
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshihiro Mitaka | -- | 5420 | 2023-12-18 09:16:16 | | | |
| 2 | Mona Zou | + 347 word(s) | 5767 | 2023-12-21 02:01:49 | | |
Video Upload Options
Mature hepatocytes (MHs) in an adult rodent liver are categorized into the following three subpopulations based on their proliferative capability: type I cells (MH-I), which are committed progenitor cells that possess a high growth capability and basal hepatocytic functions; type II cells (MH-II), which possess a limited proliferative capability; and type III cells (MH-III), which lose the ability to divide (replicative senescence) and reach the final differentiated state. These subpopulations may explain the liver’s development and growth after birth. Generally, small-sized hepatocytes emerge in mammal livers. The cells are characterized by being morphologically identical to hepatocytes except for their size, which is substantially smaller than that of ordinary MHs. We initially discovered small hepatocytes (SHs) in the primary culture of rat hepatocytes.
1. SHs In Vitro
1.1. Culture Medium
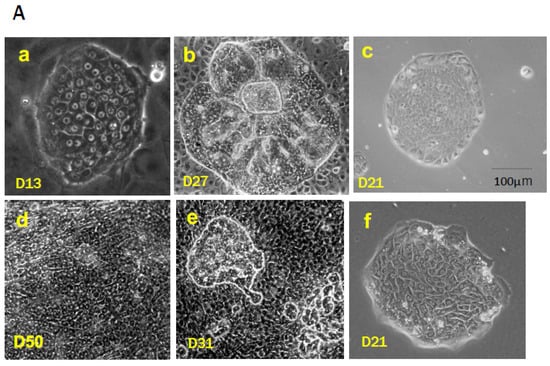
