
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Noelle Abbott | -- | 5008 | 2023-12-13 18:53:45 | | | |
| 2 | Peter Tang | + 10 word(s) | 5018 | 2023-12-14 02:02:31 | | |
Video Upload Options
Developmental language disorder (DLD) is a heterogenous neurodevelopmental disorder that affects a child’s ability to comprehend and/or produce spoken and/or written language, yet it cannot be attributed to hearing loss or overt neurological damage. The link between brain development and language outcomes in children with DLD is unclear, and this lack of connection is apparent when reviewing the DLD neuroimaging literature. Over the past 50 years, there have been fewer than 60 neuroimaging studies (excluding EEG studies) with children diagnosed with DLD. The majority of these studies have focused on structural brain differences when compared to language-unimpaired (neurotypical) children or children with other neurodevelopmental language disorders, such as children diagnosed with ASD and concomitant language impairment. Though there are some consistencies differences in participant selection and inclusion, diagnostic criteria, methodology, and analyses used underlie the disparate findings to date. As such, comparing the results across studies and evaluating how structural brain abnormalities contribute to language impairment in children with DLD is challenging. Nonetheless, the researchers provide a general overview of structural neuroimaging findings in DLD and highlight consistent patterns of results.
1. Introduction
2. Structural Brain Differences
2.1. Global Brain Volume
2.2. Total Gray Matter Volume
3. Regional Brain Differences

3.1. Planum Temporale
3.2. Inferior Frontal Gyrus

3.3. Caudate Nucleus
4. White Matter Pathways
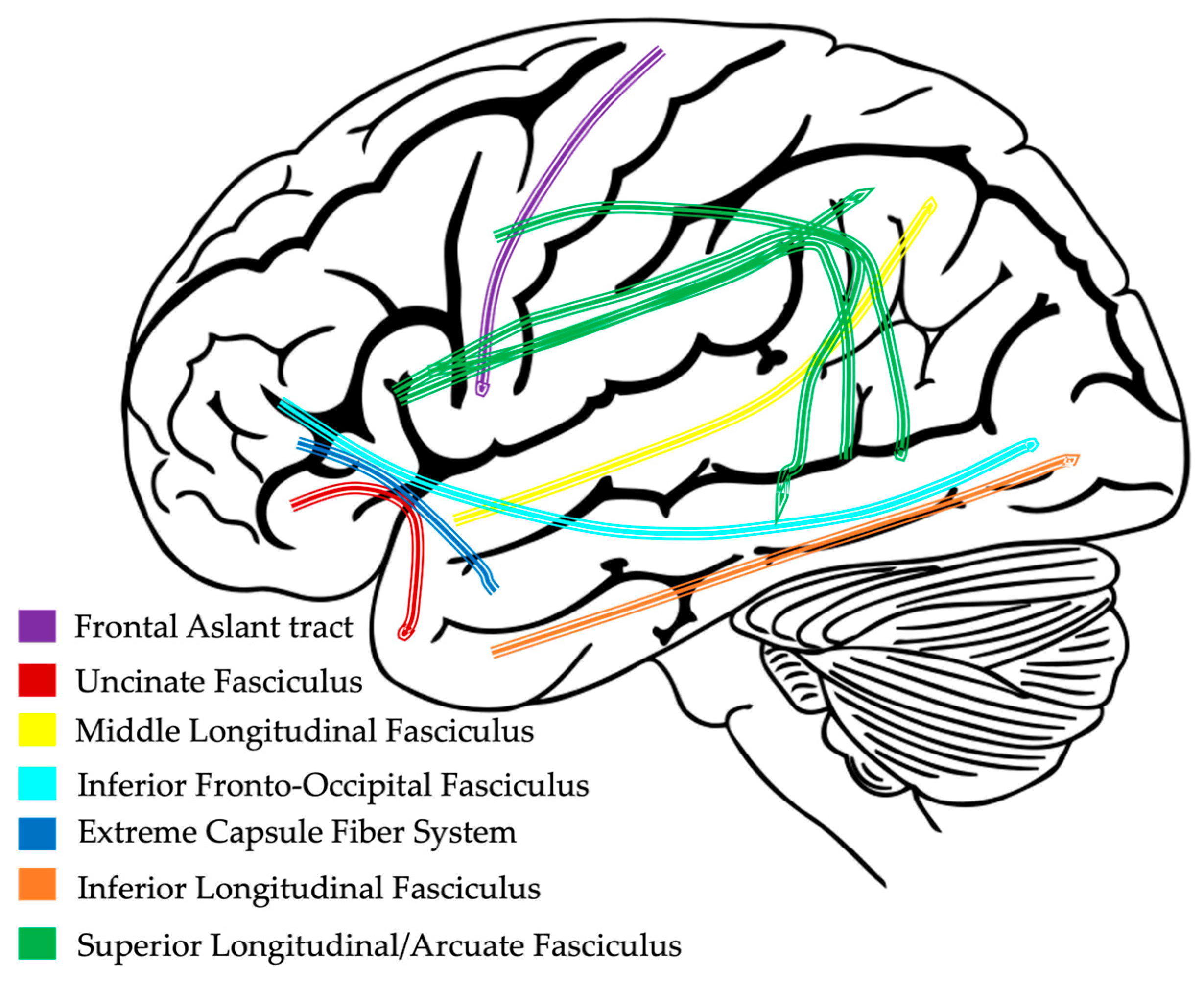
4.1. White Matter Volume
4.2. White Matter Diffusivity in Child Language-Impaired Populations
4.3. Dorsal and Ventral Language Pathways
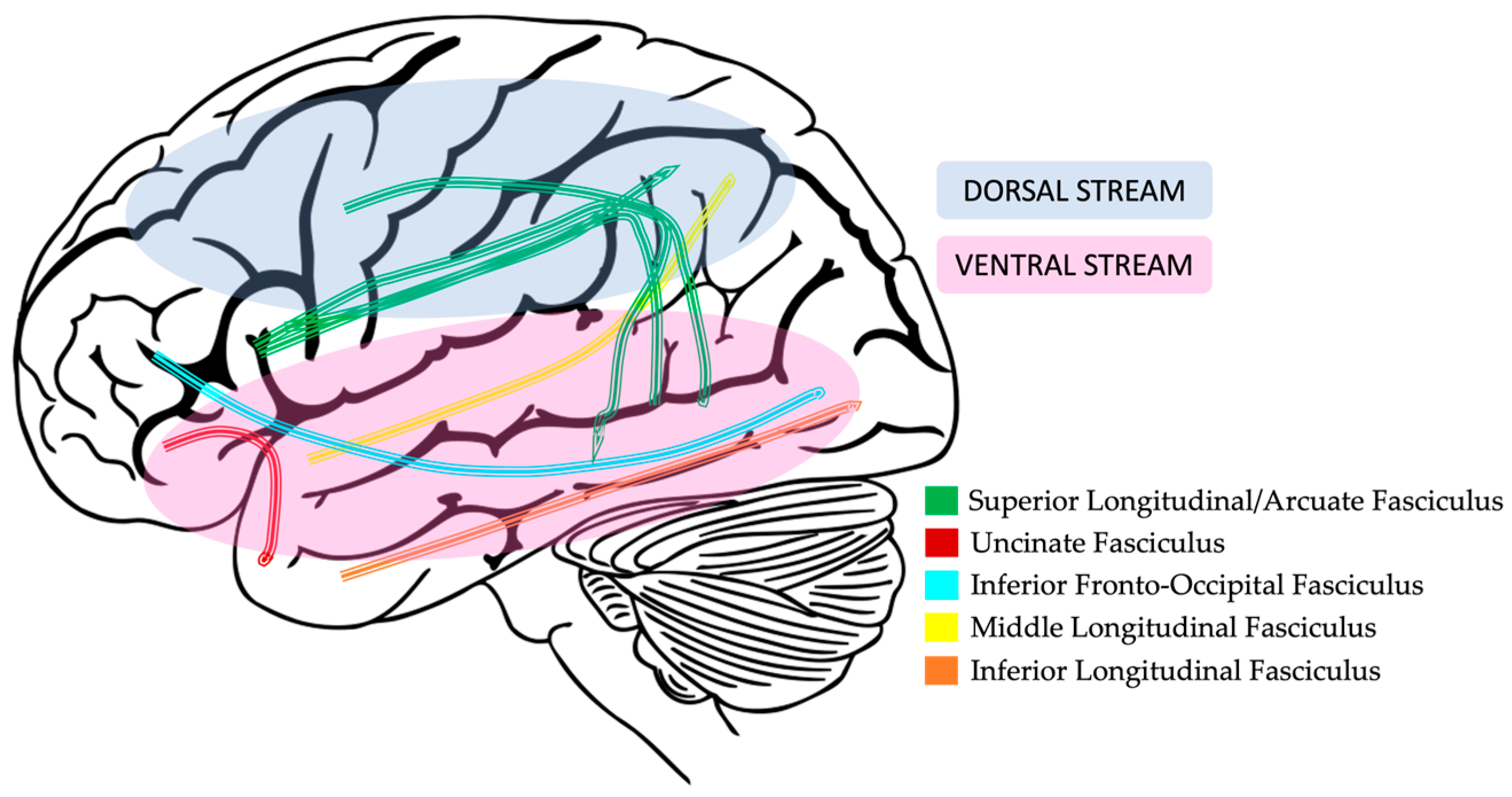
4.4. Dorsal Pathway Findings in DLD
4.5. Ventral Pathway Findings in DLD
References
- Tomblin, J.B.; Records, N.L.; Buckwalter, P.; Zhang, X.; Smith, E.; O’Brien, M. Prevalence of Specific Language Impairment in Kindergarten Children. J. Speech Lang. Hear. Res. 1997, 40, 1245–1260.
