Pectin consists of many active functional groups of polysaccharides, enabling them to have much more excellent modification properties than other biopolymers. Pectin is a hydrophilic natural polymer that can absorb or retain much water and exhibit swelling properties. Hydrogels and composite materials can be formed by crosslinking and other techniques, and the matrix structure can be incorporated with various bioactive compounds. Pectin-based smart composites with physical-sensitive (light, temperature, electricity), chemical-sensitive (pH, redox, glucose), and biological-sensitive (enzymes) properties are suitable in the delivery system of bioactive compounds in addition to their suitable biodegradable and biocompatible properties. Due to its broad availability, pectin has become a prominent branch of the research and development of nature-based biomedical and healthcare areas.
1. Introduction
Recent studies have gained attention on the natural polymer pectin due to its lower price and biological properties that enable it to be used in various pharmacological and biomedical applications
[1]. Various developments of simple and more innovative in vitro and in vivo testing to influence immunity, together with manufacturing, purification, and characterization techniques, have immensely contributed to the ongoing research of pectin and pectin-based composites in the food, healthcare, and cosmetic industries for their low toxicity and therapeutic effects. Commercially available pectin satisfies the required specifications and is approved by several Food and Agriculture Organizations for specific applications. Pectin is commercially obtained from the residual part of the plant materials after the extraction of juice (citrus or apple) and sugar (sugar beet). Pectin is an essential part of the cell wall that is needed for the development of plants. Pectin can be efficiently used for drug delivery and tissue engineering through gel beads or microspheres, 3D scaffolds, and membranes.
In all primary cell walls, there exist three significant types of pectic polysaccharides: (i) homogalacturonan, (ii) rhamnogalacturonan-I, and (iii) rhamnogalacturonan-II
[2][3][4]—pectin-modifying enzymes and endomembrane system biosynthesis cause the structural complexity and the pectin domains’ heterogeneity. The enzyme pectin methyl esterase can modulate Homogalacturonan. Rhamnogalacturonan-I contains extremely distinct functionally regulated polymers; however, rhamnogalacturonan-II shows a highly stable pectin matrix
[2]. Structurally, pectin is classified in a multifunctional family of covalently linked D-galacturonic acid-rich polysaccharides found in terrestrial plants’ primary cell walls
[5]. The covalently linked 1-4-alpha-D-galacturonic units are interchangeable with 1-2 attached alpha-L-rhamnopyranosyl remnants that carry saccharide polymers
[6]. The galacturonic remnants found in pectin are typically present as salts or methyl esters. The precise chemical structure of pectin is complex to deduce. It depends on the source and conditions they extract in location and other surrounding factors, making their chemical arrangement different. Commercially, pectin is extracted from plant materials such as citrus peel, apple pomace, and sugar beets. The polysaccharide helps to provide intercellular adhesion, rigidity, and mechanical resistance for the cell walls of plants. This support is needed to help plants living in harmful environments related to temperature, pollutants, and other environmental stressors survive. The multifunctional component of pectin has allowed it to provide numerous target sites for chemical modifications
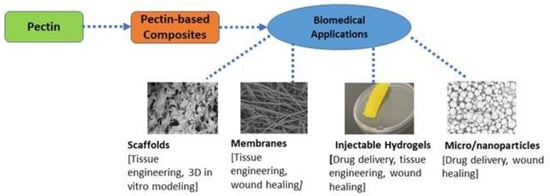
[7]. The properties of pectin, such as its nontoxicity, emulsion behavior, diverse chemical composition, biocompatibility, and high stability, enable it to be a commonly used biopolymer. Industrially, pectin is used for various applications such as food manufacturing and biomedical engineering. Biomedical applications of pectin primarily include drug delivery, tissue engineering, and wound healing (
Figure 1).
Figure 1. Several biomedical applications of pectin and pectin-based composites.
The structure of pectin differs and depends on the type of plants and cell types that it develops in. Based on the source that the pectin emerges from, the polymer will vary in size, acetylation type, the degree to which it is esterified, and other variables that are controlled by the differences between the galacturonic acid that lead to the homogalacturonan chain and the side chain type of the rhamnogalacturonan-1
[8]. Rhamnogalacturonan-1 generally forms the branched regions of the pectin polysaccharide, which are the primary carbohydrate chains. Interestingly, Homogalacturonan forms the linear fragment of the polysaccharide, and sometimes, the chain forms the component that rhamnogalacturonan-1 generally makes up (
Figure 2). The pectin polysaccharide varies based on the source from which it is groomed and the conditions from which it is extracted
[9]. Regardless of the diversity found within the pectin polysaccharide, the structure is classified as canonical. Though pectin was discovered over two hundred years ago, the design of its composition has yet to be ultimately interpreted.