1.2. Purification of SHs
1.3. Self-Renewal of Hepatic Progenitor Cells
1.4. Characteristics of HPPCs
2. SH in Liver Lobules
2.1. The 2-AAF/PH Model
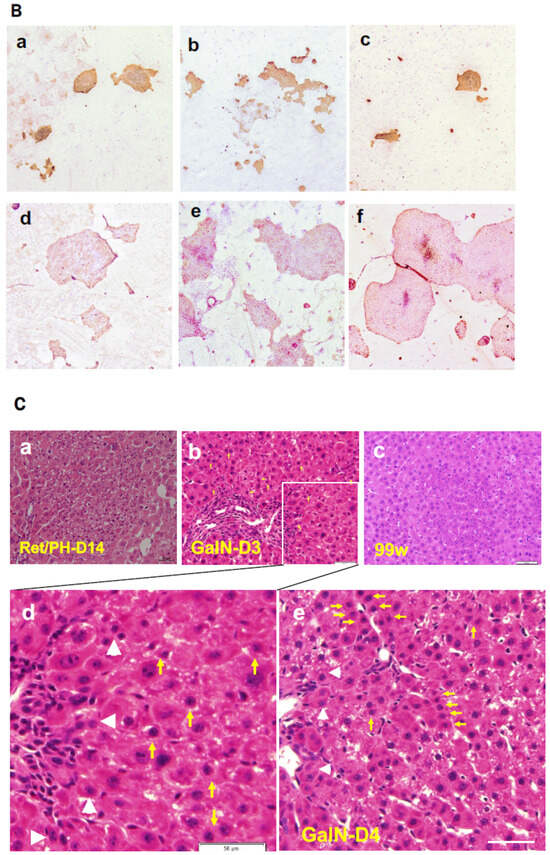
2.2. d-Galactosamine (GalN) Model
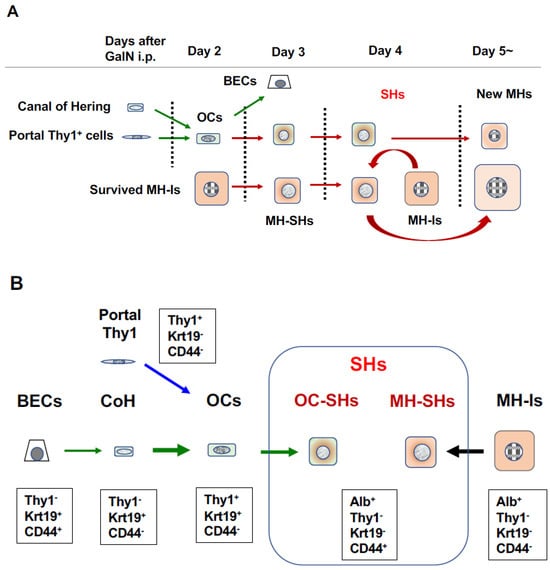
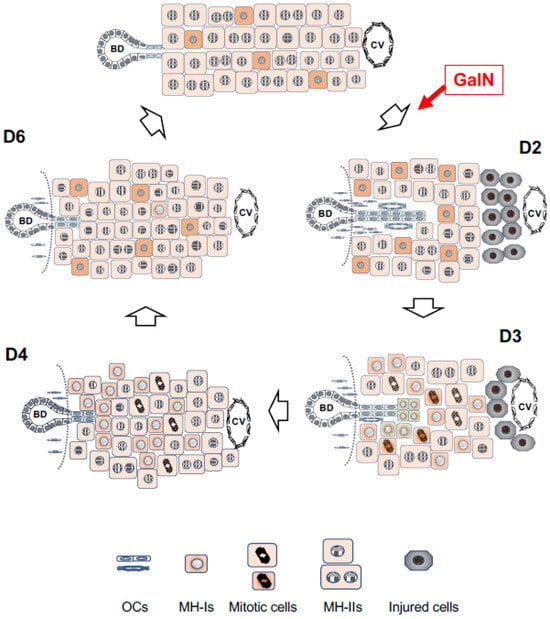
2.3. Retrorsine/PH Model
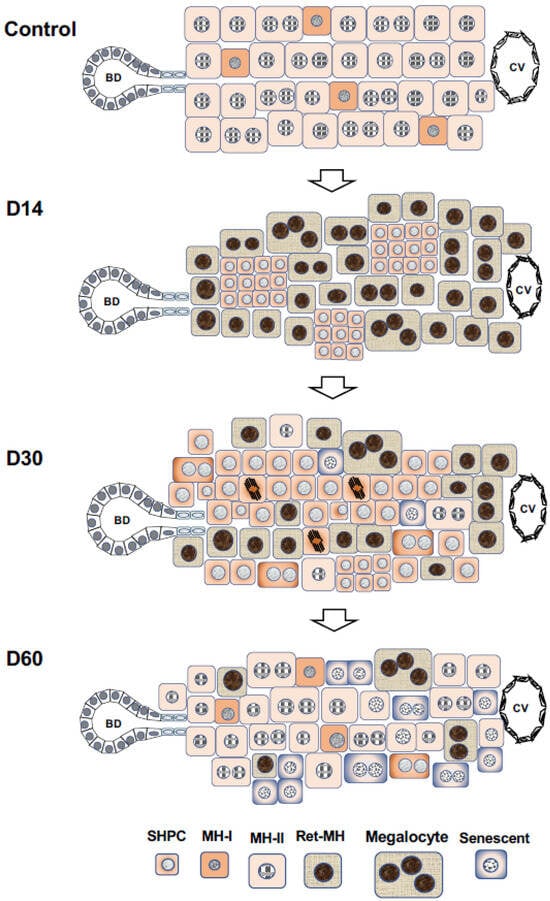
2.4. Appearance of Intermediate Cells in the Human Liver
References
- Mitaka, T.; Mikami, M.; Sattler, G.L.; Pitot, H.C.; Mochizuki, Y. Small cell colonies appear in the primary culture of adult rat hepatocytes in the presence of nicotinamide and epidermal growth factor. Hepatology 1992, 16, 440–447.
- Block, G.D.; Locker, J.; Bowen, W.C.; Petersen, B.E.; Katyal, S.; Strom, S.C.; Riley, T.; Howard, T.A.; Michalopoulos, G.K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996, 132, 1133–1149.
- Wu, J.C.; Merlino, G.; Cveklova, K.; Mosinger, B., Jr.; Fausto, N. Autonomous growth in serum-free medium and production of hepatocellular carcinomas by differentiated hepatocyte lines that overexpress transforming growth factor alpha 1. Cancer Res. 1994, 54, 5964–5973.
