Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paul Scheel | -- | 2158 | 2023-12-13 08:42:51 | | | |
| 2 | Mona Zou | Meta information modification | 2158 | 2023-12-14 09:20:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maniar, Y.; Gilotra, N.A.; Scheel, P.J. Arrhythmogenic Cardiomyopathy across the Spectrum of Ventricular Involvement. Encyclopedia. Available online: https://encyclopedia.pub/entry/52658 (accessed on 08 February 2026).
Maniar Y, Gilotra NA, Scheel PJ. Arrhythmogenic Cardiomyopathy across the Spectrum of Ventricular Involvement. Encyclopedia. Available at: https://encyclopedia.pub/entry/52658. Accessed February 08, 2026.
Maniar, Yash, Nisha A. Gilotra, Paul J. Scheel. "Arrhythmogenic Cardiomyopathy across the Spectrum of Ventricular Involvement" Encyclopedia, https://encyclopedia.pub/entry/52658 (accessed February 08, 2026).
Maniar, Y., Gilotra, N.A., & Scheel, P.J. (2023, December 13). Arrhythmogenic Cardiomyopathy across the Spectrum of Ventricular Involvement. In Encyclopedia. https://encyclopedia.pub/entry/52658
Maniar, Yash, et al. "Arrhythmogenic Cardiomyopathy across the Spectrum of Ventricular Involvement." Encyclopedia. Web. 13 December, 2023.
Copy Citation
Improved disease recognition through family screening and increased life expectancy with appropriate sudden cardiac death prevention has increased the burden of heart failure in arrhythmogenic cardiomyopathy (ACM). Heart failure management guidelines are well established but primarily focus on left ventricle function. A significant proportion of patients with ACM have predominant or isolated right ventricle (RV) dysfunction. Management of RV dysfunction in ACM lacks evidence but requires special considerations across the spectrum of heart failure regarding the initial diagnosis, subsequent management, monitoring for progression, and end-stage disease management.
arrhythmogenic cardiomyopathy
heart failure
arrhythmogenic right ventricular cardiomyopathy
guideline-directed medical therapy
1. Heart Failure Diagnosis and Assessment
Heart failure is broadly defined as a clinical syndrome with characteristic signs or symptoms caused by a structural and/or functional cardiac abnormality, corroborated by laboratory, imaging, or hemodynamic abnormalities [1]. Heart failure in ACM often manifests as a predominant right-sided heart failure syndrome, though biventricular and LV syndromes are also seen. Left- and right-sided heart failure share many signs and symptoms: lower extremity swelling, unintentional weight gain, jugular venous distension with hepatojugular reflex, exertional dyspnea, and fatigue. Conversely, orthopnea and paroxysmal nocturnal dyspnea (PND) typically indicate LV failure along with exam findings of pulmonary rales and a displaced point of maximal impulse (PMI) as shown in Figure 1. RV failure may have a palpable RV heave, hepatomegaly due to congestion, or a tricuspid regurgitation murmur along with a pulsatile liver. RV failure may have more predominant abdominal distension, anorexia or early satiety from gut edema, or scrotal swelling. In one study of heart failure specifically in ARVC where nearly 50% of patients had clinical heart failure, dyspnea on exertion, fatigue, and abdominal swelling were the highest frequency symptoms [2].