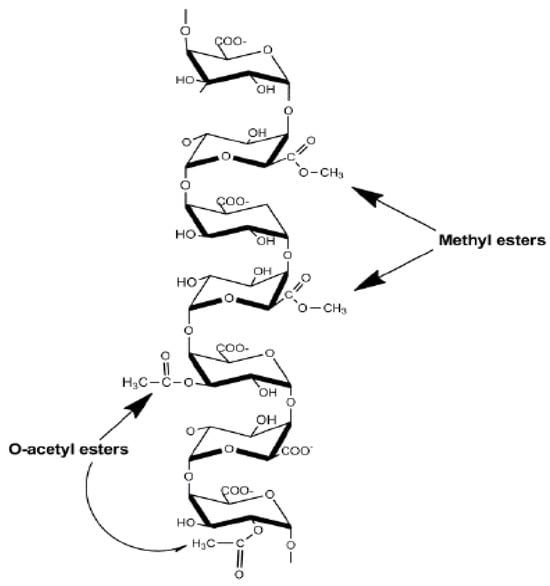
Figure 2. Homogalacturonan structure of pectin polysaccharides. Homogalacturonan is a linear polymer of α-(1,4)-D galacturonic acid with methyl-esterified at C-6 and acetyl-esterified at positions O-2 and O-3
[10].
The pectin polymer is a core structure alternating alpha-1, 4-linked D-galacturonic acid and alpha-1, 2-L-rhamnose units. The system of pectin regulates the influence that the polysaccharide has on cytokine production; this proves that the elemental characteristics that are found in the polysaccharide are related to its ability to impact cellular environmental conditions. The diversity in the structure of pectin polymers from different plant origins enables it to be used in multiple applications. Pectin extracted from various sources of plants generally has similar structural characteristics, but the structures ultimately differ based on the species and the plant’s physiological stage. With the effect of structural features, the chemical composition of pectin, such as the galacturonic acid proportion, methyl group content, and grade of acetylation, determines the polymer’s function
[1].
Immune reactivity is a factor that influences the use of pectin within biomedical and tissue engineering/drug delivery applications
[11][12][13]. In applications for pathological conditions, immunomodulators are essential to regulate the body’s distinctive immune response to foreign materials and antigens from foreign or transplanted cells. The purpose of utilizing immunomodulators such as pectin is not to eliminate the immune response but to regulate the reactivity and further the efficiency of the applications that require the modulation of the immune system. Past studies have reported that pectin can weaken inflammatory reactivity by stimulating anti-inflammatory cytokines and decreasing the assembly of proinflammatory cytokines
[14].
Pectin’s versatile properties allow it to be prospectively used in other applications, like medicine, as a carrier vehicle for drug delivery and a scaffold in tissue engineering or regenerative medicine.
2. Immunoregulatory Activity
The structural features of pectin provide a polysaccharide with biological activities such as immunomodulation. Immunomodulation is classified as a group of therapeutic interventions to regulate the immune system. Immunomodulators respond to the immune system by two different mechanisms: immunostimulation and immunosuppression. Immunosuppressive activity occurs on the backbone of pectin polysaccharides
[15]. The structural changes in the galacturonic chain of the pectin control the macromolecule’s capacity to reduce immune reactivity
[16]. The presence of a high quantity of galacturonic acid residues displays an increased immunosuppression activity. The amount of galacturonic acid residue fragments found on pectin determines the immunomodulatory effect. The injection of a glucan, zymosan, enables the pectin that contains more than 80% of the content of galacturonic acid residues to lower the production of macrophages. The polysaccharides of pectin that have 75% galacturonic acid residues or less do not reduce the gathering of macrophages stimulated by the injection of zymosan. Certain plants that produce pectin contain a significant percentage of galacturonic acid residues, while others do not. Plants with a high quantity of galacturonic acid residues include
Potamogeton natans L., pond weeds that produce the pectin called
Potamogeton Anand, and
Vaccinium oxycoccos L. This cranberry plant produces the pectin called oxycoccusan. Plants that give rise to the pectins with lower than 75% galacturonic acid are those derived from Butomus, derived from Butomaceae, and Lemna, which emerged from Araceae.
Table 1 shows pectin’s immunoregulatory activities.
