
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rudolf Armin Manz | -- | 2671 | 2023-12-13 16:38:51 | | | |
| 2 | Lindsay Dong | Meta information modification | 2671 | 2023-12-19 03:50:04 | | |
Video Upload Options
Food allergies are a growing public health concern worldwide, especially in children and young adults. Allergen-specific IgE plays a central role in the pathogenesis of food allergies, but their titers poorly correlate with allergy development. Host immune systems yield allergen-specific immunoglobulin (Ig)A, IgE and IgG subclasses with low or high affinities and differential Fc N-glycosylation patterns that can affect the allergic reaction to food in multiple ways. High-affinity IgE is required to induce strong mast cell activation eventually leading to allergic anaphylaxis, while low-affinity IgE can even inhibit the development of clinically relevant allergic symptoms. IgA and IgG antibodies can inhibit IgE-mediated mast cell activation through various mechanisms, thereby protecting IgE-positive individuals from allergy development. The production of IgE and IgG with differential allergenic potential seems to be affected by the signaling strength of individual B cell receptors, and by cytokines from T cells.
1. Introduction
2. Low and High-Affinity IgE Play Opposing Roles in Food Allergy
3. The Role of Antibody Isotypes, their Subclasses and Antibody Fc Glycosylation in Food Allergy
3.1. Mechanisms of IgG-Mediated Suppression of Allergy
3.2. Mechanisms of IgA-Mediated Suppression of Allergy
4. The Impact of Antibody Ig-Fc Glycosylation on Allergy Development
5. Development of Antibodies in Food Allergy
5.1. T Cell Activation
5.2. Production of Unmutated, Low-Affinity IgE
5.3. Production of Mutated, High-Affinity IgE
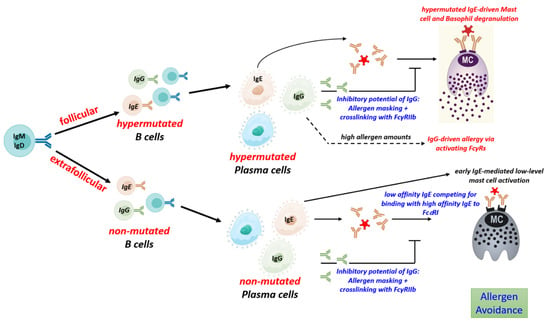
5.4. Regulation of IgG to IgE Ratios
6. Development of Differentially Glycosylated Antibodies
7. The Impact of Distinct Antibody Types in Type 1 Allergic Reactions to Aero-Allergens
8. Conclusions
References
- Jackson, K.D.; Howie, L.D.; Akinbami, O.J. Trends in Allergic Conditions among Children: United States, 1997–2011; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2013.
- Simons, F.E.R.; Ardusso, L.R.F.; Bilo, M.B.; Dimov, V.; Ebisawa, M.; El-Gamal, Y.M.; Ledford, D.K.; Lockey, R.F.; Ring, J.; Sanchez-Borges, M.; et al. 2012 Update: World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 389–399.
- Cooke, A.T.; Meize-Grochowski, R. Epinephrine Auto-Injectors for Anaphylaxis Treatment in the School Setting: A Discussion Paper. SAGE Open Nurs. 2019, 5, 2377960819845246.
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, e9–e17.
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235.
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies among US Adults. JAMA Netw. Open 2019, 2, e185630.
- Kurukulaaratchy, R.J.; Karmaus, W.; Arshad, S.H. Gender and Atopy Influences on the Natural History of Rhinitis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 7.
- Harris, D.P.; Haynes, L.; Sayles, P.C.; Duso, D.K.; Eaton, S.M.; Lepak, N.M.; Johnson, L.L.; Swain, S.L.; Lund, F.E. Reciprocal Regulation of Polarized Cytokine Production by Effector B and T Cells. Nat. Immunol. 2000, 1, 475–482.
