Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adel Razek | -- | 2156 | 2023-12-13 20:30:18 | | | |
| 2 | Sirius Huang | + 1 word(s) | 2157 | 2023-12-15 03:00:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Razek, A. Image-Guided Surgical and Pharmacotherapeutic Routines. Encyclopedia. Available online: https://encyclopedia.pub/entry/52710 (accessed on 07 February 2026).
Razek A. Image-Guided Surgical and Pharmacotherapeutic Routines. Encyclopedia. Available at: https://encyclopedia.pub/entry/52710. Accessed February 07, 2026.
Razek, Adel. "Image-Guided Surgical and Pharmacotherapeutic Routines" Encyclopedia, https://encyclopedia.pub/entry/52710 (accessed February 07, 2026).
Razek, A. (2023, December 13). Image-Guided Surgical and Pharmacotherapeutic Routines. In Encyclopedia. https://encyclopedia.pub/entry/52710
Razek, Adel. "Image-Guided Surgical and Pharmacotherapeutic Routines." Encyclopedia. Web. 13 December, 2023.
Copy Citation
Medical procedures have moved from a direct fully invasive “hand-eye” pairing process to a minimal invasive (MI) process with robot-imaging pairing in a closed-loop treatment architecture. Acts related to the invasive nature mainly concern surgical interventions (SIs) and the restricted dispensing of drugs. Currently, both of these procedures can use MI image-guided (IG) robotics, which enables patient comfort and safety, and medical staff accuracy and efficiency.
MRI-control
surgical interventions
implanted drug delivery
image-guided robotics
compliant treatment
MRI-compatibility
1. Introduction
Over the past few decades, medical procedures have moved from a direct fully invasive “hand-eye” pairing process to a minimal invasive (MI) process with robot-imaging pairing in a closed-loop treatment architecture. Acts related to the invasive nature mainly concern surgical interventions (SIs) and the restricted dispensing of drugs. Currently, both of these procedures can use MI image-guided (IG) robotics, which enables patient comfort and safety, and medical staff accuracy and efficiency. MI surgeries can be performed on most parts of the body, and the restricted drug delivery (RDD) that can be accomplished by implanted therapies, helps prevent the generalization of drugs throughout the body. Most imaging devices can be used in IG procedures. However, different imaging techniques are each tolerable for a specific situation. For example, those involving ionizing radiation would not be suitable for long interval exposure actions. In such a case, only the two imaging techniques exerting non-ionizing (NI) characteristics can be used; namely magnetic resonance imaging technique (MRI) and ultrasound imaging technique (USI).
An Imaging device is supposed to provide high-resolution 3D picturing of target configuration and neighboring material, including treatment instruments. Robotic assistance works normally within the imaging scaffold along with the target throughout imaging, permitting cooperative tasks to monitor treatment in a closed-loop mode. This can include pursuing target motion and deformation, localizing robotized equipment, and supervising therapy release. With the ever-increasing medical use of imaging technique-robot linked procedures, this presaged a new methodology to aid treatment techniques that allow medical staff to supervise patients better and more efficiently. Positioning robotic organizations within the frame of the scanner allows for a synergy of the imaging’s visual ability and the robotic assistant’s manipulation skill, resulting in closed-loop processing. With respect to imaging techniques with non-ionizing behavior, both MRI and USI reflect the characteristics mentioned above regarding imaging devices. Note that both imaging techniques can work in procedures with MI, IG, and NI for either SI or RDD operations. It should be noted that the only difference in behavior between these two imaging techniques is that the USI can only operate in airless and boneless windows. Additionally, operational precautions are required for MRI, for which the scaffolding environment must be free of electromagnetic (EM) noise. Nevertheless, MRI seems to be the universal imaging tool conditional to avoiding EM noise.
Robotic systems mainly contain actuation and sensing components. In the case of MRIs, the whole system is made of non-magnetic and non-conductive materials. This assumes that actuators and sensors contain negligible magnetic and conductive materials. Given that the scanner, is hyper sensitive to electromagnetic disturbances, questions on MRI-IG robots’ presence in this environment are of concern. Matters that do not disturb the scanner are called MRI-compatible and those that are disruptive are labelled MRI-incompatible. The latter produce artifacts deteriorating the images. The scanner compatibility relates to its functional ability and can be controlled via functional control (FC) analysis. For MRI, FC analysis is via electromagnetic compatibility (EMC) analysis.
