Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vikas Sharma | -- | 3773 | 2023-12-15 16:08:20 | | | |
| 2 | Jason Zhu | Meta information modification | 3773 | 2023-12-18 02:42:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharma, V.; Ankita, ..; Karnwal, A.; Sharma, S.; Kamal, B.; Jadon, V.S.; Gupta, S.; Sivanasen, I. Micropropagation and Its Applications of Eucalyptus Plantations. Encyclopedia. Available online: https://encyclopedia.pub/entry/52817 (accessed on 09 February 2026).
Sharma V, Ankita ., Karnwal A, Sharma S, Kamal B, Jadon VS, et al. Micropropagation and Its Applications of Eucalyptus Plantations. Encyclopedia. Available at: https://encyclopedia.pub/entry/52817. Accessed February 09, 2026.
Sharma, Vikas, . Ankita, Arun Karnwal, Shivika Sharma, Barkha Kamal, Vikash S. Jadon, Sanjay Gupta, Iyyakkannu Sivanasen. "Micropropagation and Its Applications of Eucalyptus Plantations" Encyclopedia, https://encyclopedia.pub/entry/52817 (accessed February 09, 2026).
Sharma, V., Ankita, .., Karnwal, A., Sharma, S., Kamal, B., Jadon, V.S., Gupta, S., & Sivanasen, I. (2023, December 15). Micropropagation and Its Applications of Eucalyptus Plantations. In Encyclopedia. https://encyclopedia.pub/entry/52817
Sharma, Vikas, et al. "Micropropagation and Its Applications of Eucalyptus Plantations." Encyclopedia. Web. 15 December, 2023.
Copy Citation
The genus Eucalyptus is a globally captivated source of hardwood and is well known for its medicinal uses. The hybrid and wild species of Eucalyptus are widely used as exotic plantations due to their renowned potential of adapting to various systems and sites, and rapid large-scale propagation of genetically similar plantlets, which further leads to the extensive propagation of this species. Tissue culture plays a crucial role in the preservation, propagation, and genetic improvement of Eucalyptus species. Despite unquestionable progression in biotechnological and tissue culture approaches, the productivity of plantations is still limited, often due to the low efficiency of clonal propagation from cuttings.
Eucalyptus
tissue culture
F1 hybrids
clonal propagation
1. Micropropagation and Its Applications
Achieving true-to-type plants with desirable traits using in vitro protocols is termed micropropagation (Figure 1 and Figure 2). Five critical stages need to be accomplished to successfully establish micropropagation [1]. These five stages include (i) Stage 0, defined as the preparatory stage for developing efficient and reproducible protocol; (ii) Stage 1, which aims to establish an aseptic and viable culture; (iii) Stage 2, which involves propagation without immolating objective to be achieved; (iv) Stage 3, involving large-scale propagation; and (v) Stage 4, the hardening stage of the established plant material. Each of the stages involved is discussed comprehensively in subsequent sections. Figure 3 illustrates different events of Eucalyptus micropropagation.
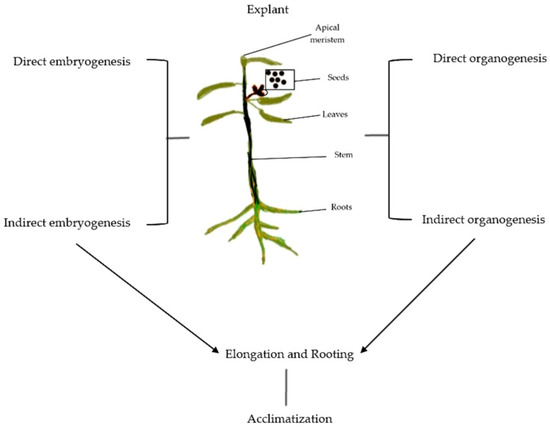
Figure 1. Schematic illustration of Eucalyptus micropropagation presenting sources of explant and basic steps involved in the protocol.
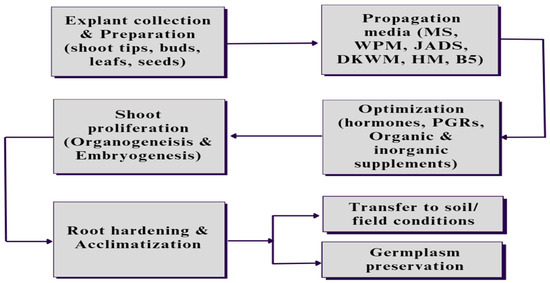
Figure 2. The steps involved in Eucalyptus micropropagation start with the collection and preparation of explants, which could be an axial leaf, shoot tips, buds, or seeds, followed by inoculating and culturing in specific media with the optimized concentration of the hormones and other nutritional supplements based on the requirement of the plant as well as environmental conditions. After shoot proliferation, the developed plants are subjected to root proliferation and root hardening in field conditions, or the germplasm of the developed plants could be preserved for future use in similar related protocols.
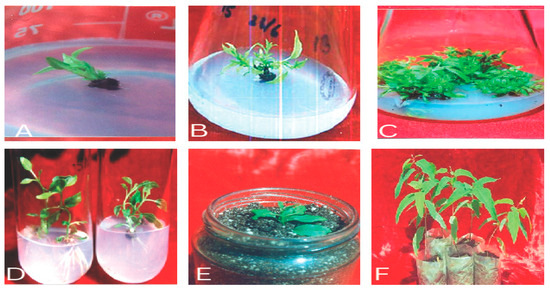
Figure 3. (A–F) Different stages in Eucalyptus micropropagation: (A) aseptic shoot/explant inoculation; (B) aseptic culture establishment; (C) shoot proliferation; (D) in vitro rhizogenesis of microshoots; (E) hardening; (F) acclimatization and field transfer.