- Song, Z.; Yuan, W.; Zheng, L.; Wang, X.; Kuchroo, V.K.; Mohib, K.; Rothstein, D.M. B Cell IL-4 Drives Th2 Responses in Vivo, Ameliorates Allograft Rejection, and Promotes Allergic Airway Disease. Front. Immunol. 2022, 13, 762390.
- Hurdayal, R.; Ndlovu, H.H.; Revaz-Breton, M.; Parihar, S.P.; Nono, J.K.; Govender, M.; Brombacher, F. IL-4–Producing B Cells Regulate T Helper Cell Dichotomy in Type 1-and Type 2-Controlled Diseases. Proc. Natl. Acad. Sci. USA 2017, 114, E8430–E8439.
- Hammad, H.; Plantinga, M.; Deswarte, K.; Pouliot, P.; Willart, M.A.M.; Kool, M.; Muskens, F.; Lambrecht, B.N. Inflammatory Dendritic Cells—Not Basophils—Are Necessary and Sufficient for Induction of Th2 Immunity to Inhaled House Dust Mite Allergen. J. Exp. Med. 2010, 207, 2097–2111.
- Looney, T.J.; Lee, J.-Y.; Roskin, K.M.; Hoh, R.A.; King, J.; Glanville, J.; Liu, Y.; Pham, T.D.; Dekker, C.L.; Davis, M.M.; et al. Human B-Cell Isotype Switching Origins of IgE. J. Allergy Clin. Immunol. 2016, 137, 579–586.
- He, J.-S.; Narayanan, S.; Subramaniam, S.; Ho, W.Q.; Lafaille, J.J.; de Lafaille, M.A.C. Biology of IgE Production: IgE Cell Differentiation and the Memory of IgE Responses. In IgE Antibodies: Generation and Function; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–19.
- Udoye, C.C.; Rau, C.N.; Freye, S.M.; Almeida, L.N.; Vera-Cruz, S.; Othmer, K.; Korkmaz, R.Ü.; Clauder, A.-K.; Lindemann, T.; Niebuhr, M.; et al. B-Cell Receptor Physical Properties Affect Relative IgG1 and IgE Responses in Mouse Egg Allergy. Mucosal Immunol. 2022, 15, 1375–1388.
- He, J.-S.; Subramaniam, S.; Narang, V.; Srinivasan, K.; Saunders, S.P.; Carbajo, D.; Wen-Shan, T.; Hidayah Hamadee, N.; Lum, J.; Lee, A.; et al. IgG1 Memory B Cells Keep the Memory of IgE Responses. Nat. Commun. 2017, 8, 641.
- Takhar, P.; Smurthwaite, L.; Coker, H.A.; Fear, D.J.; Banfield, G.K.; Carr, V.A.; Durham, S.R.; Gould, H.J. Allergen Drives Class Switching to IgE in the Nasal Mucosa in Allergic Rhinitis. J. Immunol. 2005, 174, 5024–5032.
- Hoh, R.A.; Joshi, S.A.; Lee, J.-Y.; Martin, B.A.; Varma, S.; Kwok, S.; Nielsen, S.C.A.; Nejad, P.; Haraguchi, E.; Dixit, P.S.; et al. Origins and Clonal Convergence of Gastrointestinal IgE+ B Cells in Human Peanut Allergy. Sci. Immunol. 2020, 5, eaay4209.
- Manz, R.A.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Maintenance of Serum Antibody Levels. Annu Rev. Immunol. 2005, 23, 367–386.
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The Regulation of Immunoglobulin E Class-Switch Recombination. Nat. Rev. Immunol. 2003, 3, 721–732.
- Yang, Z.; Robinson, M.J.; Chen, X.; Smith, G.A.; Taunton, J.; Liu, W.; Allen, C.D.C. Regulation of B Cell Fate by Chronic Activity of the IgE B Cell Receptor. Elife 2016, 5, e21238.
- Croote, D.; Darmanis, S.; Nadeau, K.C.; Quake, S.R. High-Affinity Allergen-Specific Human Antibodies Cloned from Single IgE B Cell Transcriptomes. Science 2018, 362, 1306–1309.