Although SI and RDD robotic actions can behave autonomously, medical staff can supervise and modify their conducts via remote control of the procedure. In other words, the robot-imaging duo that replaced the hand-eye one is still mastered in a different way.
The evolution from early hand-eye pairing of SI and RDD until robot-imaging IG controlled MI- and NI-universal procedure using MRI under FC (EMC analysis) and hand-eye supervision is schematically summarized (Memento) in Figure 1.
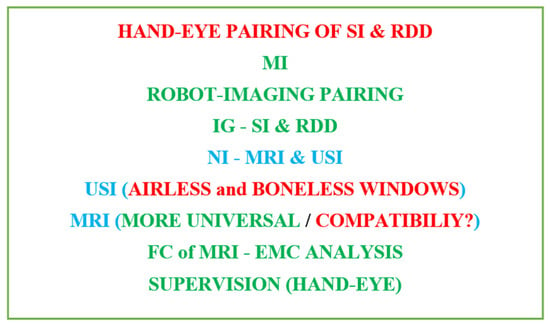
Figure 1. Summarized (Memento) successive evolution of surgical and pharmacotherapeutic routines following diligent medical treatment.
2. Image Guided Medical Procedures
As mentioned earlier, mildly intrusive automated surgical or therapeutic treatments have emerged as an important tool in current medication. This involves IG robotics of SI and implanted therapies. It characterizes the advantages of MI treatment, e.g., faster catch-up intervals for patients, and escapes many of its disadvantages for medical personnel, e.g., disturbed pointer-vision matching and lack of autonomy within the treatment of the concerned body portion of the patient. Thus, the automated MI treatment reduces the body and mental burden after the staff weigh it. Nevertheless, offering personalized and appropriate support to further improve clinical performance remains a subject of exploration. Figure 2 illustrates schematics principle of IG medical procedures, involving medical environment (surgery, implanted therapeutic, etc.), medical tools (surgical needle, drug source, etc.), and medical data (action, position, etc.).
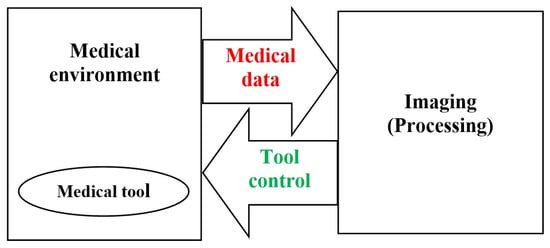
Figure 2. Schematics principle of IG medical treatment.
As mentioned earlier, choosing NI behavior involves using IRM or USI for IG operation of SI or RDD. As discussed and summarized in Figure 1, MRI appears to be suitable for all parts of the body without restriction. Due to its universal character, this text will focus on MRI analysis, knowing that the USI can be used if it is suitable for the materials examined. Note that USI reflects good maneuverability and reasonable cost while MRI, despite producing soft tissue imaging, is more complicated and expensive. Therefore, from the point of view of practical use, the choice between the two imaging techniques depends on the situation. Therefore, the USI must be used whenever possible, even for interventions. Only in cases involving bone and/or air, such as the brain and many other parts of the body, should we use MRI. Normally, surgical centers performing MRI-assisted treatments are expected to have surgical-imaging rooms and do not need to move the scanner. Regarding imaging costs, if the well-being of the patient is taken into account, the only option for non-ionizing and minimally invasive treatment regarding a brain intervention, for example, is an MRI-assisted intervention.
Indeed, MRI can deliver high-grade 3D imagining of object structure, nearby tissue, and instruments; however, there are substantial challenges in its use for effectually guiding SI or RDD procedures. These challenges consist of the use of three magnetic fields of dissimilar natures (as we will see later), allergic reactions to EM noise, and the restrained work area inside the imaging scaffold. This last drawback can be overcome by using an open structure scanner while accepting the disadvantage of a lower field intensity and therefore slower operation. On the other hand, MRI appears to be superior to other imaging strategies for different reasons. In addition to its NI behavior, it exhibits exceptional contrast allowing the visualization of tumors as well as other characters not detectable by other imaging techniques. It has true 3D-imaging potential, including multimodal detection, e.g., blood flow, temperature, biomarker tracking, etc. Under these conditions, the practice of robotic assistance by an MRI can allow an excellent SI or RDD.