1.1. Establishing Axenic Culture
For ensuring the success of micropropagation, maintaining uncontaminated cultures are necessary throughout the protocol. The foremost important step in the in vitro propagation of plants is to establish a microbial contamination-free culture because the primary explants employed are nonsterile and thus a major source of microbial contamination in the culture [2]. The nodal segments bearing axillary buds, shoot tips, lead discs, and seeds could be used as explants to initiate tissue culture. The use of seeds as explants does not lead to true-to-type propagation; however, due to the ease of decontamination and juvenility of young seedlings, this method is superior for rapid clonal propagation [3][4]. Moreover, dormant axillary buds from nodes are widely used for maintaining clonal fidelity, providing thousands of plantlets via rapid propagation with high multiplication rates. The propagation from nodal segments is also reported in the micropropagation of woody plants like bamboo [5]. Nonetheless, the explants from leaf discs and shoot tips provide true-to-type propagation, although they are least preowned due to onerous sterilization [3][5]. The initial step involves the surface sterilization of the explant, which is usually achieved by first rinsing/washing the explant with nonsterilized water, immersing it in ethyl alcohol (70%), or treating it with chlorine-based sterilant such as calcium hypochlorite (Ca(OCl)2, mercuric chloride (HgCl2), and sodium hypochlorite (NaOCl), followed by rinsing with sterile distilled water. To prevent drying and increase the interaction between the explant surface and sterilant, a drop of wetting agents such as Tween 20 is often added. Although chlorine-based sterilants can efficiently sterilize the explants, their use is associated with toxic effects on living cells. Mercuric chloride has been reported to cause mammalian toxicity. So, the use of either calcium hypochlorite or sodium hypochlorite is recommended [6][7]. The basic explant sterilization of Eucalyptus is achieved by treating it with sodium hypochlorite at 67–1340 mM concentrations. However, the concentrations of sodium hypochlorite must be optimized before experimentation as the concentration affects the overall success of micropropagation. For instance, as reported, the seed germination of Corymbia citridora and Corymbia torelliana hybrid was decreased when the concentration of NaOCl was increased from 134 to 670 mM [8]. The optimization of the sterilization protocol of five different clones of eucalypt was performed using 1% sodium hypochlorite, 0.1% mercuric chloride, 1 mg/mL of rifampicin, and 70% ethanol as sterilant; the study suggested that, along with 1% sodium hypochlorite and 70% ethanol, using 0.1% mercuric chloride for 3 min was optimum, and using 1 mg/mL of rifampicin for 5 min was optimum for effective sterilization [9].
Leaching out of phenolics from cultured explants is also a main concern that prevents the establishment of in vitro cultures to a large extent. Reports suggest that nodal segments cultured on MS medium exudated phenolics. The number of phenolics exudated by nodal segments and bud-break response varied with the month of the collection of explants. Nodal segments were reported to be collected every month from January to December. It was found that explants collected during April and July to September showed the least phenolic exudation and better bud-break response comparatively and were best for in vitro studies. The phenolic exudation was high from October to January and May to June with poor bud-break responses [10].
1.2. In Vitro Proliferation of Shoot
1.2.1. Organogenesis
Organogenesis refers to the process through which shoot regeneration occurs via the differentiation and development of new shoots from pre-existing meristematic cells or tissues. In this pathway, the initial step involves the induction of adventitious shoots from explants such as leaf, stem, or root segments. The explants are cultured on a nutrient medium supplemented with specific plant growth regulators, particularly cytokinin, which stimulate the formation of shoot primordia. These primordia then undergo further growth and differentiation to develop into shoots with organized structures, including leaves and stems. Organogenesis is often characterized by the initiation of multiple shoots, referred to as shoot proliferation or multiplication [11]. The formation of new organs directly from explants is termed direct organogenesis, while the formation of new organs from cell cultures or suspensions, tissues, or calluses is termed indirect organogenesis [11][12][13]. Furthermore, organogenesis also includes the regeneration of roots from explants, which is known as direct root organogenesis, wherein new roots are directly developed from explants. The development of new organs depends on a variety of factors, such as hormones and type of media [14]. The differential media and nutrients employed in Eucalyptus micropropagation are discussed in the next section. Mostly basal MS salts and Murashige Skoog media of different strengths are used for establishing organogenesis in Eucalyptus. Besides these, Woody Plant Medium; Driver and Kuniyaki Walnut medium; Juan, Antonio, Diva, and Salvin medium; Schenk and Hildebrandt medium; and B5 medium have also been extensively used [11][15][16].
1.2.2. Somatic Embryogenesis
The development of bipolar embryos from somatic cells/tissues asexually is termed somatic embryogenesis [17]. It is a pathway for shoot, root, and plantlet regeneration that mimics the process of embryo development in plants that involves the dedifferentiation of somatic cells to a totipotent state, where they regain the ability to form an embryonic structure. Somatic embryos can be formed via two pathways, direct embryogenesis in which the embryo develops from pre-embryonic cells, or indirect embryogenesis in which the embryo develops from a callus grown on culture media. Pre-embryonic cells are undifferentiated cells that have the potency to differentiate into any kind of cells. Generally, apical meristems, hypocotyls, and epicotyls serve as a source of pre-embryonic cells as these contain undifferentiated cells [11][17]. A protocol for somatic embryogenesis has been reported by [18], suggesting that the leaves from adult trees are better for inducing somatic embryogenesis than floral tissues. The dedifferentiated cells progress through various stages of embryo development, including the formation of a proembryo, globular embryo, heart-shaped embryo, and mature embryo. Shoots arise from these embryogenic structures and can be further multiplied through subsequent subculturing. However, the inability of somatic embryos to reach the maturation stage is an adverse limitation of clonal propagation, the success of which is directly dependent upon the optimization of PGRs used, the age of tissue used, and the type of media used for establishment. Typically, semi-solid MS media supplemented with sucrose are used to initiate Eucalyptus somatic embryogenesis; however, B5 media supplemented with sucrose have also been reported to induce somatic embryogenesis in C. citridora. Moreover, various PGRs have also been reported to induce embryogenesis in Eucalyptus; for instance, a hormone-free medium for inducing somatic embryogenesis was also suggested [19][20][21][22].
