Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kameliya Kirilova Anichina | -- | 2319 | 2023-12-15 13:16:19 | | | |
| 2 | Wendy Huang | Meta information modification | 2319 | 2023-12-16 02:20:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Anichina, K.K.; Georgiev, N.I. 2-Substituted Benzimidazoles as Photo-Protective Agents. Encyclopedia. Available online: https://encyclopedia.pub/entry/52812 (accessed on 07 February 2026).
Anichina KK, Georgiev NI. 2-Substituted Benzimidazoles as Photo-Protective Agents. Encyclopedia. Available at: https://encyclopedia.pub/entry/52812. Accessed February 07, 2026.
Anichina, Kameliya K., Nikolai I. Georgiev. "2-Substituted Benzimidazoles as Photo-Protective Agents" Encyclopedia, https://encyclopedia.pub/entry/52812 (accessed February 07, 2026).
Anichina, K.K., & Georgiev, N.I. (2023, December 15). 2-Substituted Benzimidazoles as Photo-Protective Agents. In Encyclopedia. https://encyclopedia.pub/entry/52812
Anichina, Kameliya K. and Nikolai I. Georgiev. "2-Substituted Benzimidazoles as Photo-Protective Agents." Encyclopedia. Web. 15 December, 2023.
Copy Citation
The modern trend in sunscreen products is towards the development of UV filters with multi-functional properties, to provide a broad shielding against ultraviolet radiation, antioxidant activity, and the prevention of skin cancer. Additionally, they should also be safe for humans as well as the environment. The benzimidazole heterocycle is a suitable platform for the development of such multifunctional molecules with potential application in cosmetic formulations, due to their ability to act as both UV protectors and reactive pharmacophores.
2-arylbenzimidazoles
UV filters
antioxidant activity
antiproliferative activity
multifunctional molecules
sunscreen product
2-substituted benzimidazole
1. Introduction
Benzimidazoles have long been a research focus as UV-protective agents owing to their significant merits, such as UV filtering ability, a simple molecular structure, easy production on an industrial scale, high water solubility, and a good safety profile. The popular benzimidazole-based commercial UV filters, widely used in sunscreen and cosmetics formulations, are Ensulizole® (INCI: 2-phenyl-1H-benzimidazole-5-sulphonic acid, PBSA), UVB filter, and Bisdisulizole, Neo Heliopan® AV (INN: Sodium salt of 2,2′-bis(1,4-phenylene)-1H-benzimidazole-4,6-disulfonic acid, DPDT). Ensulizole® is a UVB filter, while Bisdisulizole absorbs mainly in the UVA range (Figure 1).
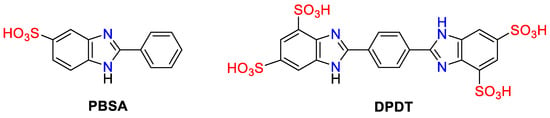
Figure 1. Structures of benzimidazole-based commercial UV filters.
2. 2-Substituted Benzimidazoles as Photo-Protective Agents: Discovery and Development
Since the discovery of 2-phenyl-1H-benzimidazole-5-sulfonic acid (Scheme 1) in 1933 [10] as a UV filter, multiple benzimidazole compounds have been designed and synthesized to form a new class of sunscreen molecules.