2.1. Image Guided Surgical Interventions
Intraoperative imaging has fashioned a necessity to elaborate medical tools that satisfy the requests of diverse imaging techniques. Imaging backgrounds are demanding and they intensely influence the shape of the utensils used there. Advances in image resolution and disjunction capability have made interventions possible during the imaging procedure. 3D-imaging technique provides actual faithful descriptions of the human tissues while the instrument is being operated within a specified space, by tracking the coordinates of the image. In IG intraoperative procedures and MI-NI SI, MRI is increasingly more utilized due to its consistency, precision, and security. Due to its superior ability to differentiate tumor tissue from normal tissue, MRI is employed in SI for biopsies or tumor abstractions, for example [1][2][3][4][5][6][7]. The employed tools should be compatible with MRI. This compatibility has steered advancement of novel materials adapted for such tools. The use of an MRI-compatible robot to facilitate the approach to the body tissues inside the imaging scaffold permits the patient to remain within the scaffold during the entire SI time. Such IG association permits an important reduction of the SI duration, a higher SI accuracy, and faster recovery progression. Currently, the more accurate the IG association, the more recognizable the MRI-compatible strategies will be. Figure 3 schematically summarizes the representation of IG-SI, involving the interventional device in the MRI scaffold, the surgical data (action and location), the MRI imaging processing, and the device control.
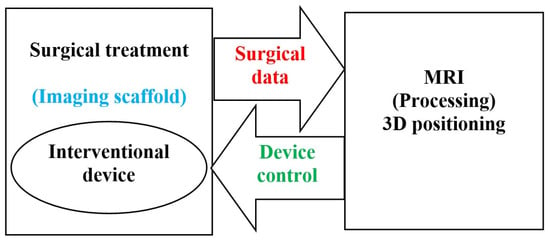
Figure 3. Schematics of an MRI IG-SI.
2.2. Implantable Drug Delivery Schemes
Operational drug delivery (DD) devices are able to deliver drugs to the target location and maintain drug intensity within a curatively relevant range. The dosage provided should deliver the drug for a specific duration necessary to achieve the greatest positive effect with the least adverse side consequences to adjacent healthy matters. Classical DD by means of discontinuous oral or intravenous delivery can result in high and rapid blood drug intensities soon after administration, thus intolerable harmful consequence may occur for patients. Another problem with these delivery modes is that they undergo first-pass metabolic rate, resulting in a significant reduction in drug concentration by the liver before attaining normal spread and hence often multiple deliveries are needed. DD made locally, sustainably, and supervised, can have the least adverse side effects possible. The use of DD implants enables such behavior.
The most used DD schemes comprise polymer reservoir-based structures, pumps (peristaltic, infusion, and osmotic), and micro and nano electromechanical structures (MEMS and NEMS), see e.g., [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]. Reservoir structures utilize drug release by dispersion via a film structure. The different pumps are used according to the type of disorder, the location of the implant, and the DD permanence. Larger ones are more suited to prolonged illnesses, while smaller pumps like the osmotic pump will be more suited to local-specific particular effects using DD of uniform release. Regarding MEMS and NEMS, they behave at micro and nano levels of DD without any significant danger due to a precipitous onset of the drug.
Even though implants present sophisticated and effectual ways for controlled DD, all of them necessitate placing by medical staff. The corresponding SIs differ according to the implant location and are connected to possible defies and unfavorable outcomes. Even if side consequences are generally slight, in certain conditions they can be substantial. It may be noted that the convolution and restrictions of SI for implant placing and withdrawing have weighty influences on the technology tolerability by the patient. Intrinsically, upcoming mechanism layout should utilize MI methodologies and reduced implant sizes, and less introduction-withdrawal processes can fully influence their capacity.
Future implants could be mainstream DD methodology, if thoughtfully designed with miniature size for MI insertion and continuous DD avoiding inclusion-exclusion with ease of drug filling. In fact, all of the implants described in the last paragraph are made from non-biodegradable materials and require the disposal of SI. Two next challenges should be acknowledged: The first concerns the facilitation of self-contained implanted DD systems addressing simple and spatially homogeneous problems using constant drug release and biodegradable structures that facilitate disposal of implanted systems [24][25][26][27][28][29][30][31][32][33][34][35]. The second concerns the treatment of spatially complicated cases requiring non-uniform DD, focusing only on diseased areas and avoiding healthy areas. This requires mobile implants with actions in restricted areas. Such a problem is closely similar to that of a complex SI requiring precise spatial assistance, as the IG assistance discussed in the last section. This implanted therapy will be MI-NI-IG-RDD [36][37][38][39][40][41][42][43][44]. The implant structure may be of non-biodegradable material but must be MRI-compatible when using such imaging techniques [45][46]. This procedure is similar to the MI-NI-IG-SI replacing the assisted actuated-robot with an assisted actuated-RDD implant. Wireless driving and monitoring transmission entails conveying strength and signals from an outside supply to the embedded device free of physical connection [45][46]. Figure 4 shows a schematically summarized representation of an MRI IG-implanted DD, including therapeutic device (DD source) in MRI scaffold, DD data (treated zone location), MRI processing, and device control.