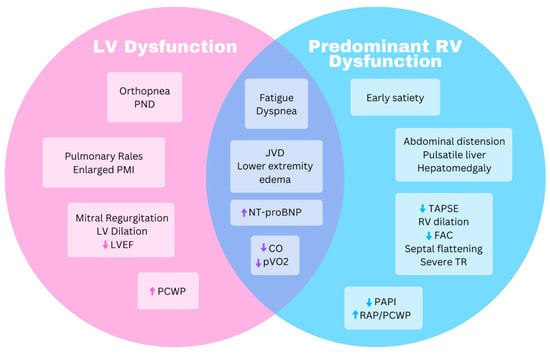
Figure 1. Venn diagram highlighting similarities and differences for symptoms and objective data between predominant right ventricle and left ventricle dysfunction. CO, cardiac output; FAC, fractional area change; JVD, jugular venous distension; LV, left ventricle; LVEF, left ventricle ejection fraction; NT-proBNP, N-terminal prohormone brain natriuretic peptide; PAPI, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PND, paroxysmal nocturnal dyspnea; PMI, point of maximal impulse; pVO2, peak oxygen uptake; RAP, right atrial pressure; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Not all ACM patients will develop heart failure but gene carriers and affected patients should be regularly assessed for the more subtle signs of RV dysfunction as outlined in Figure 2. Symptom identification is further complicated by recommendations for exercise restriction to prevent or slow progression in a population of patients that were disproportionately very physically active prior to the diagnosis [3]. Substantial exercise restriction limits symptom opportunity and residual conditioning, especially in younger patients, and may compensate for progressive cardiac dysfunction. Therefore, patients may remain asymptomatic until a significant portion of their cardiac reserve is lost and thereby present at a later stage of disease. Serial assessments of cardiac structure and function, as discussed later, aid in identification of progression. Progressive morphologic changes or any symptoms or subtle signs of heart failure warrant referral to a heart failure specialist for evaluation.
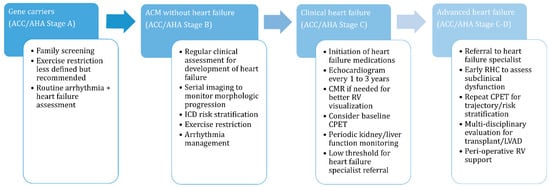
Figure 2. Heart failure management considerations across the arrhythmogenic cardiomyopathy disease spectrum. ACM, arrhythmogenic cardiomyopathy; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise test; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; RHC, right heart catheterization; RV, right ventricle.
Laboratory tests supplement a heart failure diagnosis in ACM and novel markers may assist risk stratification. N-terminal pro-brain natriuretic peptide (NT-proBNP) correlates with RV dysfunction and RV dilation on cardiac magnetic resonance imaging (CMR) as well as with clinical outcomes including heart transplant and death [4][5]. Increased testosterone in males and decreased estradiol in females were associated with adverse cardiovascular outcomes in ACM in one study, offering a possible explanation for observed sex differences [6]. Serum inflammatory markers such as complement system components have been associated with structural changes and heart failure severity in ACM [7][8]. Many other novel serologic biomarkers including soluble ST2 protein, galectin-3, and extracellular matrix metalloproteinases 2 and 9 are under investigation, but to date have limited clinical availability and according to a 2023 study, provide less prognostic value than NT-proBNP [4].
Echocardiography is often the first-line imaging test in heart failure, as it can provide a rapid, noninvasive appraisal of both right and left ventricular structure and function. RV regional akinesis or dyskinesis, an aneurysm of the RV free wall, and RV outflow tract dilation are all structural changes included in diagnostic criteria for ARVC [9]. Right atrial size and degree of tricuspid regurgitation along with RV functional measurements including fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), peak systolic excursion velocity (S’), RV early diastolic myocardial velocity (e’), and an RV strain analysis can be assessed and are often abnormal in RV-predominant ACM (Figure 1) [10]. The degree of RV dysfunction on an echocardiogram correlates with the likelihood of clinical heart failure in ACM patients [2], and FAC and TAPSE in particular have been shown to predict adverse outcomes [11][12]. Additionally, septal flattening can suggest RV pressure and/or volume overload, and the inferior vena cava (IVC) diameter and respiratory variation assess volume status.
LV dilation and dysfunction are also easily assessed on echocardiography and may be the primary abnormality in some patients with ACM. LV dysfunction is well recognized as a poor prognostic factor in ARVC [13][14] and an LV ejection fraction (LVEF) < 50% in conjunction with RV dysfunction correlates with an increased risk of death or heart transplant [11]. Echocardiography also provides an easily accessible method for serial assessment to monitor progression of both LV and RV morphologic changes, which may precede worsening heart failure symptoms [15][16]. Repeat echocardiography is recommended every 1 to 3 years to monitor these changes (Figure 2) [17]. The main limitation of echocardiography in general is variable image quality but in ACM, this is further compounded by the geometric layout of the RV often hampering an echocardiographic analysis.