Table 1. Pectin’s immunoregulatory activities: source and mechanism of action.
| Pectin Source |
Uses and Mechanism of Action |
Reference |
| i. Lemon Pectin |
The physical-chemical characteristics of lemon pectin, for example, the degree of methyl esterification and the extent of polymerization, influence the immunostimulatory properties. It is significantly essential to utilize pectins to improve immune response. |
[17][18] |
| ii. Sumbuci floss or elderflower |
Used to heal various diseases linked with the immune system, for example, influenza, chill, or pyrexia. Extracts from S. nigra flowers have stimulation effects on macrophages. In vitro studies reported that the biological activity of rhamnogalacturonan I (RG-1) comprising polysaccharides of elderflowers contributes to higher immunomodulation activity and enhanced macrophage-stimulating effects. |
[1][19] |
| iii. Tomato Pectin |
Pectic oligosaccharides in sour raw tomatoes demonstrated potential as an anticancer on a gastric cancer cell line in vitro. |
[20] |
| iv. Lycium ruthenium |
Polysaccharides in L. ruthenium suppressed proinflammatory cytokines in lipopolysaccharide-stimulated macrophages and exhibited antifatigue, antioxidation, and hypoglycemic activity. |
[21][22] |
3. Anti-Inflammatory Activity
Different degrees of methyl esterification affect the inflammatory properties of pectin. The various degrees of pectin methyl esterification play a role in determining the polysaccharide’s capacity to prevent the functional activity of white blood cells and leukocytes. In observing the influence of methyl esterification on pectin macromolecules, it is essential to analyze the makeup and characteristics of the pectin progenitor’s raw materials and the methods used to isolate the pectin. Table 2 summarizes the anti-inflammatory properties of pectin.
Table 2. Anti-inflammatory properties of pectin.
| Pectin Source |
Mechanism of Action |
Reference |
| i. Star fruit (Averrhoa carambola L.) |
In vivo, the study reported that the polysaccharides from starfruit exhibited antinociceptive and anti-inflammatory properties and were beneficial for controlling inflammatory pain. |
[23][24] |
| ii. Suaeda fruiticosa (L.) Forssk |
Polysaccharides, phenolic compounds, and bioactive flavonoids from S. fruticose, comprising free radical scavenging and lipid peroxidation, function as an anti-inflammatory agent and analgesic or antioxidant. |
[25][26] |
| iii. Citrus pectin |
An in vivo study demonstrated that low methyl-esterified pectin from citrus fruits inhibited systemic and local inflammation, whereas a high degree of esterification inhibited intestinal inflammation. |
[15][27] |
| iv. Sweet pepper fruits |
Both native and modified pectin possessed the inherent activity to control THP-1 macrophages. Due to the availability of lipopolysaccharides, anti-inflammatory properties occur by inhibiting proinflammatory and promoting anti-inflammatory cytokines. |
[28][29] |
4. Antibacterial Activity
Biomedical applications of antimicrobial natural systems have gained much attention in recent years. Biodegradable natural products based on pectin, pectin-linoleate, pectin-oleate, and pectin palmitate were reported to inhibit the microbial effect on several bacterial strains, including E. coli and S. aureus. Table 3 shows the reported data on the antibacterial properties of pectin. Table 3 exhibits the antibacterial properties of various pectin.
Table 3. Antibacterial properties of pectin-based composite materials.
| Pectin-Based System |
Mechanism of Action |
Reference |
| i. Citrus pectin-coated Ag nanoparticles (NPs) |
Citrus pectin-coated Ag NPs exhibited great antibacterial activities toward Gram-negative E. coli and Gram-positive S. Aureus. |
[30] |
| ii. Pectin–cadmium sulfide nanocomposite (Pc/CSNC); pectin–zirconium (IV) silicophosphate nanocomposite (Pc/ZSPNC) |
Pc/CSNC exhibited a significant effect of antibacterial activity against E. coli. PC/ZSPNC showed substantial antibacterial activity towards E. coli and S. aureus. |
[31][32] |
| iii. Citrus pectin–MgO nanocomposites |
Pectin–MgO showed significant antibacterial activity against clinical pathogens lactobacillus and Bacillus subtills. |
[33] |
| iv. Pectin/lysozymes layer-by-layer nanofibrous mats |
Pectin/lysosome nanofibrous mats exhibited significant antibacterial effects against E. coli and S. aureus. |
[34] |
| v. Essential oils (EOs)/Pectin nanoemulsion |
EOs/Pectin nanoemulsion exhibited antibacterial activity towards E. coli and L. innocua populations. |
[35][36] |
5. Anticancer Activity of Pectin and Pectin-Based Composites
Effective cancer treatment, a significant global disease, is highly challenging. Even though there is a substantial advancement in surgery, gene therapy, immunotherapy, chemotherapy, and radiotherapy, the mortality rate due to metastatic cancer is still alarming. Drug resistance of cancer tumor cells and adverse side effects of chemotherapies have been considered the critical drawbacks of cancer treatment. Several in vitro and in vivo studies reported the anti-tumor activity of pectin that showed a decrease in tumor cell adhesion and proliferation and stimulation of cell apoptosis
[37].