- Mitaka, T.; Sattler, G.L.; Pitot, H.C. Amino acid-rich medium (Leibovitz L-15) enhances and prolongs proliferation of primary cultured rat hepatocytes in the absence of serum. J. Cell. Physiol. 1991, 147, 495–504.
- Leibovitz, A. THE GROWTH AND MAINTENANCE OF TISSUE–CELL CULTURES IN FREE GAS EXCHANGE WITH THE ATMOSPHERE1. Am. J. Epidemiology 1963, 78, 173–180.
- Seglen, P.O.; Gordon, P.B.; Schwarze, P.E. Autophagy and protein degradation in rat hepatocytes. In Isolation, Characterization, and Use of Hepatocytes; Harris, R.A., Cornell, N.W., Eds.; Elsevier: New York, NY, USA, 1983; pp. 153–163.
- Mitaka, T. The current status of primary hepatocyte culture. Int. J. Exp. Pathol. 2002, 79, 393–409.
- Cable, E.E.; Isom, H.C. Exposure of primary rat hepatocytes in long-term DMSO culture to selected transition metals induces hepatocyte proliferation and formation of duct-like structures. Hepatology 1997, 26, 1444–1457.
- Isom, H.C.; Secott, T.; Georgoff, I.; Woodworth, C.; Mummaw, J. Maintenance of differentiated rat hepatocytes in primary culture. Proc. Natl. Acad. Sci. USA 1985, 82, 3252–3256.
- Mitaka, T.; Sato, F.; Mizuguchi, T.; Yokono, T.; Mochizuki, Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology 1999, 29, 111–125.
- Mitaka, T. Reconstruction of hepatic organoid by hepatic stem cells. J. Hepato-Biliary-Pancreatic Surg. 2002, 9, 697–703.
- Mitaka, T.; Kojima, T.; Mizuguchi, T.; Mochizuki, Y. Growth and Maturation of Small Hepatocytes Isolated from Adult Rat Liver. Biochem. Biophys. Res. Commun. 1995, 214, 310–317.
- Sugimoto, S.; Mitaka, T.; Ikeda, S.; Harada, K.; Ikai, I.; Yamaoka, Y.; Mochizuki, Y. Morphological changes induced by extracellular matrix are correlated with maturation of rat small hepatocytes. J. Cell. Biochem. 2002, 87, 16–28.
- Ikeda, S.; Mitaka, T.; Harada, K.; Sugimoto, S.; Hirata, K.; Mochizuki, Y. Proliferation of rat small hepatocytes after long-term cryopreservation. J. Hepatol. 2002, 37, 7–14.
- Ooe, H.; Kon, J.; Miyamoto, S.; Ozone, Y.; Ninomiya, S.-I.; Mitaka, T. Cytochrome P450 Expression of Cultured Rat Small Hepatocytes after Long-Term Cryopreservation. Drug Metab. Dispos. 2006, 34, 1667–1671.
- Kon, J.; Ooe, H.; Oshima, H.; Kikkawa, Y.; Mitaka, T. Expression of CD44 in rat hepatic progenitor cells. J. Hepatol. 2006, 45, 90–98.
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189–196.
- Ichinohe, N.; Tanimizu, N.; Ooe, H.; Nakamura, Y.; Mizuguchi, T.; Kon, J.; Hirata, K.; Mitaka, T. Differentiation capacity of hepatic stem/progenitor cells isolated from D -galactosamine-treated rat livers. Hepatology 2012, 57, 1192–1202.
- Kon, J.; Ichinohe, N.; Ooe, H.; Chen, Q.; Sasaki, K.; Mitaka, T. Thy1-Positive Cells Have Bipotential Ability to Differentiate into Hepatocytes and Biliary Epithelial Cells in Galactosamine-Induced Rat Liver Regeneration. Am. J. Pathol. 2009, 175, 2362–2371.
- Hansen, B.; Longati, P.; Elvevold, K.; Nedredal, G.-I.; Schledzewski, K.; Olsen, R.; Falkowski, M.; Kzhyshkowska, J.; Carlsson, F.; Johansson, S.; et al. Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Exp. Cell Res. 2005, 303, 160–173.