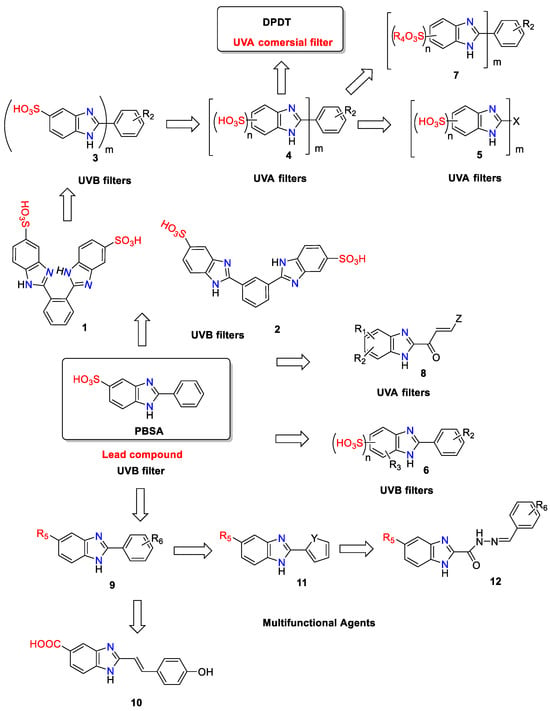
Scheme 1. Evolution of the 2-substituted benzimidazole derivatives as UV filtering agents. Where R1 = COOH; COOC1-C6-Alkyl; COCl; COBr or CN; R2 = one or more C1-C6-Alkyl; C1-C6-Alkoxy; OH; F; Br; R3 = C1-C8-Alkyl; C1-C8-Alkoxy; R4 = linear, branched or cyclic C16-C50-Alkyl; R5 = H; SO3H; CN; COOH; R6 = one or more OH; OCH3; Cl; Br; N(Et)2; X = (un)substituted naphthalene; (un)substituted 1,1′-biphenyl; (un)substituted thiophene; (un)substituted furan; (un)substituted pyrrole; Y = O; S; NH; Z = X or R6-substituted phenyl; m = 1–3; n = 2–3.
PBSA is a water-soluble white powder with a high melting point that absorbs strongly at UV-B wavelengths. It has a moderate to high extinction coefficient of 26,000 and λmax about 310 nm. However, PBSA has also been reported to generate a variety of free radicals and exhibit photosensitizing activity, raising the risk of phototoxic damage to DNA and other cellular components [11][12][13].
This necessitates the additional inclusion of antioxidants in the sunscreen product or the development of dualistic molecules able to act as photoprotective agents and inhibitors of the formation of radical species [14].
DE1282855 describes the use of 1,2-bis-(5-sulfobenzimidazol-2-yl)-benzene 1 and 1,3-bis-(5-sulfobenzimidazol-2-yl)-benzene 2 (Scheme 1) as water-soluble UVB filters [15]. Patent application WO93/15061 [16] was related to an improved process for preparing arylbenzimidazoles with general formula 3 (Scheme 1). The compounds containing at least two benzimidazole rings in combination with a sulfo group in each of the heterocycles are useful as UVB absorbers in sunscreen products. Surprisingly, it was found that after introducing two or more sulfo groups into the bis-benzimidazole-benzenes, the compounds 4 (Scheme 1) had an absorption maximum in the range UV-A-II and protected against the dangerous short-wave UVA rays through strong absorption [17]. In addition, these UVA filters have excellent light fastness, good thermal stability, toxicological and dermatological harmlessness, and good solubility in cosmetic solvents. The commercial UVA filter DPDT belongs to this family of polysulfonated bis-benzimidazole analogs.
More benzimidazole derivative 5 (Scheme 1), characterized by different substitution patterns at the five-membered heterocyclic (thiophene, furan, or pyrrole) ring at position 2 of benzimidazole moiety, were also prepared. Some compounds 5 absorbing in the UVA range were obtained with a naphthalene or biphenyl linker between the benzimidazole cores [17].
Although they possess desirable photoprotective properties, water-soluble benzimidazole filters can be formulated in an alkaline environment since sulfonic acid precipitates at pH values below 7. It has been found that some polysulfonated benzimidazoles 6 (Scheme 1) or their salts can be easily incorporated into cosmetic formulations while avoiding the precipitation problem [18]. 2-Phenyl-1H-benzimidazole-4,6-disulfonic acid in the form of betaine salt is particularly preferred in this case.
The water-soluble salts of aryl benzimidazole sulfonic acids are insoluble in organic solvent systems. As a result, they must be incorporated into the aqueous phase of a sunscreen formulation, where they have the potential to be washed off the skin by perspiration or exposure to water. Furthermore, the use of salts constrains the cosmetic formulators to produce waterproof sunscreens with acceptable aesthetics. For example, the effectiveness of carbomers, as well as some waterproofing polymers such as Diglycol/CHDM/Isophthalates/SIP Copolymer, is diminished in the presence of various levels of salt [19]. A solution to overcome the water resistance challenge of benzimidazole filters in sunscreen formulation is the use of aryl benzimidazole sulfonic acid esters 7 (Scheme 1) with alcohols and/or silicones with a branched, linear, or cyclic C16-C50 chain. These compounds show increased substantiality, water resistance, sweat resistance, friction resistance, and improved solubility in the oil phase of cosmetic compositions. Due to the high extinction coefficient of the C16-C50 alkyl esters of the aryl benzimidazole sulfonic acids, high levels of photoprotection can be achieved with small amounts of sunscreen.