1.3. Adventitious Root Formation and Root Hardening
The bipolar structure formed after somatic embryogenesis can directly germinate by using nutrients from basal media for the shoot and root proliferation. Contrastingly, the unipolar structure needs the proliferation of adventitious roots at the base of their shoots for the development of plantlets. This is usually achieved via semi-solid media; often, activated charcoal is also introduced in these media as it regulates the pH of the media and is also reported to adsorb the inhibitory compounds from the media, in addition to reducing irradiance at the base of the shoots [23][24][25]. Improved in vitro rooting in E. grandis × E. nitens was reported by reducing the strength of MS media from full to half-strength and decreasing the concentration of sucrose in shooting media from 20 to 15 g L−1. Additionally, it was observed that increasing the concentration of NAA from 0.1 mg L−1 to 0.5 mg L−1 increased the average percentage of adventitious roots [7]. Also, increasing the IBA concentration from 0.1 mg L−1 to 0.5 mg L−1 increased root hair formation. Similar studies on E. erythronema × E. stricklandii suggested 8 weeks of continuous exposure to IBA on roots resulted in the longest root length [26]. Light studies on E. grandis × E. urophylla suggested the use of red–blue light to be superior for rooting and showed the highest mean number of roots [27][28].
Before adventitious root formation, the acclimatization of shoots is necessary for their future success in nursery conditions. Micropropagated plantlets were hardened using a liquid MS medium (1/4 strength) with 2% sucrose. Furthermore, for supporting roots, adsorbent cotton was soaked in this liquid medium. After maintaining for 2 weeks, the plantlets were transferred to mist bags containing a 1:1:1 ratio of soil, manure, and sand and then transferred to a net house. Finally, the plantlets were transferred to field conditions and showed 85–95% success rates in field conditions [15]. However, in another study, 58% of the success rate of plantlets in field conditions was due to the loss of some plantlets during handling [7]. Another innovative approach for acclimatization was reported, where the shoots were maintained in photoautotrophic culture at a high concentration of carbon dioxide and a low sugar concentration. These conditions promote carbon fixation and transpiration. Notably, 86–96% of success rate for E. camaldulensis and 100% success rate for E. grandis × E. urophylla have been achieved with this method [29][30][31][32]. A similar study suggested that increasing the temperature from 18–13 °C to 33–28 °C increased the number of root cuttings per stock plant [33]. Moreover, improved rooting efficiency was achieved in clones of E. urophylla via in vitro rejuvenation/reinvigoration [34].
2. Factors Affecting the Efficiency of Micropropagation
2.1. Role of Plant Growth Regulators
Plant growth regulators or plant growth hormones are chemical compounds widely recognized to alter the growth of plants, for instance, to suppress the growth of shoots, boost the growth of shoots, or alter the maturity of the fruit. Indole-3-acetic acid (IAA) is a natural auxin in plants, and indole-3-butyric acid (IBA) is the analog of the auxin found in plants. Both IAA and IBA are synthesized via tryptophan-dependent or tryptophan-independent pathways [35][36]. Both IAA and IBA can be quickly metabolized in tissues of Eucalyptus plants. Since auxins play a crucial role in the regulation of cell division, as well as the elongation of plants and many other phases of their development, these are stored by plants as either IAA or IBA, which is converted into IAA whenever required [37][38].
Plant growth regulators have been reported to, directly and indirectly, influence plantlet proliferation, including the differentiation of embryos and different organs. For instance, optimal concentrations of cytokinin–auxin necessary for inducing organogenesis in E. cloeziana micropropagation were suggested by [39]. Moreover, the highest rate of somatic embryogenesis was also reported by introducing 0.1 mg L−1 NAA and 0.5 mg L−1 BA [40]. Increased shoot multiplication via the addition of 0.5 mg L−1 BAP in WPM and ½ MS medium was also reported [41]. It is important to note that the optimization of PGRs in culture media is necessary as they could negatively affect the growth of plantlets [41][42]. Similar studies on auxin types in E. salgina and E. globulus were reported, suggesting the best rooting obtained with IBA than IAA. Best rooting was achieved in both species when treated with IBA; the possible explanation supporting this IAA is that it is highly susceptible to enzymatic degradation and is also 5 times more susceptible to photo-oxidation than IBA. In support of this, a similar study on E. sideroxylon micorcuttings was reported by comparing IBA and NAA using different concentrations of both auxins from 0 to 10 μM. A high frequency of callus induction was reported by culturing cotyledon explants on MS media supplemented with 1 mg L−1 NAA + 0.5 mg L−1 BA. The same study also suggested that MS media supplemented with 0.5 mg L−1 NAA + 1 mg L−1 BA + 1 mg L−1 GA3, as well as ½ MS media supplemented with 0.5–1 mg L−1, resulted in high-frequency adventitious root formation in E. bosistoana. Furthermore, 100% survival of preacclimatized plantlets was obtained [43]. A similar study revealed best shoot elongation by supplementing the media with 0.05 mg L−1 BAP + 1 mg L−1 NAA and 0.05 mg L−1 BAP + 1 mg L−1 NAA + 1 mg L−1 IBA−1 [44]. The results showed increased callus induction in micorcuttings exposed to IBA compared with the micorcuttings exposed to NAA. However, the responses to auxins may vary from species to species based on their differential affinities, uptake, and metabolization of auxins [45][46].