- Maenner, M.J.; Shaw, K.A.; Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1.
- Norbury, C.F.; Gooch, D.; Wray, C.; Baird, G.; Charman, T.; Simonoff, E.; Vamvakas, G.; Pickles, A. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. J. Child Psychol. Psychiatry 2016, 57, 1247–1257.
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811.
- Clegg, J.; Hollis, C.; Mawhood, L.; Rutter, M. Developmental language disorders—A follow-up in later adult life. Cognitive, language and psychosocial outcomes. J. Child Psychol. Psychiatry 2005, 46, 128–149.
- Maggio, V.; Grañana, N.E.; Richaudeau, A.; Torres, S.; Giannotti, A.; Suburo, A.M. Behavior problems in children with specific language impairment. J. Child Neurol. 2014, 29, 194–202.
- Sansavini, A.; Favilla, M.E.; Guasti, M.T.; Marini, A.; Millepiedi, S.; Di Martino, M.V.; Vecchi, S.; Battajon, N.; Bertolo, L.; Capirci, O.; et al. Developmental Language Disorder: Early Predictors, Age for the Diagnosis, and Diagnostic Tools. A Scoping Review. Brain Sci. 2021, 11, 654.
- Bishop, D.V. Cerebral asymmetry and language development: Cause, correlate, or consequence? Science 2013, 340, 1230531.
- Schwartz, R.G. Handbook of Child Language Disorders, 2nd ed.; Psychology Press: London, UK, 2017.
- Bishop, D.V.; Norbury, C.F. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. J. Child Psychol. Psychiatry 2002, 43, 917–929.
- Lancaster, H.S.; Camarata, S. Reconceptualizing developmental language disorder as a spectrum disorder: Issues and evidence. Int. J. Lang. Commun. Disord. 2019, 54, 79–94.
- Tager-Flusberg, H. Do autism and specific language impairment represent overlapping language disorders? In Developmental Language Disorders; Psychology Press: London, UK, 2004; pp. 42–63.
- Lebel, C.; Deoni, S. The development of brain white matter microstructure. Neuroimage 2018, 182, 207–218.
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348.
- Yap, Q.J.; Teh, I.; Fusar-Poli, P.; Sum, M.Y.; Kuswanto, C.; Sim, K. Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. J. Neural Transm. 2013, 120, 1369–1395.
- Jernigan, T.L.; Gamst, A.C. Changes in volume with age—Consistency and interpretation of observed effects. Neurobiol. Aging 2005, 26, 1271–1274.
- Pirozzi, F.; Nelson, B.; Mirzaa, G. From microcephaly to megalencephaly: Determinants of brain size. Dialogues Clin. Neurosci. 2018, 20, 267–282.
- Bayard, F.; Nymberg Thunell, C.; Abé, C.; Almeida, R.; Banaschewski, T.; Barker, G.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; Quinlan, E.B.; et al. Distinct brain structure and behavior related to ADHD and conduct disorder traits. Mol. Psychiatry 2020, 25, 3020–3033.
- Brieber, S.; Neufang, S.; Bruning, N.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Fink, G.R.; Konrad, K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2007, 48, 1251–1258.
- Hasan, K.M.; Molfese, D.L.; Walimuni, I.S.; Stuebing, K.K.; Papanicolaou, A.C.; Narayana, P.A.; Fletcher, J.M. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012, 25, 1263–1270.