- Mouta Carreira, C.; Nasser, S.M.; di Tomaso, E.; Padera, T.P.; Boucher, Y.; Tomarev, S.I.; Jain, R.K. LYVE-1 is not restricted to the lymph vessels: Expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001, 61, 8079–8084.
- Chen, Q.; Kon, J.; Ooe, H.; Sasaki, K.; Mitaka, T. Selective proliferation of rat hepatocyte progenitor cells in serum-free culture. Nat. Protoc. 2007, 2, 1197–1205.
- Ishii, M.; Kino, J.; Ichinohe, N.; Tanimizu, N.; Ninomiya, T.; Suzuki, H.; Mizuguchi, T.; Hirata, K.; Mitaka, T. Hepatocytic parental progenitor cells of rat small hepatocytes maintain self-renewal capability after long-term culture. Sci. Rep. 2017, 7, 46177.
- Kleinman, H.K.; McGarvey, M.L.; Liotta, L.A.; Robey, P.G.; Tryggvason, K.; Martin, G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 1982, 21, 6188–6193.
- Nishiuchi, R.; Takagi, J.; Hayashi, M.; Ido, H.; Yagi, Y.; Sanzen, N.; Tsuji, T.; Yamada, M.; Sekiguchi, K. Ligand-binding specificities of laminin-binding integrins: A comprehensive survey of laminin–integrin interactions using recombinant α3β1, α6β1, α7β1 and α6β4 integrins. Matrix Biol. 2006, 25, 189–197.
- Yamada, M.; Sekiguchi, K. Molecular basis of laminin-integrin interactions. Curr. Top. Membr. 2015, 76, 197–229.
- Kikkawa, Y.; Mochizuki, Y.; Miner, J.H.; Mitaka, T. Transient expression of laminin α1 chain in regenerating murine liver: Restricted localization of laminin chains and nidogen-1. Exp. Cell Res. 2005, 305, 99–109.
- Kino, J.; Ichinohe, N.; Ishii, M.; Suzuki, H.; Mizuguchi, T.; Tanimizu, N.; Mitaka, T. Self-Renewal Capability of Hepatocytic Parental Progenitor Cells Derived From Adult Rat Liver Is Maintained Long Term When Cultured on Laminin 111 in Serum-Free Medium. Hepatol. Commun. 2019, 4, 21–37.
- Tanimizu, N.; Kikkawa, Y.; Mitaka, T.; Miyajima, A. α1- and α5-containing Laminins Regulate the Development of Bile Ducts via β1 Integrin Signals. J. Biol. Chem. 2012, 287, 28586–28597.
- Takayama, K.; Nagamoto, Y.; Mimura, N.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Hayakawa, T.; Kawabata, K.; Mizuguchi, H. Long-Term Self-Renewal of Human ES/iPS-Derived Hepatoblast-like Cells on Human Laminin 111-Coated Dishes. Stem Cell Rep. 2013, 1, 322–335.
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615.
- Suzuki, A.; Zheng, Y.-W.; Kaneko, S.; Onodera, M.; Fukao, K.; Nakauchi, H.; Taniguchi, H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 2002, 156, 173–184.
- Tanimizu, N.; Nishikawa, M.; Saito, H.; Tsujimura, T.; Miyajima, A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003, 116, 1775–1786.
- Tanimizu, N.; Saito, H.; Mostov, K.; Miyajima, A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J. Cell Sci. 2004, 117, 6425–6434.
- Tanimizu, N.; Ichinohe, N.; Ishii, M.; Kino, J.; Mizuguchi, T.; Hirata, K.; Mitaka, T. Liver Progenitors Isolated from Adult Healthy Mouse Liver Efficiently Differentiate to Functional Hepatocytes In Vitro and Repopulate Liver Tissue. Stem Cells 2016, 34, 2889–2901.
- Kubota, H.; Reid, L.M. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl. Acad. Sci. USA 2000, 97, 12132–12137.
- Font-Burgada, J.; Shalapour, S.; Ramaswamy, S.; Hsueh, B.; Rossell, D.; Umemura, A.; Taniguchi, K.; Nakagawa, H.; Valasek, M.A.; Ye, L.; et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015, 162, 766–779.