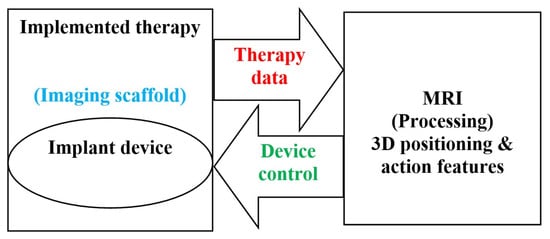
Figure 4. Summarized schematics of MRI IG-implanted DD therapeutic.
2.3. The Necessary Specifications of Surgeries and Drug Deliveries
For the well-being of patient, treatment should be precisely limited to the touched region in SI and RDD. Such precision is associated with the actuation accuracy of the mechanism and is greatly connected to the correctness of the space tracing. Consequently, the necessary condition for such high quality topological following is the image-controlled position localization. The summary of these necessary specifications are highlighted in Figure 5.

Figure 5. Summary of requests needed for MI-IG-SI and RDD.
Such a scheme may entail a collaborative arrangement functioning self-sufficiently as shown for example in the case of RDD in Figure 6. Figure 6a shows a collaborative self-governed IG-RDD scheme including the scanner, the body troubled zone, the implant, the control system and the supply. Figure 6b illustrates two scanned brains, a safe brain, (left) and an affected brain (right) with a troubled indicated zone.
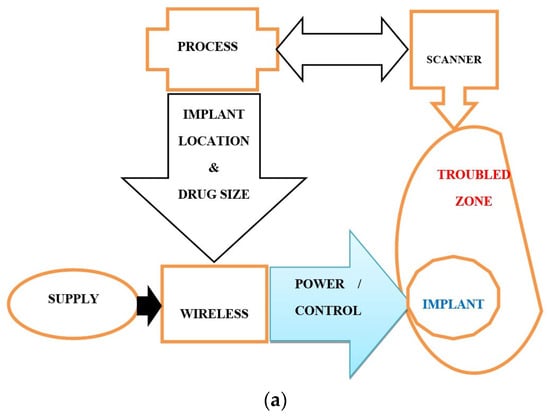
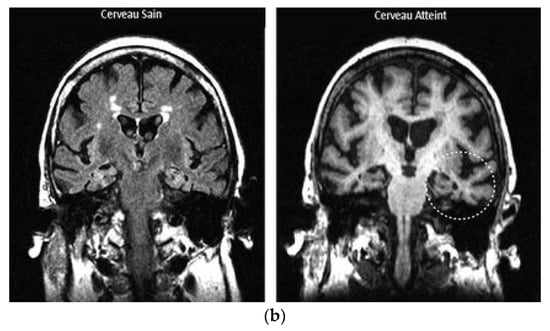
Figure 6. Representation of IG-RDD: (a) a collaborative self-governed IG-RDD scheme, (b) scanned safe brain (left), and affected brain (right) with a troubled indicated zone (the circle on the right side).
References
- Jia, X.; Zhang, Y.; Du, H.; Yu, Y. Experimental Study of Double Cable-Conduit Driving Device for Mri Compatible Biopsy Robots. J. Mech. Med. Biol. 2021, 21, 2140014.
- Li, X.; Young, A.S.; Raman, S.S.; Lu, D.S.; Lee, Y.H.; Tsao, T.C.; Wu, H.H. Automatic needle tracking using Mask R-CNN for MRI-guided percutaneous interventions. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1673–1684.
- Publicover, J.; Gallop, D.; Elliott, C.; Abed, J. A Navigation Strategy for Patient-Specific Needle Deflection in Transperineal MRI-Guided Prostate Interventions. Brachytherapy 2011, 10, S65.