A new series of benzimidazoles 8 (Scheme 1) was synthesized and assayed with the goal of obtaining new compounds that protect the skin and hair against UVA rays. The new derivatives differ from the previous benzimidazole sunscreen molecules in the side chain linked at the C-2 position. 2-(3’-Arylacryloyl)benzimidazole derivatives have good photostability and molar extinction coefficients between 25,000 and 40,000 [20].
The introduction of hydroxyl groups to the C-2 phenyl part of PBSA and substituent R5 in position 5 of the benzimidazole ring led to the discovery of a series of hydroxy-phenyl-1H-benzimidazoles 9 (Scheme 1). These molecules were found to act as radical scavengers as well as UV-protective agents [14].
In general, all compounds 9 have demonstrated an antioxidant efficacy greater than PBSA. Derivatives 9a, 9b, and 9c (Figure 2), with hydroxyl at positions 3 and 4 of the phenyl core, showed good filtering and high antioxidant power (also in the cosmetic formulation). Among them, 9a had the highest level of sun protection factor (SPF) 9.83, which is twice the SPF of PBSA. The SPF is a quantitative measure of the efficacy of sun protection products, defined for the first time by the chemist Franz Greiter in 1962 [21], as the quotient between the minimal erythema dose (MED) with applied sunscreen and the MED without sunscreen. However, since SPF does not consider the protection against UVA radiation, prolonged exposure to the sun even when using high SPF products can leave the skin defenseless against UVA radiation. To ensure that sunscreens will protect consumers against both UVB and UVA radiation, the European Commission (EC) issued a recommendation in 2006 [22] for including UVA protection in their composition. It is recommended that the minimum degree of protection provided by sunscreen products be as follows: SPF 6 against UVB; UVA protective factor/SPF ratio of at least 1:3, i.e., the protection against UVA radiation is at least one-third of the protection against UVB radiation (SPF); and a critical wavelength (λc) of 370 nm. The critical wavelength is defined as the wavelength at which the integral of the spectral absorbance curve reaches 90% of the area under the curve from 290 to 400 nm [23].
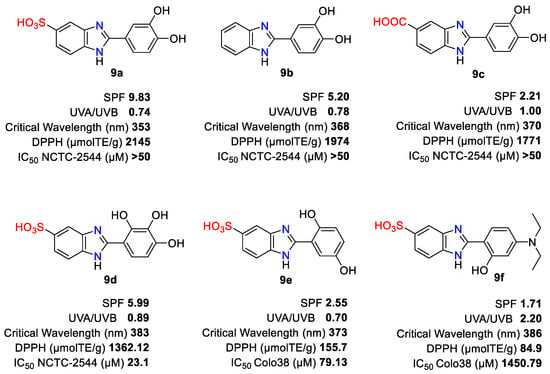
Figure 2. Chemical structure of the compounds of 2-arylbenzimidazole family with the best multifunctional profile.
In comparison with 9a, molecules 9b and 9c presented a higher UVA/UVB ratio and critical wavelength. 2-(2,3,4-Trihydroxy-phenyl)-1H-benzimidazole-5-sulfonic acid 9d had good UVA filtering parameters, its critical wavelength was the highest (λc = 383 nm), and its SPF was higher than PBSA. The cytotoxicity and phototoxicity assays using a specific cell line of human keratinocytes (NCTC-2544) showed that derivative 9d was the most cytotoxic among all the tested compounds. Molecule 9c achieved the best results regarding UV-filtering and antioxidant capacity, cytotoxicity, phototoxicity, stability, and photostability. It cannot be defined as a sunscreen filter but is an example of a booster molecule that provided very potent antioxidant activity and is capable of improving the activity of a known sunscreen.