- Krain, A.L.; Castellanos, F.X. Brain development and ADHD. Clin. Psychol. Rev. 2006, 26, 433–444.
- Nickl-Jockschat, T.; Habel, U.; Maria Michel, T.; Manning, J.; Laird, A.R.; Fox, P.T.; Schneider, F.; Eickhoff, S.B. Brain structure anomalies in autism spectrum disorder-a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012, 33, 1470–1489.
- Xia, Z.; Hoeft, F.; Zhang, L.; Shu, H. Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia 2016, 81, 68–78.
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.D.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.S.; Fletcher, P.T.; et al. Longitudinal Volumetric Brain Changes in Autism Spectrum Disorder Ages 6–35 Years. Autism Res. 2015, 8, 82–93.
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533.
- Gauger, L.M.; Lombardino, L.J.; Leonard, C.M. Brain morphology in children with specific language impairment. J. Speech Lang. Hear. Res. 1997, 40, 1272–1284.
- Girbau-Massana, D.; Garcia-Marti, G.; Marti-Bonmati, L.; Schwartz, R.G. Gray–white matter and cerebrospinal fluid volume differences in children with specific language impairment and/or reading disability. Neuropsychologia 2014, 56, 90–100.
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540.
- Lee, J.C.; Nopoulos, P.C.; Tomblin, J.B. Abnormal subcortical components of the corticostriatal system in young adults with DLI: A combined structural MRI and DTI study. Neuropsychologia 2013, 51, 2154–2161.
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Kennedy, D.N.; Filipek, P.A.; Caviness, V.S., Jr. Larger brain and white matter volumes in children with developmental language disorder. Dev. Sci. 2003, 6, F11–F22.
- Soriano-Mas, C.; Pujol, J.; Ortiz, H.; Deus, J.; López-Sala, A.; Sans, A. Age-related brain structural alterations in children with specific language impairment. Hum. Brain Mapp. 2009, 30, 1626–1636.
- Badcock, N.A.; Bishop, D.V.; Hardiman, M.J.; Barry, J.G.; Watkins, K.E. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012, 120, 310–320.
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral Lobes in Autism: Early Hyperplasia and Abnormal Age Effects. NeuroImage 2002, 16, 1038–1051.
- Wierenga, L.M.; Langen, M.; Oranje, B.; Durston, S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 2014, 87, 120–126.
- Bahar, N.; Cler, G.J.; Krishnan, S.; Asaridou, S.S.; Smith, H.J.; Willis, H.E.; Healy, M.P.; Watkins, K.E. Differences in cortical surface area in developmental language disorder. bioRxiv 2023.
- White, T.; Su, S.; Schmidt, M.; Kao, C.-Y.; Sapiro, G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010, 72, 36–45.
- Fedorenko, E.; Thompson-Schill, S.L. Reworking the language network. Trends Cogn. Sci. 2014, 18, 120–126.
- Friederici, A.D.; Chomsky, N.; Berwick, R.C.; Moro, A.; Bolhuis, J.J. Language, mind and brain. Nat. Hum. Behav. 2017, 1, 713–722.
- Hertrich, I.; Dietrich, S.; Ackermann, H. The margins of the language network in the brain. Front. Commun. 2020, 5, 519955.
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88.
- Mayes, A.K.; Reilly, S.; Morgan, A.T. Neural correlates of childhood language disorder: A systematic review. Dev. Med. Child Neurol. 2015, 57, 706–717.
- Evans, J.L.; Brown, T.T. Specific language impairment. In Neurobiology of Language; Elsevier: Amsterdam, The Netherlands, 2016; pp. 899–912.
- Ocklenburg, S.; Friedrich, P.; Fraenz, C.; Schlüter, C.; Beste, C.; Güntürkün, O.; Genç, E. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 2018, 4, eaar6830.
- Galuske, R.A.; Schlote, W.; Bratzke, H.; Singer, W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science 2000, 289, 1946–1949.
- Dorsaint-Pierre, R.; Penhune, V.B.; Watkins, K.E.; Neelin, P.; Lerch, J.P.; Bouffard, M.; Zatorre, R.J. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain 2006, 129, 1164–1176.
- Foundas, A.L.; Leonard, C.M.; Gilmore, R.; Fennell, E.; Heilman, K.M. Planum temporale asymmetry and language dominance. Neuropsychologia 1994, 32, 1225–1231.
- Geschwind, N.; Levitsky, W. Human brain: Left-right asymmetries in temporal speech region. Science 1968, 161, 186–187.