- Scheer, J.K.; Hamelin, T.; Chang, L.; Lemkuil, B.; Carter, B.S.; Chen, C.C. Real-time Magnetic Resonance Imaging-Guided Biopsy Using SmartFrame® Stereotaxis in the Setting of a Conventional Diagnostic Magnetic Resonance Imaging Suite. Oper. Neurosurg. 2017, 13, 329–337.
- Wartenberg, M.; Schornak, J.; Carvalho, P.; Patel, N.; Iordachita, I.; Tempany, C.; Hata, N.; Tokuda, J.; Fischer, G.S. Closed-loop Autonomous Needle Steering during Cooperatively Controlled Needle Insertions for MRI-guided Pelvic Interventions. In The Hamlyn Symposium; Imperial College: London, UK, 2017.
- Wartenberg, M.; Patel, N.; Li, G.; Gregory, S.; Fischer, G.S. Towards synergistic control of hands-on needle insertion with automated needle steering for MRI-guided prostate interventions. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: Piscataway, NJ, USA, 2016.
- Wu, D.; Li, G.; Patel, N.; Yan, J.; Hu Kim, G.; Monfaredi, R.; Cleary, K.; Iordachita, I. Remotely Actuated Needle Driving Device for MRI-Guided Percutaneous Interventions: Force and Accuracy Evaluation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019.
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47.
- Minko, T. Drug delivery systems. In Martin’s Physical Pharmacy and Pharmaceutical Sciences; Sinko, P.J., Ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2006; pp. 629–680.
- Stewart, S.A.; Dominguez-Robles, J.; Donnelly, R.F.; Larraneta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379.
- Santos, A.; Sinn, A.W.M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182.
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8.
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162.
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10.
- Yang, W.W.; Pierstorff, E. Reservoir-based polymer drug delivery systems. J. Lab. Autom. 2012, 17, 50–58.
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview. In Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu, A.M., Ed.; William Andrew Applied Science Publishers: Oxford, UK, 2018; pp. 473–511.
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103.
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138.
- Lee, H.J.; Choi, N.; Yoon, E.S.; Cho, I.J. MEMS devices for drug delivery. Adv. Drug Deliv. Rev. 2018, 128, 132–147.
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the functionalized carbon nanotubes as smart nanocarriers for drug delivery applications. In Fullerens, Graphenes and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–133.
- Antonino, R.S.C.M.Q.; Ruggiero, M.; Song, Z.; Nascimento, T.H.; Lima, E.M.; Bohr, A.; Knopp, M.M.; Löbmann, K. Impact of drug loading in mesoporous silica-amorphous formulations on the physical stability of drugs with high recrystallization tendency. Int. J. Pharm. X 2019, 1, 100026.
- Yang, P.M.; Mu, H.Z.; Zhang, Y.S.; Wang, W.C.; Liu, C.; Zhang, S.Y. Sequential release of immunomodulatory cytokines binding on nano-hydroxyapatite coated titanium surface for regulating macrophage polarization and bone regeneration. Med. Hypotheses 2020, 144, 110241.
- Häner, J.D.; Räber, L.; Windecker, S. Biodegradable vs. permanent polymer drug-eluting stents: The need for a new nomenclature to classify drug-eluting stent technology. Eur. Heart J. 2019, 40, 2616–2619.
- Traverson, M.; Heiden, M.; Stanciu, L.A.; Nauman, E.A.; Jones-Hall, Y.; Breur, G.J. In Vivo Evaluation of Biodegradability and Biocompatibility of Fe30Mn Alloy. Vet. Comp. Orthop. Traumatol. 2018, 31, 10–16.
- Wang, Y.; Venezuela, J.; Dargusch, M. Biodegradable shape memory alloys: Progress and prospects. Biomaterials 2021, 279, 121215.
- Li, H.; Lin, G.; Wang, P.; Huang, J.; Wen, C. Nutrient alloying elements in biodegradable metals: A review. J. Mater. Chem. B 2021, 9, 9806–9825.
- Rabeeh, V.P.M.; Hanas, T. Progress in manufacturing and processing of degradable Fe-based implants: A review. Prog. Biomater. 2022, 11, 163–191.
- Babacan, N.; Kochta, F.; Hoffmann, V.; Gemming, T. Effect of silver additions on the microstructure, mechanical properties and corrosion behavior of biodegradable Fe-30Mn-6Si. Mater. Today Commun. 2021, 28, 102689.