2.2. Effect of Culture Media
The initiation of shoot proliferation is usually achieved by culturing explant on a semi-solid medium that comprises gelling agents such as 1.5–4.0 g/L of gelrite, 4–8 g/L of agar, or 1.5–4.5 g/L of phytagel, and the pH is adjusted between 5 and 6. Also, the use of liquid media has been reported for the initiation and proliferation of nodes and shoots [47][48]. The culture of the shoot depends on the ability to encourage the development of axillary and accessory buds that are present at the base of each leaf axil. In previous research attempts at Eucalyptus micropropagation, the basal media used include Murashige and Skoog media (different strengths ½, ¼); JADS (Juan, Antonio, Diva, and Silvian) medium, WPM (Woody Plant Medium), and DKW (Driver and Kuniyaki Walnut) medium [49]. Additionally, the form of medium, whether a liquid suspension medium or a semi-solid medium, affects the growth of plantlets in vitro. Reportedly, better shoot multiplication of Eucalyptus has been observed in liquid media than in semi-solid media [50]. WPM was reported as the optimal medium for the micropropagation of E. benthamii [51]. Similarly, the JADS medium was observed to be optimal for the trunk base shoot elongation of E. grandis. The DKW medium was used as an alternative for the micropropagation of E. nitens [52]. The adjustment of the pH of the medium to 5.8 prior to autoclaving at 121 °C for 15 min has been recommended. Temperature incubation at 25 ± 2 °C and 16 h photoperiod with the photon flux density of 2500 lux from white fluorescent tubes is the recommended cultured condition for Eucalyptus spp. For improving the survival of the explant, polyvinyl pyrrolidone, activated charcoal, and ascorbate have also been added to culture media. However, the type of media and the response of plantlets vary among species of Eucalyptus and their hybrids. Moreover, some drawbacks such as chlorosis, tissue browning, and oxidation have been reported in almost all types of media used for Eucalyptus micropropagation.
2.3. Importance of Organic and Inorganic Elements
Organic and inorganic elements play a crucial role in plant micropropagation media, which are used for the propagation and growth of plants under sterile conditions. Elements like calcium (Ca), nitrogen (N), phosphorous (P), and boron (B) serve as macro- and micronutrients essential for nourishing plant growth. These have been introduced in in vitro cultures to promote the proliferation and differentiation of organs from the shoot. An appropriate balance and concentration of these organic and inorganic elements are crucial to ensure the successful propagation and growth of plants in vitro [53]. The composition of the medium can be adjusted based on the specific requirements of different plant species and their growth stages. The elements required by plants in concentrations lower than 0.5 mM/L are referred to as micronutrients, and the elements with more than this concentration are referred to as macronutrients [54][55][56]. Magnesium (Mg), calcium (Ca), hydrogen (H), sulfur (S), potassium (K), nitrogen (N), phosphorous (P), and oxygen (O) serve as macronutrients. Calcium act as an important cofactor and cellular messenger involved in various signal transduction pathways and is well known to play various important roles in plant stress [57]. For instance, in E. urophylla and E. grandis, calcium has been reported to trigger organogenesis [58]. Manganese (Mn), chlorine (Cl), iron (Fe), zinc (Zn), boron (B), sodium (Na), iodine (I), and copper (Cu) serve as the microelements among which iron is the most critical element. Also, it was reported that the deficiency of boron in media led to necrosis and callus accumulation, further inhibiting seedling growth in E. grandis [59]. Furthermore, a study suggested that calcium, in the form of calcium chloride in agitated liquid media, decreases the hyperhydricity in E. saligna. However, due to the toxicity caused by chlorine, it had been not effective in completely eliminating hyperhydricity [60]. Calcium chloride dihydrate was also reported to induce shoot elongation and decrease vitrification [61]. Nonetheless, efforts in improving the optimization and choice of organic/inorganic elements have been increased, although they are insufficient in considering the individual role of vast available macro- and micronutrients.
2.4. The Role of Carbohydrates
Carbohydrates are important biomolecules that provide biofuel and serve as a carbon source for cell growth. Different reducing and nonreducing sugars are available and have been employed in micropropagation like glucose, fructose, sucrose, and galactose, among which sucrose is still the most preferable in Eucalyptus micropropagation due to its ease of translocation in plant tissues. Sucrose is a nonreducing sugar, specifically a disaccharide composed of fructose and glucose. Some of the reports suggest that increased sucrose concentrations can hinder water and nutrient uptake in plants and inhibit photosynthesis by influencing photosynthetic enzymes. However, contrastingly, some studies suggest that plants remain uninfluenced by high sucrose concentrations. Furthermore, in E. cloiziana, high glucose and sucrose concentrations were reported to decrease the shoot length (conc. > 15 g/mL in media) [62]. For instance, studies reported different concentrations of sucrose (1–6%) in MS media for in vitro shoot proliferation. The best results were reported using 3% sucrose in MS media with a 6–7-fold increased shoot multiplication. Similar findings reported that media devoid of sucrose result in the inhibition of shoot multiplication, and the leaves and shoots turned to a pale green color [63][64]. The results are in line with those of several studies that used 3% sucrose as a carbohydrate source to promote the growth of shoots in a variety of Eucalyptus species. However, similar findings on many other woody plants have also been reported; for instance, in bamboo shoots, successful multiplication was observed when media were supplemented with 2% sucrose [65][66]. It has also been reported that an increase in sucrose levels from 3 to 4% does not cause any effect on shoots but results in albinism. Similarly, at 1% sucrose concentration, thin shoots and leaves were developed that were inappropriate for subculturing. An investigation was conducted on myo-inositol to determine its role in in vitro shoot multiplication. MS media supplemented with 100 mg L−1 of myo-inositol yielded the best shoot multiplication rates, while MS media devoid of myo-inositol showed decreased shoot multiplication. Moreover, MS media supplemented with excess myo-inositol (more than 150 mg L−1) not only decreased shoot multiplication but also had detrimental effects on shoots. For maximizing shoot multiplication rate and growth, 100 mg L−1 myo-inositol was supplemented in culture media for all trials [63][67].