- Laffleur, B.; Duchez, S.; Tarte, K.; Denis-Lagache, N.; Péron, S.; Carrion, C.; Denizot, Y.; Cogné, M. Self-Restrained B Cells Arise Following Membrane IgE Expression. Cell Rep. 2015, 10, 900–909.
- MacGlashan, D., Jr. IgE Receptor and Signal Transduction in Mast Cells and Basophils. Curr. Opin. Immunol. 2008, 20, 717–723.
- Metzger, H. The Receptor with High Affinity for IgE. Immunol. Rev. 1992, 125, 37–48.
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704.
- Mita, H.; Yasueda, H.; Akiyama, K. Affinity of IgE Antibody to Antigen Influences Allergen-induced Histamine Release. Clin. Exp. Allergy 2000, 30, 1583–1589.
- Chang, X. Low-Affinity but High-Avidity Interactions May Offer an Explanation for IgE-Mediated Allergen Cross-Reactivity. Allergy 2021, 76, 2565–2574.
- Xiong, H.; Dolpady, J.; Wabl, M.; Curotto de Lafaille, M.A.; Lafaille, J.J. Sequential Class Switching Is Required for the Generation of High Affinity IgE Antibodies. J. Exp. Med. 2012, 209, 353–364.
- Suzuki, R.; Leach, S.; Liu, W.; Ralston, E.; Scheffel, J.; Zhang, W.; Lowell, C.A.; Rivera, J. Molecular Editing of Cellular Responses by the High-Affinity Receptor for IgE. Science 2014, 343, 1021–1025.
- Kelleher, M.M.; Phillips, R.; Brown, S.J.; Cro, S.; Cornelius, V.; Carlsen, K.C.L.; Skjerven, H.O.; Rehbinder, E.M.; Lowe, A.J.; Dissanayake, E.; et al. Skin Care Interventions in Infants for Preventing Eczema and Food Allergy. Cochrane Database Syst. Rev. 2022, 11, CD013534.
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T Follicular Helper Cell Subset That Drives Anaphylactic IgE. Science 2019, 365, eaaw6433.
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of Asthma with Serum IgE Levels and Skin-Test Reactivity to Allergens. N. Engl. J. Med. 1989, 320, 271–277.
- Strait, R.T.; Posgai, M.T.; Mahler, A.; Barasa, N.; Jacob, C.O.; Köhl, J.; Ehlers, M.; Stringer, K.; Shanmukhappa, S.K.; Witte, D.; et al. IgG1 Protects against Renal Disease in a Mouse Model of Cryoglobulinaemia. Nature 2015, 517, 501–504.
- Khodoun, M.V.; Kucuk, Z.Y.; Strait, R.T.; Krishnamurthy, D.; Janek, K.; Clay, C.D.; Morris, S.C.; Finkelman, F.D. Rapid Desensitization of Mice with Anti-FcγRIIb/FcγRIII mAb Safely Prevents IgG-Mediated Anaphylaxis. J. Allergy Clin. Immunol. 2013, 132, 1375–1387.
- Beutier, H.; Gillis, C.M.; Iannascoli, B.; Godon, O.; England, P.; Sibilano, R.; Reber, L.L.; Galli, S.J.; Cragg, M.S.; van Rooijen, N.; et al. IgG Subclasses Determine Pathways of Anaphylaxis in Mice. J. Allergy Clin. Immunol. 2017, 139, 269–280.
- Strait, R.T.; Morris, S.C.; Yang, M.; Qu, X.-W.; Finkelman, F.D. Pathways of Anaphylaxis in the Mouse. J. Allergy Clin. Immunol. 2002, 109, 658–668.
- Kanagaratham, C.; Ansari, Y.S.E.; Lewis, O.L.; Oettgen, H.C. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front. Immunol. 2020, 11, 3000.
- Santos, A.F.; James, L.K.; Bahnson, H.T.; Shamji, M.H.; Couto-Francisco, N.C.; Islam, S.; Houghton, S.; Clark, A.T.; Stephens, A.; Turcanu, V.; et al. IgG4 Inhibits Peanut-Induced Basophil and Mast Cell Activation in Peanut-Tolerant Children Sensitized to Peanut Major Allergens. J. Allergy Clin. Immunol. 2015, 135, 1249–1256.