- Pu, W.; Zhang, H.; Huang, X.; Tian, X.; He, L.; Wang, Y.; Zhang, L.; Liu, Q.; Li, Y.; Li, Y.; et al. Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nat. Commun. 2016, 7, 13369.
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185.
- Zhao, L.; Jin, Y.; Donahue, K.; Tsui, M.; Fish, M.; Logan, C.Y.; Wang, B.; Nusse, R. Tissue Repair in the Mouse Liver Following Acute Carbon Tetrachloride Depends on Injury-Induced Wnt/β-Catenin Signaling. Hepatology 2019, 69, 2623–2635.
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250.
- Lin, S.; Nascimento, E.M.; Gajera, C.R.; Chen, L.; Neuhöfer, P.; Garbuzov, A.; Wang, S.; Artandi, S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018, 556, 244–248.
- Planas-Paz, L.; Orsini, V.; Boulter, L.; Calabrese, D.; Pikiolek, M.; Nigsch, F.; Xie, Y.; Roma, G.; Donovan, A.; Marti, P.; et al. The RSPO–LGR4/5–ZNRF3/RNF43 module controls liver zonation and size. Nature 2016, 18, 467–479.
- Ang, C.H.; Hsu, S.H.; Guo, F.; Tan, C.T.; Yu, V.C.; Visvader, J.E.; Chow, P.K.H.; Fu, N.Y. Lgr5+ pericentral hepatocytes are self-maintained in normal liver regeneration and susceptible to hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 19530–19540.
- He, L.; Pu, W.; Liu, X.; Zhang, Z.; Han, M.; Li, Y.; Huang, X.; Han, X.; Li, Y.; Liu, K.; et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 2021, 371, 905.
- Matsumoto, T.; Wakefield, L.; Tarlow, B.D.; Grompe, M. In Vivo Lineage Tracing of Polyploid Hepatocytes Reveals Extensive Proliferation during Liver Regeneration. Cell Stem Cell 2019, 26, 34–47.e3.
- May, S.; Müller, M.; Livingstone, C.R.; Skalka, G.L.; Walsh, P.J.; Nixon, C.; Hedley, A.; Shaw, R.; Clark, W.; Voorde, J.V.; et al. Absent expansion of AXIN2+ hepatocytes and altered physiology in Axin2CreERT2 mice challenges the role of pericentral hepatocytes in homeostatic liver regeneration. J. Hepatol. 2023, 78, 1028–1036.
- Reid, L.M. Paradoxes in studies of liver regeneration: Relevance of the parable of the blind men and the elephant. Hepatology 2015, 62, 330–333.
- Sun, T.; Pikiolek, M.; Orsini, V.; Bergling, S.; Holwerda, S.; Morelli, L.; Hoppe, P.S.; Planas-Paz, L.; Yang, Y.; Ruffner, H.; et al. AXIN2+ Pericentral Hepatocytes Have Limited Contributions to Liver Homeostasis and Regeneration. Cell Stem Cell 2019, 26, 97–107.e6.
- Wei, Y.; Wang, Y.G.; Jia, Y.; Li, L.; Yoon, J.; Zhang, S.; Wang, Z.; Zhang, Y.; Zhu, M.; Sharma, T.; et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021, 371, 906.
- Mitaka, T.; Norioka, K.; Sattler, G.L.; Pitot, H.C.; Mochizuki, Y. Effect of age on the formation of small-cell colonies in cultures of primary rat hepatocytes. Cancer Res. 1993, 53, 3145–3148.
- Sigal, S.H.; Gupta, S.; Gebhard, D.F., Jr.; Holst, P.; Neufeld, D.; Reid, L.M. Evidence for a terminal differentiation process in the liver. Differentiation 1995, 59, 35–42.
- Tateno, C.; Takai-Kajihara, K.; Yamasaki, C.; Sato, H.; Yoshizato, K. Heterogeneity of growth potential of adult rat hepatocytesin vitro. Hepatology 2000, 31, 65–74.
- Asahina, K.; Shiokawa, M.; Ueki, T.; Yamasaki, C.; Aratani, A.; Tateno, C.; Yoshizato, K. Multiplicative mononuclear small hepatocytes in adult rat liver: Their isolation as a homogeneous population and localization to periportal zone. Biochem. Biophys. Res. Commun. 2006, 342, 1160–1167.