- Rojas, D.C.; Camou, S.L.; Reite, M.L.; Rogers, S.J. Planum temporale volume in children and adolescents with autism. J. Autism Dev. Disord. 2005, 35, 479–486.
- Eckert, M.A.; Leonard, C.M. Structural imaging in dyslexia: The planum temporale. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 198–206.
- Plante, E.; Swisher, L.; Vance, R.; Rapcsak, S. MRI findings in boys with specific language impairment. Brain Lang. 1991, 41, 52–66.
- Cohen, M.; Campbell, R.; Yaghmai, F. Neuropathological abnormalities in developmental dysphasia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989, 25, 567–570.
- Galaburda, A.M.; Sherman, G.F.; Rosen, G.D.; Aboitiz, F.; Geschwind, N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1985, 18, 222–233.
- Jernigan, T.L.; Hesselink, J.R.; Sowell, E.; Tallal, P.A. Cerebral structure on magnetic resonance imaging in language-and learning-impaired children. Arch. Neurol. 1991, 48, 539–545.
- Preis, S.; Jäncke, L.; Schittler, P.; Huang, Y.; Steinmetz, H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia 1998, 36, 849–855.
- De Fossé, L.; Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; McGrath, L.; Steele, S.; Ziegler, D.A.; Herbert, M.R.; Frazier, J.A.; et al. Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 2004, 56, 757–766.
- Rogalsky, C.; Matchin, W.; Hickok, G. Broca’s area, sentence comprehension, and working memory: An fMRI study. Front. Hum. Neurosci. 2008, 2, 237.
- Grodzinsky, Y. The neurology of syntax: Language use without Broca’s area. Behav. Brain Sci. 2000, 23, 1–21.
- Martin, R.C. Language processing: Functional organization and neuroanatomical basis. Annu. Rev. Psychol. 2003, 54, 55–89.
- Hope, T.M.; Prejawa, S.; Parker Jones, Ō.; Oberhuber, M.; Seghier, M.L.; Green, D.W.; Price, C.J. Dissecting the functional anatomy of auditory word repetition. Front. Hum. Neurosci. 2014, 8, 246.
- Lee, J.C.; Dick, A.S.; Tomblin, J.B. Altered brain structures in the dorsal and ventral language pathways in individuals with and without developmental language disorder (DLD). Brain Imaging Behav. 2020, 14, 2569–2586.
- Watkins, K.E.; Vargha-Khadem, F.; Ashburner, J.; Passingham, R.E.; Connelly, A.; Friston, K.J.; Frackowiak, R.S.; Mishkin, M.; Gadian, D.G. MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain 2002, 125, 465–478.
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.; O’Brien, L.M.; Kennedy, D.N.; Filipek, P.; Bakardjiev, A.; Hodgson, J.; Takeoka, M.; Makris, N. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain 2005, 128, 213–226.
- Booth, J.R.; Wood, L.; Lu, D.; Houk, J.C.; Bitan, T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007, 1133, 136–144.
- Crosson, B.; Benefield, H.; Cato, M.A.; Sadek, J.R.; Moore, A.B.; Wierenga, C.E.; Gopinath, K.; Soltysik, D.; Bauer, R.M.; Auerbach, E.J.; et al. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J. Int. Neuropsychol. Soc. 2003, 9, 1061–1077.
- Tan, A.P.; Ngoh, Z.M.; Yeo, S.S.P.; Koh, D.X.P.; Gluckman, P.; Chong, Y.S.; Daniel, L.M.; Rifkin-Graboi, A.; Fortier, M.V.; Qiu, A.; et al. Left lateralization of neonatal caudate microstructure affects emerging language development at 24 months. Eur. J. Neurosci. 2021, 54, 4621–4637.
- Thibault, S.; Py, R.; Gervasi, A.M.; Salemme, R.; Koun, E.; Lövden, M.; Boulenger, V.; Roy, A.C.; Brozzoli, C. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science 2021, 374, eabe0874.
- Wahl, M.; Marzinzik, F.; Friederici, A.D.; Hahne, A.; Kupsch, A.; Schneider, G.-H.; Saddy, D.; Curio, G.; Klostermann, F. The Human Thalamus Processes Syntactic and Semantic Language Violations. Neuron 2008, 59, 695–707.
- Krishnan, S.; Watkins, K.E.; Bishop, D.V.M. Neurobiological Basis of Language Learning Difficulties. Trends Cogn. Sci. 2016, 20, 701–714.