2.5. Effects of Radiation and Light Exposure
Light is a critical external aspect influencing the different phases of plant growth. Light hour durations and intensity are directly linked to plants’ photosynthetic rates [68]. Many hybrids have been studied that suggest the effects of light and radiation on the success of micropropagation. The effects of five sources of lights, namely fluorescent lamps, white LEDs, red LEDs, blue LEDs, and red–blue LEDs, on E. grandis × E. urophylla hybrid were studied, and red–blue LEDs and florescent lights were found to be superior for E. grandis × E. urophylla micropropagation. The response of Eucalyptus to micropropagation varies among genotypes. Moreover, a low level of irradiation triggered rooting in E. globulus; contrastingly, some studies confirmed that low-level irradiations hindered root proliferation in E. globulus [28][69][70]. Also, studies on E. salgina and E. globulus were conducted for the effect of light on rooting capacity using white fluorescent lamps. E. globulus did not show any effect from exposure and was found to be dependent only on exogenous auxin concentration for rooting, while E. salgina cuttings showed increased root density per rooted cutting upon exposure to light combined with exogenous auxin application [71]. A similar study suggested that preservation under low light intensity effectively preserved cultures for 3 months [72]. Besides these, increases in light intensity and carbon dioxide content have been shown to increase the growth of explants photoautotrophically [73]. In a similar study, the effect of light quality on the clone of E. urophylla in the photoautotrophic system was assessed, focusing on the stomatal density, carotenoid content, chlorophyll content, the number of shoots, and the longest shoot. The results indicated that blue LED resulted in fewer shoots, while high production of carotenoids was observed under white light [74]. In another study on Eucalyptus dunnii and Eucalyptus grandis × E. urophylla, it was observed that the use of white light was associated with increased buds per plant, decreased tissue oxidation, and longer shoot length. In E. dunnii, blue, red, and yellow light resulted in increased chlorophyll a and b content. Also, blue, white, purple, and red light increased stomatal densities. Moreover, a previous study revealed that irrespective of light spectra, E. dunnii showed decreased adventitious rooting [75]. Another similar study on E. grandis × E. urophylla clone was carried out to assess the impact of five different light sources, namely fluorescent lamps as well as blue, green, red, and yellow cellophane light in a bioreactor system. Yellow and blue light sources were found to be more suitable for the clone as less hyperhydricity was observed along with spongy parenchymatic tissue, thicker mesophyll, increased shoot length, and more shoots per explant [76]. Moreover, another study suggests that for the in vitro multiplication of E. pilularis, white light was more suitable, and for the E. urograndis clone, blue light was more suitable because it increased the number of buds, shoots length, and fresh weight per explant [77].
References
- Debergh, P.C.; Read, P.E. Micropropagation. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1991; pp. 1–13.
- Cassells, A.C. Pathogen and Biological Contamination Management in Plant Tissue Culture: Phytopathogens, Vitro Pathogens, and Vitro Pests. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 57–80.
- Wendling, I.; Brooks, P.R.; Trueman, S.J. Topophysis in Corymbia torelliana × C. citriodora Seedlings: Adventitious Rooting Capacity, Stem Anatomy, and Auxin and Abscisic Acid Concentrations. New For. 2015, 46, 107–120.
- Bag, N.; Chandra, S.; Palni, L.M.S.; Nandi, S.K. Micropropagation of Dev-Ringal —A Temperate Bamboo, and Comparison between in Vitro Propagated Plants and Seedlings. Plant Sci. 2000, 156, 125–135.
- Giri, C.C.; Shyamkumar, B.; Anjaneyulu, C. Progress in Tissue Culture, Genetic Transformation and Applications of Biotechnology to Trees: An Overview. Trees 2004, 18, 115–135.
- Ansar, S.; Iqbal, M. Effect of Dietary Antioxidant on Mercuric Chloride Induced Lung Toxicity and Oxidative Stress. Toxin Rev. 2015, 34, 168–172.
- Keret, R.; Nakhooda, M.; Jones, N.B.; Hills, P.N. Optimisation of Micropropagation Protocols for Temperate Eucalypt Hybrids in South Africa, with a Focus on Auxin Transport Proteins. South. For. J. For. Sci. 2021, 83, 254–263.
- Trueman, S.J.; Richardson, D.M. In Vitro Propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via Cytokinin-Free Node Culture. Aust. J. Bot. 2007, 55, 471.