- Akdis, C.A.; Akdis, M. Mechanisms of Allergen-Specific Immunotherapy and Immune Tolerance to Allergens. World Allergy Organ. J. 2015, 8, 17.
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J.J. The Role of Allergen-specific IgE, IgG and IgA in Allergic Disease. Allergy 2021, 76, 3627–3641.
- Ansari, Y.S.E.; Kanagaratham, C.; Burton, O.T.; Santos, J.V.; Hollister, B.-M.A.; Lewis, O.L.; Renz, H.; Oettgen, H.C. Allergen-Specific IgA Antibodies Block IgE-Mediated Activation of Mast Cells and Basophils. Front. Immunol. 2022, 13, 881655.
- Strait, R.T.; Morris, S.C.; Finkelman, F.D. IgG-Blocking Antibodies Inhibit IgE-Mediated Anaphylaxis in Vivo through Both Antigen Interception and FcγRIIb Cross-Linking. J. Clin. Investig. 2006, 116, 833–841.
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut Oral Immunotherapy Modifies IgE and IgG4 Responses to Major Peanut Allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134.
- Coker, H.A.; Durham, S.R.; Gould, H.J. Local Somatic Hypermutation and Class Switch Recombination in the Nasal Mucosa of Allergic Rhinitis Patients. J. Immunol. 2003, 171, 5602–5610.
- Li, T.; DiLillo, D.J.; Bournazos, S.; Giddens, J.P.; Ravetch, J.V.; Wang, L.-X. Modulating IgG Effector Function by Fc Glycan Engineering. Proc. Natl. Acad. Sci. USA 2017, 114, 3485–3490.
- Yeo, S.C.; Cheung, C.K.; Barratt, J. New Insights into the Pathogenesis of IgA Nephropathy. Pediatr. Nephrol. 2018, 33, 763–777.
- Epp, A.; Hobusch, J.; Bartsch, Y.C.; Petry, J.; Lilienthal, G.-M.; Koeleman, C.A.M.; Eschweiler, S.; Möbs, C.; Hall, A.; Morris, S.C.; et al. Sialylation of IgG Antibodies Inhibits IgG-Mediated Allergic Reactions. J. Allergy Clin. Immunol. 2018, 141, 399–402.
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319.
- Izadi, N.; Luu, M.; Ong, P.Y.; Tam, J.S. The Role of Skin Barrier in the Pathogenesis of Food Allergy. Children 2015, 2, 382–402.
- Jakwerth, C.A.; Ordovas-Montanes, J.; Blank, S.; Schmidt-Weber, C.B.; Zissler, U.M. Role of Respiratory Epithelial Cells in Allergic Diseases. Cells 2022, 11, 1387.
- van Splunter, M.; Liu, L.; van Neerven, R.J.J.; Wichers, H.J.; Hettinga, K.A.; Jong, N.W.D. Mechanisms Underlying the Skin-Gut Cross Talk in the Development of IgE-Mediated Food Allergy. Nutrients 2020, 12, 3830.
- Asero, R.; Antonicelli, L. Does Sensitization to Foods in Adults Occur Always in the Gut? Int. Arch. Allergy Immunol. 2010, 154, 6–14.
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of Food Allergy Development and Suppression of Established Food Allergy by Neutralization of Thymic Stromal Lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179.
- Divekar, R.; Kita, H. Recent Advances in Epithelium-Derived Cytokines (IL-33, IL-25 and TSLP) and Allergic Inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98.
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105.
- MacLennan, I.C.M.; Toellner, K.-M.; Cunningham, A.F.; Serre, K.; Sze, D.M.-Y.; Zúñiga, E.; Cook, M.C.; Vinuesa, C.G. Extrafollicular Antibody Responses. Immunol. Rev. 2003, 194, 8–18.
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150.