- Nagy, P.; Thorgeirsson, S.S.; Paku, S.; Kopper, L. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: Confocal and electron microscopic studies. Hepatology 2004, 39, 1353–1361.
- Alison, M.R.; Golding, M.; Sarraf, C.; Edwards, R.; Lalani, E. Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology 1996, 110, 1182–1190.
- Dezső, K.; Papp, V.; Bugyik, E.; Hegyesi, H.; Sáfrány, G.; Bödör, C.; Nagy, P.; Paku, S. Structural analysis of oval-cell-mediated liver regeneration in rats. Hepatology 2012, 56, 1457–1467.
- Evarts, R.P.; Nagy, P.; Marsden, E.; Thorgeirsson, S.S. In situ hybridization studies on expression of albumin and a-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987, 47, 5469–5475.
- Sarraf, C.; Lalani, E.N.; Golding, M.; Anilkumar, T.V.; Poulsom, R.; Alison, M. Cell behavior in the acetylaminofluorene-treated regenerating rat liver. Light. and electron microscopic observations. Am. J. Pathol. 1994, 145, 1114–1126.
- DeBaun, J.R.; Rowley, J.Y.; Miller, E.C.; Miller, J.A. Sulfotransferase Activation of N-Hydroxy-2-acetylaminofluorene in Rodent Livers Susceptible and Resistant to this Carcinogen. Exp. Biol. Med. 1968, 129, 268–273.
- Petersen, B.E.; Goff, J.P.; Greenberger, J.S.; Michalopoulos, G.K. Hepatic oval cells express the hematopoietic stem cell marker thy-1 in the rat. Hepatology 1998, 27, 433–445.
- Petersen, B.E.; Bowen, W.C.; Patrene, K.D.; Mars, W.M.; Sullivan, A.K.; Murase, N.; Boggs, S.S.; Greenberger, J.S.; Goff, J.P. Bone Marrow as a Potential Source of Hepatic Oval Cells. Science 1999, 284, 1168–1170.
- Dezső, K.; Jelnes, P.; László, V.; Baghy, K.; Bödör, C.; Paku, S.; Tygstrup, N.; Bisgaard, H.C.; Nagy, P. Thy-1 Is Expressed in Hepatic Myofibroblasts and Not Oval Cells in Stem Cell-Mediated Liver Regeneration. Am. J. Pathol. 2007, 171, 1529–1537.
- Dudas, J.; Mansuroglu, T.; Batusic, D.; Saile, B.; Ramadori, G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007, 329, 503–514.
- Bachmann, W.; Harms, E.; Hassels, B.; Henninger, H.; Reuitter, W. Studies on rat liver plasma membrane. Altered protein and phospholipid metabolism after injection of d-galactosamine. Biochem. J. 1977, 166, 455–462.
- Decker, K.; Keppler, D. Galactosamine hepatitis: Key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 1974, 71, 78–106.
- Lesch, R.; Reutter, W.; Keppler, D.; Decker, K. Liver restitution after acute galactosamine hepatitis: Autoradiographic and biochemical studies in rats. Exp. Mol. Pathol. 1970, 12, 58–69.
- Medline, A.; Schaffner, F.; Popper, H. Ultrastructural features in galactosamine-induced hepatitis. Exp. Mol. Pathol. 1970, 12, 201–211.
- Ichinohe, N.; Kon, J.; Sasaki, K.; Nakamura, Y.; Ooe, H.; Tanimizu, N.; Mitaka, T. Growth Ability and Repopulation Efficiency of Transplanted Hepatic Stem Cells, Progenitor Cells, and Mature Hepatocytes in Retrorsine-Treated Rat Livers. Cell Transplant. 2012, 21, 11–22.
- Fiegel, H.C.; Park, J.J.H.; Lioznov, M.V.; Martin, A.; Jaeschke-Melli, S.; Kaufmann, P.M.; Fehse, B.; Zander, A.R.; Kluth, D. Characterization of cell types during rat liver development. Hepatology 2003, 37, 148–154.
- Lázaro, C. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology 2003, 38, 1095–1106.