- Kuppusamy, S.; Ramanathan, S.; Sengodagounder, S.; Senniappan, C.; Shanmuganathan, R.; Brindhadevi, K.; Kaliannan, T. Optimizing the Sterilization Methods for Initiation of the Five Different Clones of the Eucalyptus Hybrid Species. Biocatalaysis Agric. Biotechnol. 2019, 22, 101361.
- Kamal, B.; Arya, I.D.; Gupta, S. In-Vitro Regeneration of Interspecific F1 Hybrid (Eucalyptus citriodora and Eucalyptus torelliana) of Eucalyptus. J. Mt. Res. 2022, 17, 125–130.
- Aggarwal, D.; Kumar, A.; Reddy, M.S. Shoot Organogenesis in Elite Clones of Eucalyptus tereticornis. Plant Cell Tiss. Organ. Cult 2010, 102, 45–52.
- Girijashankar, V. In Vitro Regeneration of Eucalyptus camaldulensis. Physiol. Mol. Biol. Plants 2012, 18, 79–87.
- Fernando, S.C.; Goodger, J.Q.D.; Gutierrez, S.S.; Johnson, A.A.T.; Woodrow, I.E. Plant Regeneration through Indirect Organogenesis and Genetic Transformation of Eucalyptus polybractea R.T. Baker. Ind. Crops Prod. 2016, 86, 73–78.
- Sluis, A.; Hake, S. Organogenesis in Plants: Initiation and Elaboration of Leaves. Trends Genet. 2015, 31, 300–306.
- Singh, D.; Kaur, S.; Kumar, A. In Vitro Drought Tolerance in Selected Elite Clones of Eucalyptus tereticornis Sm. Acta Physiol. Plant 2020, 42, 17.
- Oberschelp, G.P.J.; Gonçalves, A.N. Assessing the Effects of Basal Media on the in Vitro Propagation and Nutritional Status of Eucalyptus dunnii Maiden. In Vitro Cell. Dev. Biol.—Plant 2016, 52, 28–37.
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77.
- Martínez, M.T.; del San-José, M.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm Oak Somatic Embryogenesis: Current Status and Future Perspectives. Front Plant Sci 2019, 10, 239.
- Pinto, G.; Silva, S.; Park, Y.-S.; Neves, L.; Araújo, C.; Santos, C. Factors Influencing Somatic Embryogenesis Induction in Eucalyptus globulus Labill.: Basal Medium and Anti-Browning Agents. Plant Cell Tiss. Organ Cult. 2008, 95, 79–88.
- Pinto, G.; Park, Y.-S.; Neves, L.; Araújo, C.; Santos, C. Genetic Control of Somatic Embryogenesis Induction in Eucalyptus globulus Labill. Plant Cell Rep. 2008, 27, 1093–1101.
- Pinto, G.; Park, Y.-S.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Factors Affecting Maintenance, Proliferation, and Germination of Secondary Somatic Embryos of Eucalyptus globulus Labill. Plant Cell Tiss. Organ. Cult. 2008, 95, 69–78.
- Pinto, G.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Histocytological Changes and Reserve Accumulation during Somatic Embryogenesis in Eucalyptus globulus. Trees 2010, 24, 763–769.
- Thomas, T.D. The Role of Activated Charcoal in Plant Tissue Culture. Biotechnol. Adv. 2008, 26, 618–631.
- Jones, N.B.; van Staden, J. Micropropagation and Establishment of Eucalyptus grandis Hybrids. S. Afr. J. Bot. 1994, 60, 122–126.
- Sapaeing, A.; Sutthinon, P.; Hilae, A.; Wattanapan, N. Effects of BA, NAA, and Activated Charcoal on Micropropagation of Nepenthes Mirabilis (Lour.) Druce. Acta Hortic. 2020, 1298, 281–286.
- Glocke, P.; Delaporte, K.; Collins, G.; Sedgley, M. Micropropagation of Juvenile Tissue of Eucalyptus erythronema × Eucalyptus stricklandii Cv. ‘Urrbrae Gem’. In Vitro Cell. Dev. Biol.—Plant 2006, 42, 139–143.
- Souza, D.M.S.C.; Fernandes, S.B.; Avelar, M.L.M.; Frade, S.R.D.P.; Molinari, L.V.; Gonçalves, D.S.; Pinto, J.E.B.P.; Brondani, G.E. Light Quality in Micropropagation of Eucalyptus grandis × Eucalyptus urophylla. Sci. For. 2020, 48, e3329.
- Xu, Y.; Liang, Y.; Yang, M. Effects of Composite LED Light on Root Growth and Antioxidant Capacity of Cunninghamia lanceolata Tissue Culture Seedlings. Sci. Rep. 2019, 9, 9766.
- Tanaka, M.; Giang, D.T.T.; Murakami, A. Application of a Novel Disposable Film Culture System to Photoautotrophic Micropropagation of Eucalyptus uro-grandis (Urophylia × grandis). In Vitro Cell. Dev. Biol.—Plant 2005, 41, 173–180.
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor Systems for Micropropagation of Plants: Present Scenario and Future Prospects. Front. Plant Sci. 2023, 14, 1159588.
- Zobayed, S. Mass Propagation of Eucalyptus camaldulensis in a Scaled-up Vessel Under In Vitro Photoautotrophic Condition. Ann. Bot. 2000, 85, 587–592.
- Kozai, T.; Afreen, F.; Zobayed, S.M.A. (Eds.) . Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Springer: Berlin/Heidelberg, Germany, 2005.
- Trueman, S.J.; McMahon, T.V.; Bristow, M. Production of Eucalyptus cloeziana Cuttings in Response to Stock Plant Temperature. J. Trop. For. Sci. 2013, 25, 60–69.