2. Heart Failure Management
2.1. Medical Management
Medical management of heart failure aims to stop or slow progression and manage fluid balance to alleviate symptoms as outlined in Figure 3. ACM patients with LV dysfunction should be treated with guideline-directed medical therapy (GDMT) per consensus recommendations [18][19]. These include a β-blocker (βB), mineralocorticoid receptor antagonist (MRA), sodium/glucose cotransporter-2 inhibitor (SGTL2i), and angiotensin receptor/neprilysin inhibitor (ARNI). Second-line for patients who cannot tolerate ARNI therapy is an angiotensin converting enzyme inhibitor (ACEi) or angiotensin II receptor block (ARB) therapy. Similar evidence to guide management of patients with isolated or predominant RV dysfunction is lacking and mostly extrapolated from studies of LV dysfunction and smaller studies [20].
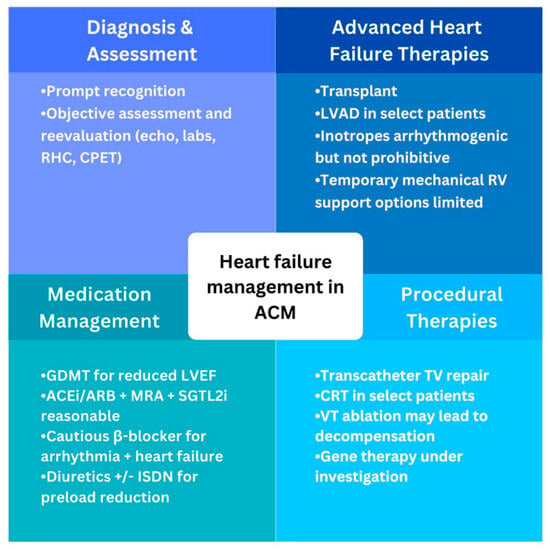
Figure 3. Global overview of heart failure management in arrhythmogenic cardiomyopathy. ACEi, angiotensin converting enzyme inhibitor; ACM, arrhythmogenic cardiomyopathy; ARB, angiotensin receptor blocker; CPET, cardiopulmonary exercise test; CRT, cardiac rexynchronization therapy; GDMT, guideline-directed medical therapy; ISDN, isosorbide dinitrate; LVAD, left ventricular assist device; LVEF, left ventricle ejection fraction; MRA, mineralocorticoid receptor antagonist; RV, right ventricle; SGLT2i, sodium/glucose cotransporter-2 inhibitor; TV, tricuspid valve; VT, ventricular tachycardia.
ACEi or ARB therapy was associated with slower reductions in TAPSE, RV dilation, and ventricular arrhythmias in one retrospective study [21]. Preemptive initiation of ACEi in mouse models of ARVC type 5 (an aggressive autosomal dominant form) led to reductions in myocardial fibrosis and mortality along with less severe reduction in LV dysfunction [22]. Initiation of ACEi or ARB should be considered in patients with symptomatic RV dysfunction, recognizing the subtle symptoms of heart failure in ACM and the benefit of early initiation, which may aid myocardial remodeling. ARNI therapy is an alternative but may be limited by hypotension and currently no clear evidence exists to recommend this preferentially over ACEi/ARB unless LV dysfunction is present. MRA similarly should be considered in symptomatic RV dysfunction due to beneficial remodeling effects seen in LV dysfunction.
