoid composition and antioxidant properties in Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 31, 7041–7050.
- Takahashi, A.; Ohnishi, T. The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biol. Sci. Space 2004, 18, 255–260.
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265.
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321.
- Salata, A.; Nurzynska-Wierdak, R.; Kalisz, A.; Kunicki, E.; Ibáñez-Asensio, S.; Moreno-Ramón, H. Effects of organic cropping on phenolic compounds and antioxidant capacity of globe artichoke herbs. Agronomy 2022, 12, 192.
- Ribes-Moya, A.M.; Adalid, A.M.; Raigon, M.D.; Hellin, P.; Fita, A.; Rodríguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223.
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809.
- Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248.
- Hodaei, Μ.; Rahimmalek, Μ.; Arzania, A.; Talebib, Μ. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304.
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of drought stress and UV-B radiation—Impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197.
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254.
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762.
- Shafeiee, M.; Ehsanzadeh, P. Physiological and biochemical mechanisms of salinity tolerance in several fennel genotypes: Existence of clearly-expressed genotypic variations. Ind. Crops Prod. 2019, 132, 311–318.
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M. Salinity stress alters ion homeostasis, antioxidant activities and the production of rosmarinic acid, luteolin and apigenin in Dracocephalum kotschyi Boiss. Biologia 2020, 75, 2147–2158.
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212.
- Abdallah, S.B.; Aung, B.; Amyot, L.; Lalin, I.; Lachaal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72.
- El-Shafey, N.M.; AbdElgawad, H. Luteolin, a bioactive flavone compound extracted from Cichorium endivia L. subsp. divaricatum alleviates the harmful effect of salinity on maize. Acta Physiol. Plant. 2012, 34, 2165–2177.
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320.
- Papageorgiou, V.; Mallouchos, A.; Komaitis, M. Investigation of the antioxidant behavior of air- and freeze-dried aromatic plant materials in relation to their phenolic content and vegetative cycle. J. Agric. Food Chem. 2008, 56, 5743–5752.
- Skoula, M.; Grayer, R.J.; Kite, G.C.; Veitch, N.C. Exudate flavones and flavanones in Origanum species and their interspecific variation. Biochem. Syst. Ecol. 2008, 36, 646–654.
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoran (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402.
- Theoharides, T.C.; Kempuraj, D.; Iliopoulou, B.P. Mast cells, T cells, and inhibition by luteolin: Implications for the pathogenesis and treatment of multiple sclerosis. Immune-Mediat. Dis. Theory Ther. 2007, 601, 423–430.
- Kempuraj, D.; Tagen, M.; Iliopoulou, B.P.; Clemons, A.; Vasiadi, M.; Boucher, W.; House, M.; Wolfberg, A.; Theoharides, T.C. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br. J. Pharmacol. 2008, 155, 1076–1084.
- Theoharides, T.C. Luteolin as a therapeutic option for multiple sclerosis. J. Neuroinflammation 2009, 6, 29.
- Kritas, S.K.; Saggini, A.; Varvara, G.; Murmura, G.; Caraffa, A.; Antinolfi, P.; Toniato, E.; Pantalone, A.; Neri, G.; Frydas, S.; et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 955–959.
- Lee, M.N.; Lee, Y.; Wu, D.; Pae, M. Luteolin inhibits NLRP3 inflammasome activation via blocking ASC oligomerization. J. Nutr. Biochem. 2021, 92, 108614.
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108.
- Shen, R.; Ma, L.; Zheng, Y. Anti-inflammatory effects of luteolin on acute gouty arthritis rats via TLR/MyD88/NF-kappaB pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 115–122.
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Hsiao, C.Y.; Wu, S.J. Luteolin Attenuates IL-1beta-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-kappaB and MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9421340.
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008.
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849.
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1alpha pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020, 141, 111436.
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711.
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366.
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503.
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29.
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208.
- Tan, L.; Liang, C.; Wang, Y.; Jiang, Y.; Zeng, S.; Tan, R. Pharmacodynamic effect of luteolin micelles on alleviating cerebral ischemia reperfusion injury. Pharmaceutics 2018, 10, 248.
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and evaluations of transdermally delivered luteolin loaded cationic nanoemulsion: In vitro and ex vivo evaluations. Pharmaceutics 2021, 13, 1218.
- Koromili, M.; Kapourani, A.; Barmpalexis, P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers 2023, 15, 169.
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11.
- Pang, J.; Yang, F.; Zhang, Z.; Yang, W.; Li, Y.; Xu, H. The role of luteolin nanocomposites in rheumatoid arthritis treatment. Mater. Express 2021, 11, 303–309.
- Dia, V.P.; Pangloli, P. Epithelial-to-mesenchymal transition in paclitaxel-resistant ovarian cancer cells is downregulated by luteolin. J. Cell. Physiol. 2017, 232, 391–401.
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66.
- Gilani, S.J.; Bin-Jumah, M.; Rizwanullah, M.; Imam, S.S.; Imtiyaz, K.; Alshehri, S.; Rizvi, M.M.A. Chitosan coated luteolin nanostructured lipid carriers: Optimization, In vitro-Ex vivo assessments and cytotoxicity study in breast cancer cells. Coatings 2021, 11, 158.
- Chatzopoulos, G.S.; Karakostas, P.; Kavakloglou, S.; Assimopoulou, A.; Barmpalexis, P.; Tsalikis, L. Clinical Effectiveness of Herbal Oral Care Products in Periodontitis Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10061.
- Gutiérrez-Venegas, G.; Kawasaki-Cárdenas, P.; Maldonado-Frías, S. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. Eur. J. Pharmacol. 2006, 541, 95–105.
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The effect of luteolin in prevention of periodontal disease in Wistar rats. J. Periodontol. 2019, 90, 1481–1489.
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 1–10.
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid Med. Cell Longev. 2022, 6, 9966750.
- Kariu, T.; Hamada, N.; Lakshmyya, K. Luteolin inhibits Porphyromonas gingivalis growth and alleviates alveolar bone destruction in experimental murine periodontitis. Biosci. Biotechnol. Biochem. 2023, zbad137.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine; Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
630
Revisions:
2 times
(View History)
Update Date:
19 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