- Mendonça, E.G.; Batista, T.R.; Stein, V.C.; Balieiro, F.P.; de Abreu, J.R.; Pires, M.F.; de Souza, P.A.; Paiva, L.V. In Vitro Serial Subculture to Improve Rooting of Eucalyptus urophylla. New For. 2020, 51, 801–816.
- Leva, A. (Ed.) Recent Advances in Plant In Vitro Culture; InTech: London, UK, 2012.
- Zhao, Y. Auxin Biosynthesis. Arab. Book 2014, 12, e0173.
- Frick, E.M.; Strader, L.C. Roles for IBA-Derived Auxin in Plant Development. J. Exp. Bot. 2018, 69, 169–177.
- Strader, L.C.; Bartel, B. Transport and Metabolism of the Endogenous Auxin Precursor Indole-3-Butyric Acid. Mol. Plant. 2011, 4, 477–486.
- De Oliveira, L.S.; Brondani, G.E.; Molinari, L.V.; Dias, R.Z.; Teixeira, G.L.; Gonçalves, A.N.; De Almeida, M. Optimal Cytokinin/Auxin Balance for Indirect Shoot Organogenesis of Eucalyptus cloeziana and Production of Ex Vitro Rooted Micro-Cuttings. J. For. Res. 2022, 33, 1573–1584.
- Prakash, M.G.; Gurumurthi, K. Effects of Type of Explant and Age, Plant Growth Regulators and Medium Strength on Somatic Embryogenesis and Plant Regeneration in Eucalyptus camaldulensis. Plant Cell Tiss. Organ Cult. 2010, 100, 13–20.
- Brondani, G.E.; Dutra, L.F.; Wendling, I.; Grossi, F.; Hansel, F.A.; Araujo, M.A. Micropropagation of an Eucalyptus hybrid (Eucalyptus benthamii × Eucalyptus dunnii). Acta Sci. Agron. 2011, 33, 8317.
- Nazirah, A.; Nor-Hasnida, H.; Mohd-Saifuldullah, A.W.; Muhammad-Fuad, Y.; Ahmad-Zuhaidi, Y.; Rozidah, K. Development of an Efficient Micropropagation Protocol for Eucalyptus Hybrid (E. urophylla × E. grandis) through axillary shoot proliferation. J. Trop. For. Sci. 2021, 33, 391–397.
- Shwe, S.S.; Leung, D.W.M. Plant Regeneration from Eucalyptus bosistoana Callus Culture. In Vitro Cell. Dev.Biol.—Plant 2020, 56, 718–725.
- Faria, J.C.T.; Ribeiro-Kumara, C.; Costa, R.S.D.R.; Nieri, E.M.; De Carvalho, D.; Pinto, J.E.B.P.; Neto, A.R.D.S.; Brondani, G.E. Use of Biodegradable Polyester-Based Microvessels for Micropropagation of Mature Eucalyptus microcorys. N. Z. J. For. Sci. 2022, 52, 1–13.
- Skůpa, P.; Opatrný, Z.; Petrášek, J. Auxin Biology: Applications and the Mechanisms Behind. In Applied Plant Cell Biology; Nick, P., Opatrny, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 22, pp. 69–102.
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in Action: Signalling, Transport and the Control of Plant Growth and Development. Nat. Rev. Mol. Cell. Biol. 2006, 7, 847–859.
- Bunn, E. Development of in Vitro Methods for Ex Situ Conservation of Eucalyptus impensa, an Endangered Mallee from Southwest Western Australia. Plant Cell. Tiss. Organ. Cult. 2005, 83, 97–102.
- Kaur, S. In Vitro Regeneration of Shoots From Nodal Explants of Dendrobium chrysotoxum Lindl. J. Hortic. Res. 2017, 25, 27–34.
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735.
- Chen, J.; Ziv, M. The Effect of Ancymidol on Hyperhydricity, Regeneration, Starch and Antioxidant Enzymatic Activities in Liquid-Cultured Narcissus. Plant Cell Rep. 2001, 20, 22–27.
- Brondani, G.E.; de Wit Ondas, H.W.; Baccarin, F.J.B.; Gonçalves, A.N.; de Almeida, M. Micropropagation of Eucalyptus benthamii to Form a Clonal Micro-Garden. In Vitro Cell. Dev. Biol.—Plant 2012, 48, 478–487.
- Gomes, F.; Canhoto, J.M. Micropropagation of Eucalyptus nitens Maiden (Shining Gum). In Vitro Cell. Dev. Biol.—Plant 2003, 39, 316–321.
- Ngomuo, M.; Mneney, E.; Ndakidemi, P.A. The In Vitro Propagation Techniques for Producing Banana Using Shoot Tip Cultures. Am. J. Plant Sci. 2014, 05, 1614–1622.
- Al-Aizari, A.A.; Al-Obeed, R.S.; Mohamed, M.A.H. Improving Micropropagation of Some Grape Cultivars via Boron, Calcium and Phosphate. Electron. J. Biotechnol. 2020, 48, 95–100.
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. (Eds.) The Components of Plant Tissue Culture Media I: Macro- and Micro-Nutrients. In Plant Propagation by Tissue Culture; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 65–113.
- Pérez-Tornero, O.; Burgos, L. Different Media Requirements for Micropropagation of Apricot Cultivars. Plant Cell Tissue Organ Cult. 2000, 63, 133–141.
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511.
- Al-Mayahi, A.M.W. Effect of Calcium and Boron on Growth and Development of Callus and Shoot Regeneration of Date Palm ‘Barhee’. Can. J. Plant Sci. 2020, 100, 357–364.