In an attempt to obtain more potent multifunctional compounds, a three series of 2-arylbenzimidazoles 9 were synthesized by different substitution patterns C-5 (R5 = CN, COOH and SO3H) in the benzimidazole ring and were assayed for UV-filtering and antioxidant and antiproliferative activity [24]. In general, 2-arylbenzimidazole-5-sulphonic acids showed the best broad-spectrum solar protection against UVA and UVB rays. In particular, the presence of a tertiary amino group on the aromatic ring (compound 9f) promotes the shift of the maximum absorption peak to the right in the spectrum (bathochromic shift). The most interesting data showed 9f and its ciano analog (R5 = CN), with the best UVA protection factor value of 15.77 and 14.30, respectively. Compound 9e maintained its filtering profile even when incorporated into formulations for topical use and was chosen as a lead compound for further development. The UV absorption spectra of all tested 2-arylbenzimidazoles showed λmax shifted toward longer wavelengths as compared to the reference PBSA. The derivatives bearing 5-cyano or 5-carboxyl groups have shown medium to high antioxidant activity, while the presence of a sulfo group at the 5-position of the benzimidazole nucleus is the least favorable in terms of antioxidant activity.
The 2-styryl-benzimidazole 10 (Scheme 1) was the best in terms of broad-spectrum filtering activity [24]. Furthermore, the same compound was the best in antiproliferative activity on human melanoma Colo38 cells with an IC50 value 6.20 μM.
In the search for scaffolds for multifunctional compounds, Djuidje and co-workers [25] synthesized benzimidazole derivatives 11 bearing a five-membered ring and tested their photoprotective profile against UV rays, their in vitro antioxidant capacity against different radicals (DPPH and FRAP test), the antifungal inhibitory activity, and the antiviral and antiproliferative activity. According to the results from the in vitro photoprotective activity, none of the compounds 11 had a broad-spectrum profile (λc < 370 nm). The highest SPF value was shown by the derivative characterized by the presence of furan in position 2 of the benzimidazole ring and without a functional group in position 5. The SPF value was decreased by replacing the furan with the pyrrole. Thus, by keeping the group present in position-5 of benzimidazole and by varying the five-membered ring at position-2, the order of protection against UVB is as follows—furan > pyrrole > thiophene. Furthermore, the photoprotective activity against the UVB radiation of the benzimidazoles 11 does not depend only on the substitution in the 2-position of the benzimidazole ring, but also on the groups present in position 5. In this regard, according to the R5 group, the structure–activity relationship of this group of compounds is -H > -COOH > -SO3H.
Based on the results of the biological tests, the best multifunctional molecule of this series is the 2-(1H-pyrrol-2-yl)-1H-benzimidazole, which was slightly more UVB protective than other tested compounds, but with good antioxidant (IC50 = 64.098 µg/mL in 1,1-diphenyl-2-picryl-hydroxyl radical (DPPH) assay), antifungal (IC50 values in the range of 0.97–3.80 µg/mL), and antiproliferative (IC50 = 9.7 µM against human melanoma SK-Mel 5 cells) activity.
In the search for new multifunctional molecules with improved UV protection parameter values and antioxidant and anti-proliferative activity (in particular against melanoma cells), Baidisserotto A. et al. [26] investigated a series of benzimidazole-containing hydrazone derivatives 12 (Scheme 1), in which the benzimidazole scaffold was connected by a hydrazone linker and an aromatic nucleus with different substituents, in particular, hydroxy, methoxy, and diethylamino groups.