- Tai, C.-C.; Lo, H.-L.; Liaw, C.-K.; Huang, Y.-M.; Huang, Y.-H.; Yang, K.-Y.; Huang, C.-C.; Huang, S.-I.; Shen, H.-H.; Lin, T.-H.; et al. Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model. Int. J. Mol. Sci. 2021, 22, 7368.
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hadisi, Z.; Iqbal, N. Characterization and biological properties of nanostructured clinoenstatite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2021, 259, 123969.
- Sun, Y.; Chen, L.; Liu, N.; Wang, H.; Liang, C. Laser-modified fe–30 mn surfaces with promoted biodegradability and biocompatibility toward biological applications. J. Mater. Sci. 2021, 56, 13772–13784.
- Saliba, L.; Sammut, K.; Tonna, C.; Pavli, F. FeMn and FeMnag Biodegradable Alloys: An In Vitro and In Vivo Investigation. Available online: https://ssrn.com/abstract=4325636 (accessed on 1 November 2023).
- Hao, S.; Yang, T.; Zhang, A.; Wang, P.; Jiang, H.; Shen, D.; Guo, L.; Ye, M. Evaluation of Biodegradable Alloy Fe30Mn0.6N in Rabbit Femur and Cartilage through Detecting Osteogenesis and Autophagy. Biomed Res. Int. 2023, 18, 3626776.
- Biffi, C.A.; Fiocchi, J.; Bregoli, C.; Gambaro, S.; Copes, F.; Mantovani, D.; Tuissi, A. Ultrashort Laser Texturing for Tuning Surface Morphology and Degradation Behavior of the Biodegradable Fe–20Mn Alloy for Temporary Implants. Adv. Eng. Mater. 2022, 24, 2101496.
- Putra, N.E.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zhou, J.; Zadpoor, A.A. Extrusion-based 3D printing of ex situ-alloyed highly biodegradable MRI-friendly porous iron-manganese scaffolds. Acta Biomater. 2021, 134, 774–790.
- Williams, R.P.; Karzova, M.M.; Yuldashev, P.V.; Kaloev, A.Z.; Nartov, F.A.; Khokhlova, V.A.; Cunitz, B.W.; Morrison, K.P.; Khokhlova, T.D. Dual-Mode 1-D Linear Ultrasound Array for Image-Guided Drug Delivery Enhancement without Ultrasound Contrast Agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 693–707.
- Williams, R.P.; Karzova, M.M.; Yuldashev, P.V.; Kaloev, A.Z.; Nartov, F.A.; Khokhlova, V.A.; Cunitz, B.W.; Morrison, K.P.; Khokhlova, T.D. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043.
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040.
- Nazary Abrbekoh, F.; Salimi, L.; Saghati, S.; Amini, H.; Fathi Karkan, S.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390.
- Arno, M.C.; Simpson, J.D.; Blackman, L.D.; Brannigan, R.P.; Thurecht, K.J.; Dove, A.P. Enhanced drug delivery to cancer cells through a pH-sensitive polycarbonate platform. Biomater. Sci. 2023, 11, 908–915.
- Chen, W.; Cheng, C.A.; Zink, J.I. Spatial, temporal, and dose control of drug delivery using noninvasive magnetic stimulation. ACS Nano 2019, 13, 1292–1308.
- Huang, J.; Xiao, K. Nanoparticles-based strategies to improve the delivery of therapeutic small interfering RNA in precision oncology. Pharmaceutics 2022, 14, 1586.
- Jia, M.; Zhang, D.; Zhang, C.; Li, C. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J. Nanobiotechnol. 2021, 19, 384.
- Zhao, J.F.; Zou, F.L.; Zhu, J.F.; Huang, C.; Bu, F.; Zhu, Z.; Yuan, R. Nano-drug delivery system for pancreatic cancer: A visualization and bibliometric analysis. Front. Pharmacol. 2022, 13, 1025618.
- Razek, A. Towards an image-guided restricted drug release in friendly implanted therapeutics. Eur. Phys. J. Appl. Phys. 2018, 82, 31401.
- Razek, A. Assessment of Supervised Drug Release in Cordial Embedded Therapeutics. Athens J. Technol. Eng. 2019, 6, 77–91.
More
Information
Subjects:
Physics, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
405
Revisions:
2 times
(View History)
Update Date:
15 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