- Brunoni, F.; Rolli, E.; Dramis, L.; Incerti, M.; Abarca, D.; Pizarro, A.; Diaz-Sala, C.; Ricci, A. Adventitious Rooting Adjuvant Activity of 1,3-Di(BenzoOxazol-5-Yl)Urea and 1,3-Di(BenzoOxazol-6-Yl)Urea: New Insights and Perspectives. Plant Cell Tiss. Organ Cult. 2014, 118, 111–124.
- Lopes da Silva, A.L.; Gollo, A.; Brondani, G.; Horbach, M.; Oliveira, L.; Machado, M.; Lima, K.; Costa, J. Micropropagation of Eucalyptus saligna Sm. from Cotyledonary Nodes. Pak. J. Bot. 2015, 47, 311–318.
- Sharma, S.; Ramamurthy, V. Micropropagation of 4-Year-Old Elite Eucalyptus tereticornis Trees. Plant Cell Rep. 2000, 19, 511–518.
- Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semi-solid Medium and Temporary Immersion Bioreactors. Forests 2021, 12, 1408.
- Joshi, I.; Bisht, P.; Sharma, V.K.; Uniyal, D.P. In Vitro Clonal Propagation of Mature Eucalyptus F1 Hybrid (Eucalyptus tereticornis Sm. x E. grandis Hill Ex. Maiden). Silvae Genet. 2003, 52, 110–113.
- Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of Sucrose on Growth and Stress Status of Castanea sativa × C. crenata Shoots Cultured in Liquid Medium. Plants 2022, 11, 965.
- Sandhu, M.; Wani, S.H.; Jiménez, V.M. In Vitro Propagation of Bamboo Species through Axillary Shoot Proliferation: A Review. Plant Cell Tiss Organ Cult. 2018, 132, 27–53.
- Nadgauda, R.S.; Parasharami, V.A.; Mascarenhas, A.F. Precocious Flowering and Seeding Behaviour in Tissue-Cultured Bamboos. Nature 1990, 344, 335–336.
- Arya, I.D.; Chauhan, S.S.S.; Arya, S. Micropropagation of Superior Eucalyptus Hybrids FRI-5 (Eucalyptus camaldulensis Dehn × E. tereticornis Sm) and FRI-14(Eucalyptus Torelliana F.V. Muell × E. citriodora Hook): A Commercial Multiplication and Field Evaluation. Afr. J. Biotechnol. 2009, 8, 5718–5726.
- Shen, G.; Tan, S.; Sun, X.; Chen, Y.; Li, B. Experimental Evidence for the Importance of Light on Understory Grass Communities in a Subtropical Forest. Front Plant Sci. 2020, 11, 1051.
- Mankessi, F.; Saya, A.; Baptiste, C.; Nourissier, S.; Monteuuis, O. In Vitro Rooting of Genetically Related Eucalyptus urophylla × Eucalyptus grandis Clones in Relation to the Time Spent in Culture. Trees 2009, 23, 931–940.
- Fett-Neto, A.G.; Fett, J.P.; Goulart, L.W.V.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct Effects of Auxin and Light on Adventitious Root Development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464.
- Fogaça, C.M.; Fett-Neto, A.G. Role of Auxin and Its Modulators in the Adventitious Rooting of Eucalyptus Species Differing in Recalcitrance. Plant Growth Regul. 2005, 45, 1–10.
- Watt, M.P.; Thokoane, N.L.; Mycock, D.; Blakeway, F. In Vitro Storage of Eucalyptus grandis Germplasm under Minimal Growth Conditions. Plant Cell Tissue Organ Cult. 2000, 61, 161–164.
- Xiao, Y.; Niu, G.; Kozai, T. Development and Application of Photoautotrophic Micropropagation Plant System. Plant Cell Tiss. Organ Cult. 2011, 105, 149–158.
- Miranda, N.A.; Xavier, A.; Otoni, W.C.; Gallo, R.; Gatti, K.C.; de Moura, L.C.; Souza, D.M.S.C.; Maggioni, J.H.; de Santos, S.S.O. Quality and Intensity of Light in the In Vitro Development of Microstumps of Eucalyptus urophylla in a Photoautotrophic System. For. Sci. 2020, 66, 754–760.
- Rangel Do Prado Frade, S.; Santana Costa Souza, D.M.; Fernandes, S.B.; Lopes Martins Avelar, M.; Vaz Molinari, L.; Santos Gonçalves, D.; Alves Magalhães, T.; Brondani, G.E. Spectral Quality Influence on in Vitro Morphophysiological Responses of Eucalyptus dunnii Maiden and Eucalyptus grandis W.Hill Ex Maiden × E. urophylla ST Blake. N. Z. J. For. Sci. 2023, 53, 1–16.
- Souza, D.M.S.C.; Avelar, M.L.M.; Fernandes, S.B.; Silva, E.O.; Duarte, V.P.; Molinari, L.V.; Brondani, G.E. Spectral Quality and Temporary Immersion Bioreactor for in Vitro Multiplication of Eucalytpus grandis × Eucalyptus urophylla. 3 Biotech 2020, 10, 457.
- Matheus, D.; Souza, S.C.; Martins, A.R.; Fernandes, S.B.; Lopes Martins Avelar, M.; Vaz Molinari, L.; Santos Gonçalves, D.; Brondani, G.E. In Vitro Multiplication of Eucalyptus pilularis and Eucalyptus grandis × E. urophylla (Urograndis Eucalypt): Effect of Light Quality in Temporary Immersion Bioreactor. Mindanao J. Sci. Technol. 2022, 20, 72–86.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
29 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