Regarding the photoprotective activity, in general, the hydrazone derivatives showed better UV-filtering than the reference PBSA sunscreen filter. Researchers note that the SPF parameter does not always have a direct influence in proportion to the number of hydroxy groups in the substituent. The presence of a methoxy group or the 2-hydroxy-4-(diethylamino) moiety positively influences the filtering parameters. The 4-hydroxyl-phenyl derivative had the highest SPF, of 12.32. In general, an inverse relationship is observed between the SPF value and λc, i.e., the hydrazones with the highest SPF possessed λc < 370 nm. However, among broad-spectrum derivatives, the SPF value was noticeable only for compounds 12a and 12b (Figure 3). On the other hand, of all compounds tested, only 12a and its 3-OH-phenyl analog showed a UVA/UVB ratio of less than 1/3, which is lower than the aforementioned EU recommendation. The antioxidant data deriving from the study of benzimidazolehydrazone derivatives 12 confirm what was reported by the previous series 2-arylbenzimidazoles 9. The best antioxidant activity was shown by the hydrazones with at least two hydroxy groups (12a, 12b) or a 2-hydroxy and a 4-methoxy group. Among the best antioxidant hydrazones tested against human melanoma Colo38 cells, compound 12c showed the best anti-proliferative effect with IC50 = 0.50 µM.
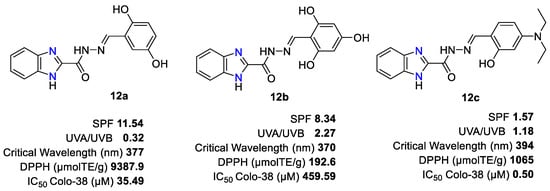
Figure 3. Chemical structure of the benzimidazolehydrazone derivatives with the best multifunctional profile.
The introduction of a hydrazone linker in 2-arylbenzimidazoles 9 resulted in more potent multifunctional molecules 12 (12a vs. 9e; 12c vs. 9f, Figure 2 and Figure 3).
Isosteric modifications of PBSA represent a strategy that has been employed by the research group of Prof. Manfredini. They designed and synthesized derivatives of PBSA in order to realize multifunctional compounds with good antioxidant activity, broad UV A-B filter capabilities, and good antineoplastic activity. For this purpose, PBSA was modified by replacing the benzimidazole core with other fused bicyclic heterocycles such as benzofuran, indole, benzoxazole, or 6-hydroxyproline (Figure 4) [25][26][27][28].
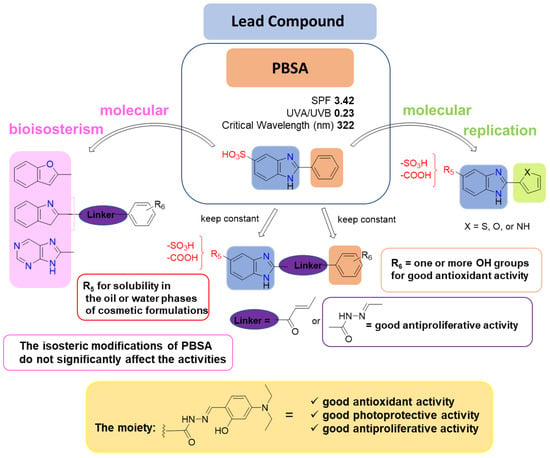
Figure 4. Modifications to the substitution pattern of PBSA that affected the multifunctional activity.
The data of structure–activity relationships (SAR) revealed a correlation between the number and position of hydroxyl groups on the arylidene portion and the antioxidant activity. With regard to the photoprotective capacity, all the hydrazone derivatives series showed better in vitro SPF profiles as compared to the commercial reference PBSA filter. The hydrazone linker was responsible for the antiproliferative activity of the compounds. In general, the isosteric modifications of PBSA did not significantly affect the activities. The investigations have shown that the presence of the 2-hydroxy-4-diethylamino moiety is related to the antioxidant, photoprotective, and antiproliferative activity in all series of hydrazones, and can therefore be considered the focus of the multifunctional profile of these derivatives (Figure 4).
References
- Tahlan, S.; Kumar, S.; Narasimhan, B. Antimicrobial potential of 1H-benzoimidazole scaffold: A review. BMC Chem. 2019, 13, 18.
- Chintakunta, R.; Meka, G. Synthesis, in silico studies and antibacterial activity of some novel 2-substituted benzimidazole derivatives. Future J. Pharm. Sci. 2020, 6, 1–6.
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236.
- Wang, X.J.; Xi, M.Y.; Fu, J.H.; Zhang, F.R.; Cheng, G.F.; Yin, D.L.; You, Q.D. Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents. Chin. Chem. Lett. 2012, 23, 707–710.