SGTL2i therapy is the newest addition to heart failure GDMT and its benefits can likely be extrapolated to RV dysfunction. An added benefit of SGLT2i is the diuretic effect which can help reduce RV preload. In a plakophilin-deficient ACM mouse model, RV preload reduction with diuretics and isosorbide dinitrate prevented RV dilation [23], and use of isosorbide dinitrate (ISDN) can be considered in patients with symptomatic RV dysfunction [20]. Diuretic therapy, typically with furosemide or torsemide, is the primary method of preload reduction and should be used for ACM patients with evidence of volume overload. In general, there is no difference in outcomes between furosemide or torsemide [24]. However, torsemide has increased bioavailability and absorption is less affected by intestinal edema, which is a predominant manifestation of RV failure. Therefore, torsemide can be considered as an alternative to furosemide for initial therapy and should be utilized in the setting of ineffective preload management with furosemide. Adequate preload reduction needs to be balanced against the preload dependence of a failing RV. Initiation and continuation of these medications should be accompanied by reassessment and monitoring of symptoms along with periodic evaluation of kidney and liver function.
2.2. Procedural Therapies
Invasive procedures for management of heart failure are potentially available when medical therapy is inadequate (Figure 3). Chronic resynchronization therapy (CRT) benefits symptomatic patients with heart failure, reduced LVEF, and a widened QRS complex [18][19] and these apply to ACM patients meeting the same criteria. The benefit of CRT or other pacemaker-related therapy in isolated RV dysfunction has not been demonstrated. Utility and selection of patients for ventricular tachycardia (VT) ablation are discussed elsewhere [20][25], and data are lacking for heart-failure-related outcomes. However, the risk of hemodynamic decompensation during VT ablation due to repeated arrhythmias, fluid loading, and extended anesthesia needs to be balanced against the benefit and probability of success.
Progressive RV dilation can eventually lead to significant secondary tricuspid regurgitation (TR), which further exacerbates RV dysfunction. Transcatheter tricuspid valve repair for patients with severe symptomatic TR was recently shown to be safe and improve quality of life but did not affect mortality [26]. It is unclear how effective this therapy would be in predominant-RV-affected ACM but may be an option for patients with significant symptoms and appropriate anatomy without other options for heart failure relief. Remote pulmonary artery pressure monitoring using an implantable sensor prevents heart failure hospitalizations [27] and could be an attractive option for assessment and trajectory monitoring of subtle heart failure symptoms in RV-predominant ACM. However, it is unclear how effective they would be as many of these patients have normal pulmonary artery pressures, a rise in which is the primary parameter being measured with these devices.
2.3. Advanced Heart Failure Surgical Therapies
Patients with ACM may progress to develop or even present with advanced or end-stage heart failure. Evidence of advanced heart failure is similar across the LV and RV functional spectrum and includes the need for inotrope therapy, persistent symptoms and/or elevated biomarkers, worsening renal or liver function, repeat hospitalizations for heart failure, an increasing diuretic requirement, and low blood pressure [28]. While typically considered a sign of advanced heart failure, a recurrent ventricular arrhythmia is less applicable in ACM where arrhythmias are inherent to the condition and often occur independent of heart failure [29][30]. Similarly, inability to up-titrate GDMT or having to decrease GDMT is another sign of advanced heart failure that may be less applicable in ACM with significant RV dysfunction and must be considered in the context of RV physiology.