- Masson, N.M.; Currie, I.S.; Terrace, J.D.; Garden, O.J.; Parks, R.W.; Ross, J.A. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am. J. Physiol. Liver Physiol. 2006, 291, G45–G54.
- Oertel, M.; Menthena, A.; Chen, Y.-Q.; Shafritz, D.A. Comparison of hepatic properties and transplantation of Thy-1+ and Thy-1− cells isolated from embryonic day 14 rat fetal liver. Hepatology 2007, 46, 1236–1245.
- McLean, E.K. The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol. Rev. 1970, 42, 429–483.
- Gordon, G.J.; Coleman, W.B.; Hixson, D.C.; Grisham, J.W. Liver Regeneration in Rats with Retrorsine-Induced Hepatocellular Injury Proceeds through a Novel Cellular Response. Am. J. Pathol. 2000, 156, 607–619.
- Dabeva, M.D.; Laconi, E.; Oren, R.; Petkov, P.M.; Hurston, E.; Shafritz, D.A. Liver regeneration and a-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998, 58, 5825–5834.
- Laconi, E.; Sarma, D.; Pani, P. Transplantation of normal hepatocytes modulates the development of chronic liver lesions induced by a pyrrolizidine alkaloid, lasiocarpinet. Carcinog. 1995, 16, 139–142.
- Laconi, E.; Oren, R.; Mukhopadhyay, D.K.; Hurston, E.; Laconi, S.; Pani, P.; Dabeva, M.D.; Shafritz, D.A. Long-Term, Near-Total Liver Replacement by Transplantation of Isolated Hepatocytes in Rats Treated with Retrorsine. Am. J. Pathol. 1998, 153, 319–329.
- Oren, R.; Dabeva, M.D.; Karnezis, A.N.; Petkov, P.M.; Rosencrantz, R.; Sandhu, J.P.; Moss, S.F.; Wang, S.; Hurston, E.; Laconi, E.; et al. Role of thyroid hormone in stimulating liver repopulation in the rat by transplanted hepatocytes. Hepatology 1999, 30, 903–913.
- Gordon, G.J.; Coleman, W.B.; Grisham, J.W. Bax-mediated apoptosis in the livers of rats after partial hepatectomy in the retrorsine model of hepatocellular injury. Hepatology 2000, 32, 312–320.
- Ichinohe, N.; Ishii, M.; Tanimizu, N.; Kon, J.; Yoshioka, Y.; Ochiya, T.; Mizuguchi, T.; Hirata, K.; Mitaka, T. Transplantation of Thy1+ Cells Accelerates Liver Regeneration by Enhancing the Growth of Small Hepatocyte-Like Progenitor Cells via IL17RB Signaling. Stem Cells 2016, 35, 920–931.
- Best, D.H.; Coleman, W.B. Cells of origin of small hepatocyte-like progenitor cells in the retrorsine model of rat liver injury and regeneration. J. Hepatol. 2008, 48, 369–371.
- Pichard, V.; Ferry, N. Origin of small hepatocyte-like progenitor in retrorsine-treated rats. J. Hepatol. 2008, 48, 368–369.
- Chen, Y.-H.; Chang, M.-H.; Chien, C.-S.; Wu, S.-H.; Yu, C.-H.; Chen, H.-L. Contribution of mature hepatocytes to small hepatocyte-like progenitor cells in retrorsine-exposed rats with chimeric livers. Hepatology 2012, 57, 1215–1224.
- Vig, P.; Russo, F.P.; Edwards, R.J.; Tadrous, P.J.; Wright, N.A.; Thomas, H.C.; Alison, M.R.; Forbes, S.J. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology 2006, 43, 316–324.
- Avril, A.; Pichard, V.; Bralet, M.-P.; Ferry, N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J. Hepatol. 2004, 41, 737–743.
- Pichard, V.; Aubert, D.; Ferry, N. Direct In Vivo Cell Lineage Analysis in the Retrorsine and 2AAF Models of Liver Injury after Genetic Labeling in Adult and Newborn Rats. PLoS ONE 2009, 4, e7267.
- Coleman, W.B.; Best, D.H. Cellular responses in experimental liver injury: Possible cellular origins of regenerative stem-like progenitor cells. Hepatology 2005, 41, 1173–1176.