- Mackay, F.; Figgett, W.A.; Saulep, D.; Lepage, M.; Hibbs, M.L. B-cell Stage and Context-dependent Requirements for Survival Signals from BAFF and the B-cell Receptor. Immunol. Rev. 2010, 237, 205–225.
- Finney, J.; Yeh, C.-H.; Kelsoe, G.; Kuraoka, M. Germinal Center Responses to Complex Antigens. Immunol. Rev. 2018, 284, 42–50.
- Shlomchik, M.J.; Weisel, F. Germinal Center Selection and the Development of Memory B and Plasma Cells. Immunol. Rev. 2012, 247, 52–63.
- Elgueta, R.; Benson, M.J.; Vries, V.C.D.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172.
- Crotty, S. Follicular Helper CD4 T Cells (Tfh). Annu. Rev. Immunol. 2011, 29, 621–663.
- Wade-Vallance, A.K.; Allen, C.D.C. Intrinsic and Extrinsic Regulation of IgE B Cell Responses. Curr. Opin. Immunol. 2021, 72, 221–229.
- Newman, R.; Tolar, P. Chronic Calcium Signaling in IgE+ B Cells Limits Plasma Cell Differentiation and Survival. Immunity 2021, 54, 2756–2771.
- Schwarz, A.; Panetta, V.; Cappella, A.; Hofmaier, S.; Hatzler, L.; Rohrbach, A.; Tsilochristou, O.; Bauer, C.-P.; Hoffmann, U.; Forster, J.; et al. IgG and IgG4 to 91 Allergenic Molecules in Early Childhood by Route of Exposure and Current and Future IgE Sensitization: Results from the Multicentre Allergy Study Birth Cohort. J. Allergy Clin. Immunol. 2016, 138, 1426–1433.
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation Converts Arthritogenic IgG into Inhibitors of Collagen-Induced Arthritis. Nat. Commun. 2016, 7, 11205.
- Bartsch, Y.C.; Eschweiler, S.; Leliavski, A.; Lunding, H.B.; Wagt, S.; Petry, J.; Lilienthal, G.-M.; Rahmöller, J.; de Haan, N.; Hölscher, A.; et al. IgG Fc Sialylation Is Regulated during the Germinal Center Reaction Following Immunization with Different Adjuvants. J. Allergy Clin. Immunol. 2020, 146, 652–666.
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L.; et al. mRNA Vaccines against SARS-CoV-2 Induce Comparably Low Long-Term IgG Fc Galactosylation and Sialylation Levels but Increasing Long-Term IgG4 Responses Compared to an Adenovirus-Based Vaccine. Front. Immunol. 2023, 13, 1020844.
- Tong, X.; Guan, C.; Ji, T.; Cao, C.; Jiang, J.; Liu, M.; Guo, Q.; Zhou, P.; Gong, F. Increased Circulating T Follicular Helper 13 Subset Correlates with High IgE Levels in Pediatric Allergic Asthma. Eur. J. Immunol. 2022, 52, 2010–2012.
- Faber, M.A.; Van Gasse, A.L.; Decuyper, I.I.; Sabato, V.; Hagendorens, M.M.; Mertens, C.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Cross-Reactive Aeroallergens: Which Need to Cross Our Mind in Food Allergy Diagnosis? J. Allergy Clin. Immunol. Pract. 2018, 6, 1813–1823.
- Liu, T.; Lai, S.; Li, W.; Jiang, Y. Prevalence of Food Allergen and Aeroallergen Sensitization among Children in Sichuan Province. Medicine 2020, 99, e21055.
- Williams, J.W.; Tjota, M.Y.; Sperling, A.I. The Contribution of Allergen-Specific IgG to the Development of Th2-Mediated Airway Inflammation. J. Allergy 2012, 2012, 236075.
- Orengo, J.; Radin, A.; Kamat, V.; Badithe, A.; Ben, L.; Bennett, B.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating Cat Allergy with Monoclonal IgG Antibodies That Bind Allergen and Prevent IgE Engagement. Nat. Commun. 2018, 9, 1421.