- Ullman, M.T.; Pierpont, E.I. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex 2005, 41, 399–433.
- Krishnan, S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Asaridou, S.S.; Healy, M.P.; Papp, D.; Watkins, K.E. Quantitative MRI reveals differences in striatal myelin in children with DLD. eLife 2022, 11, e74242.
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin development in cerebral gray and white matter during adolescence and late childhood. Neuroimage 2021, 227, 117678.
- Timmler, S.; Simons, M. Grey matter myelination. Glia 2019, 67, 2063–2070.
- Zhao, T.; Xu, Y.; He, Y. Graph theoretical modeling of baby brain networks. NeuroImage 2019, 185, 711–727.
- Dubois, J.; Dehaene-Lambertz, G.; Kulikova, S.; Poupon, C.; Hüppi, P.S.; Hertz-Pannier, L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 2014, 276, 48–71.
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 2012, 60, 340.
- Lebel, C.; Treit, S.; Beaulieu, C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019, 32, e3778.
- Kaestner, E.; Balachandra, A.R.; Bahrami, N.; Reyes, A.; Lalani, S.J.; Macari, A.C.; Voets, N.L.; Drane, D.L.; Paul, B.M.; Bonilha, L. The white matter connectome as an individualized biomarker of language impairment in temporal lobe epilepsy. NeuroImage Clin. 2020, 25, 102125.
- Langer, N.; Peysakhovich, B.; Zuk, J.; Drottar, M.; Sliva, D.D.; Smith, S.; Becker, B.L.; Grant, P.E.; Gaab, N. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex 2017, 27, 1027–1036.
- Olivé, G.; Slušná, D.; Vaquero, L.; Muchart-López, J.; Rodríguez-Fornells, A.; Hinzen, W. Structural connectivity in ventral language pathways characterizes non-verbal autism. Brain Struct. Funct. 2022, 227, 1817–1829.
- Vanderauwera, J.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Early dynamics of white matter deficits in children developing dyslexia. Dev. Cogn. Neurosci. 2017, 27, 69–77.
- Fletcher, P.T.; Whitaker, R.T.; Tao, R.; Dubray, M.B.; Froehlich, A.; Ravichandran, C.; Alexander, A.L.; Bigler, E.D.; Lange, N.; Lainhart, J.E. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage 2010, 51, 1117–1125.
- Li, M.; Wang, Y.; Tachibana, M.; Rahman, S.; Kagitani-Shimono, K. Atypical structural connectivity of language networks in autism spectrum disorder: A meta-analysis of diffusion tensor imaging studies. Autism Res. 2022, 15, 1585–1602.
- Dick, A.S.; Bernal, B.; Tremblay, P. The language connectome: New pathways, new concepts. Neuroscientist 2014, 20, 453–467.
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99.
- Saur, D.; Kreher, B.W.; Schnell, S.; Kümmerer, D.; Kellmeyer, P.; Vry, M.-S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040.
- Roberts, T.; Heiken, K.; Zarnow, D.; Dell, J.; Nagae, L.; Blaskey, L.; Solot, C.; Levy, S.; Berman, J.; Edgar, J. Left hemisphere diffusivity of the arcuate fasciculus: Influences of autism spectrum disorder and language impairment. Am. J. Neuroradiol. 2014, 35, 587–592.
- Vydrova, R.; Komarek, V.; Sanda, J.; Sterbova, K.; Jahodova, A.; Maulisova, A.; Zackova, J.; Reissigova, J.; Krsek, P.; Kyncl, M. Structural alterations of the language connectome in children with specific language impairment. Brain Lang. 2015, 151, 35–41.
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage 2014, 92, 356–368.
- Verly, M.; Gerrits, R.; Sleurs, C.; Lagae, L.; Sunaert, S.; Zink, I.; Rommel, N. The mis-wired language network in children with developmental language disorder: Insights from DTI tractography. Brain Imaging Behav. 2019, 13, 973–984.
- Liu, J.; Tsang, T.; Jackson, L.; Ponting, C.; Jeste, S.S.; Bookheimer, S.Y.; Dapretto, M. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev. Sci. 2019, 22, e12768.
- Baum, G.L.; Cui, Z.; Roalf, D.R.; Ciric, R.; Betzel, R.F.; Larsen, B.; Cieslak, M.; Cook, P.A.; Xia, C.H.; Moore, T.M.; et al. Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. USA 2020, 117, 771–778.