- Gordon, G.J.; Coleman, W.B.; Grisham, J.W. Temporal Analysis of Hepatocyte Differentiation by Small Hepatocyte-Like Progenitor Cells during Liver Regeneration in Retrorsine-Exposed Rats. Am. J. Pathol. 2000, 157, 771–786.
- Hulla, J.E.; Juchau, M.R. Occurrence and inducibility of cytochrome P 450IIIA in maternal and fetal rats during prenatal development. Biochemistry 1989, 28, 4871–4879.
- Omiecinski, C.J.; Hassett, C.; Costa, P. Developmental expression and in situ localization of the phenobarbital-inducible rat hepatic mRNAs for cytochromes CYP2B1, CYP2B2, CYP2C6, and CYP 3A1. Mol. Pharmacol. 1990, 38, 462–470.
- Rich, K.J.; Boobis, A.R. Expression and inducibility of P450 enzymes during liver ontogeny. Microsc. Res. Tech. 1997, 39, 424–435.
- Gordon, G.J.; Coleman, W.B.; Grisham, J.W. Induction of Cytochrome P450 Enzymes in the Livers of Rats Treated with the Pyrrolizidine Alkaloid Retrorsine. Exp. Mol. Pathol. 2000, 69, 17–26.
- Ichinohe, N.; Ishii, M.; Tanimizu, N.; Mizuguchi, T.; Yoshioka, Y.; Ochiya, T.; Suzuki, H.; Mitaka, T. Extracellular vesicles containing miR-146a-5p secreted by bone marrow mesenchymal cells activate hepatocytic progenitors in regenerating rat livers. Stem Cell Res. Ther. 2021, 12, 312.
- Gordon, G.J.; Butz, G.M.; Grisham, J.W.; Coleman, W.B. Isolation, short-term culture, and transplantation of small hepatocyte-like progenitor cells from retrorsine-exposed rats1. Transplantation 2002, 73, 1236–1243.
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745.
- Libbrecht, L.; Desmet, V.; Van Damme, B.; Roskams, T. Deep intralobular extension of human hepatic ‘progenitor cells’ correlates with parenchymal inflammation in chronic viral hepatitis: Can ‘progenitor cells’ migrate? J. Pathol. 2000, 192, 373–378.
- Lowes, K.N.; Brennan, B.A.; Yeoh, G.C.; Olynyk, J.K. Oval Cell Numbers in Human Chronic Liver Diseases Are Directly Related to Disease Severity. Am. J. Pathol. 1999, 154, 537–541.
- Roskams, T.; Yang, S.Q.; Koteish, A.; Durnez, A.; DeVos, R.; Huang, X.; Achten, R.; Verslype, C.; Diehl, A.M. Oxidative Stress and Oval Cell Accumulation in Mice and Humans with Alcoholic and Nonalcoholic Fatty Liver Disease. Am. J. Pathol. 2003, 163, 1301–1311.
- Van Eyken, P.; Sciot, R.; Callea, F.; Van Der Steen, K.; Moerman, P.; Desmet, V.J. The development of the intrahepatic bile ducts in man: A keratin-immunohistochemical study. Hepatology 1988, 8, 1586–1595.
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011, 458, 251–259.
- Weng, H.-L.; Cai, X.; Yuan, X.; Liebe, R.; Dooley, S.; Li, H.; Wang, T.-L. Two sides of one coin: Massive hepatic necrosis and progenitor cell-mediated regeneration in acute liver failure. Front. Physiol. 2015, 6, 178.
- Dezső, K.; Nagy, P.; Paku, S. Human liver regeneration following massive hepatic necrosis: Two distinct patterns. J. Gastroenterol. Hepatol. 2019, 35, 124–134.
- Lefkowitch, J.H. The Pathology of Acute Liver Failure. Adv. Anat. Pathol. 2016, 23, 144–158.
- Ko, S.; Russell, J.O.; Molina, L.M.; Monga, S.P. Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 23–50.
- Katoonizadeh, A.; Nevens, F.; Verslype, C.; Pirenne, J.; Roskams, T. Liver regeneration in acute severe liver impairment: A clinicopathological correlation study. Liver Int. 2006, 26, 1225–1233.