- Błaszczak-Świątkiewicz, K.; Correia Almeida, D.; De Jesus Perry, M.; Mikiciuk-Olasik, E. Synthesis, anticancer activity’ and UPLC analysis of the stability of some new benzimidazole-4,7-dione derivatives. Molecules 2014, 19, 400–413.
- Anichina, K.; Argirova, M.; Tzoneva, R.; Uzunova, V.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.; Fratev, F.; Guncheva, M.; Yancheva, D. 1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies. Chem. Biol. Interact. 2021, 345, 109540.
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868.
- Vasantha, K.; Basavaraja Swamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426.
- Veerasamy, R.; Roy, A.; Karunakaran, R.; Rajak, H. Structure–Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents: An Overview. Pharmaceuticals 2021, 14, 663.
- Merkel, E.; Wiegand, C. Light Filter. DE676103(C), 25 May 1939.
- Stevenson, C.; Davies, R.J.H. Photosensitization of guanine-specific DNA damage by 2-phenylbenzimidazole and the sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid. Chem. Res. Toxicol. 1999, 12, 38–45.
- Bastien, N.; Millau, J.F.; Rouabhia, M.; Davies, R.J.H.; Drouin, R. The sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid photosensitizes the formation of oxidized guanines in cellulo after UV-A or UV-B exposure. J. Investig. Dermatol. 2010, 130, 2463–2471.
- Inbaraj, J.J.; Bilski, P.; Chignell, C.F. Photophysical and photochemical studies of 2-phenylbenzimidazole and UVB sunscreen 2-phenylbenzimidazole-5-sulfonic acid. Photochem. Photobiol. 2002, 75, 107–116.
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537.
- Baron, H.; Kath, J.; Doeller, W. Kosmetisches Lichtschutzmittel. DE1282855(B), 14 November 1968.
- Hewang, U.; Stein, I.; Fechtel, U.; Casutt, M.; Faller, G.; Haertner, H. Method of Preparing 2-Arylbenzimidazole-5-sulphonic Acids. WO9315061(A2), 5 August 1993.
- Pelzer, R.; Langner, R.; Surburg, H.; Sommer, H.; Krempel, A.; Hopp, R. Utilization of Benzazols as UV-Absorbers, New Benzazoles and Process for Their Preparation. EP0669323(A1), 30 August 1995.
- Heywang, U.; Schwarz, M.; Pfluecker, F. 2-Phenylbenzimidazole Sulphonic Acids as UV-B Filters. EP1167358(A1), 2 January 2002.
- Gonzalez, A.; Pechko, A.; Anderson, G.T.; Kalafsky, R.E.; Lowenborg, M.V. Novel Esters of Aryl Benzimidazole Sulfonic Acids and Sunscreen Compositions Same. WO2005065154(A2), 21 July 2005.
- Ruehter, G.; Stenzel, W. Cosmetic for Makeup and Hair Treating Compsn.-Contains New 2-(3′-Aryl-Acryloxy)-Benzimidazole Compounds as UV Absorber. DE4107489(A1), 10 September 1992.
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2014, 37, 2–30.
- Regulation (EC) № 1223/2009 of the European Parliament and of the Council: Current Consolidated Version (01/03/2022). 2022, Official Journal of the European Union. 02009R1223—EN—03.12.2020—025.001—(1–389). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R1223-20201203&rid=3 (accessed on 16 August 2023).
- Addor, F.A.S.; Barcaui, C.B.; Gomes, E.E.; Lupi, O.; Reato, C.; Hélio, M.; Miot, A. Sun-screen lotions in the dermatological prescription: Review of concepts and controversies. An. Bras. Dermatol 2022, 97, 204–222.
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem. 2020, 94, 103396.
- Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System. Molecules 2020, 25, 4324.
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. In-Vitro Evaluation of Antioxidant, Antiproliferative and Photo-Protective Activities of Benzimidazolehydrazone Derivatives. Pharmaceuticals 2020, 13, 68.
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg. Chem. 2019, 85, 568–576.
- Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 579.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
653
Revisions:
2 times
(View History)
Update Date:
16 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