Cardiac transplantation is the definitive therapy for ACM, both for arrhythmia and heart failure clinical phenotypes. Transplants for ACM have increased over the last decade due to improved pre-transplant diagnoses and survival from SCD prevention [31]. A refractory ventricular arrhythmia is an indication for a transplant, but most ACM patients are transplanted because of end-stage heart failure [30][32]. Evaluation for transplant involves extensive multidisciplinary evaluation and diagnostic testing to ensure benefits outweigh risks of transplantation and the potential clinical course thereafter. Significant renal dysfunction, whether due to long-standing heart failure or other causes, may require evaluation for dual heart–kidney transplant. More unique to RV-predominant ACM is the potential for advanced liver disease due to long-standing congestion with case reports of dual heart–liver transplants in ACM [33]
2.4. Emerging Therapies
Current management of ACM only targets disease manifestations (arrhythmia and heart failure) but pre-clinical work is ongoing to target disease mechanisms for treatment [34]. Investigators developed cell culture and small animal (mouse, zebrafish) models of ACM and targeted either cellular/inflammatory signaling pathways or gene expression [34]. Inhibition of nuclear factor of kappa-B (NFκB) and glycogen synthase kinase-3β (GSK3β) in two different animal models (DSG2, JUP) improved/preserved cardiac function and decreased myocardial fibrosis, respectively [35][36]. Similarly, small-molecule targeting of peroxisome proliferator activated receptor alpha/gamma (PPARα/γ) reduced cardiac fibrosis in mice and maintained regular electrical activity and calcium handling in human-induced pluripotent stem cells [37][38]. Off-target effects limit direct application clinically but improved understanding facilitates further discovery of potential therapeutics. Alternatively, advances in gene editing technology may allow for direct targeting of gene variants. Current techniques rely on introduction of genetic material into cells via viral vectors to incorporate into genomes and more recently genome editing with discoveries like CRISPR-Cas9. Correction of DSG2-deficient cardiomyocytes from stem cells via virally introduced normal DSG2 has been demonstrated [39]. Implementation of genome-editing technology is actively being investigated in many genetic cardiomyopathies including ACM [40].
References
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure. J. Card. Fail. 2021, 27, 387–413.
- Gilotra, N.A.; Bhonsale, A.; James, C.A.; Te Riele, A.S.J.; Murray, B.; Tichnell, C.; Sawant, A.; Ong, C.S.; Judge, D.P.; Russell, S.D.; et al. Heart failure is common and under-recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ. Heart Fail. 2017, 10, e003819.
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy–associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297.
- Borowiec, K.; Woźniak, O.; Wróbel, A.; Śmigielski, W.; Skrzypczyńska-Banasik, U.; Kowalik, E.; Lutyńska, A.; Hoffman, P.; Biernacka, E.K. NT-proBNP is superior to novel plasma biomarkers for predicting adverse outcome in arrhythmogenic right ventricular cardiomyopathy. Pol. Arch. Intern. Med. 2023, 133, 16443.
- Cheng, H.; Lu, M.; Hou, C.; Chen, X.; Wang, J.; Yin, G.; Chu, J.; Zhang, S.; Prasad, S.K.; Pu, J.; et al. Relation between n-terminal pro-brain natriuretic peptide and cardiac remodeling and function assessed by cardiovascular magnetic resonance imaging in patients with arrhythmogenic right ventricular cardiomyopathy. Am. J. Cardiol. 2015, 115, 341–347.
- Akdis, D.; Saguner, A.M.; Shah, K.; Wei, C.; Medeiros-Domingo, A.; von Eckardstein, A.; Lüscher, T.F.; Brunckhorst, C.; Chen, H.V.; Duru, F. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: From a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur. Heart J. 2017, 38, 1498–1508.
- Chen, L.; Yang, F.; Chen, X.; Rao, M.; Zhang, N.-N.; Chen, K.; Deng, H.; Song, J.-P.; Hu, S.-S. Comprehensive myocardial proteogenomics profiling reveals C/EBPα as the key factor in the lipid storage of ARVC. J. Proteome Res. 2017, 16, 2863–2876.
- Ren, J.; Chen, L.; Chen, X.; Zhang, N.; Sun, X.; Song, J. Acylation-stimulating protein and heart failure progression in arrhythmogenic right ventricular cardiomyopathy. ESC Heart Fail. 2022, 10, 492–501.
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2010, 121, 1533–1541.
- Qasem, M.; Utomi, V.; George, K.; Somauroo, J.; Zaidi, A.; Forsythe, L.; Bhattacharrya, S.; Lloyd, G.; Rana, B.; Ring, L.; et al. A meta-analysis for the echocardiographic assessment of right ventricular structure and function in ARVC: A study by the research and audit committee of the british society of echocardiography. Echo Res. Pract. 2016, 3, 95–104.
- Pinamonti, B.; Dragos, A.M.; Pyxaras, S.A.; Merlo, M.; Pivetta, A.; Barbati, G.; Di Lenarda, A.; Morgera, T.; Mestroni, L.; Sinagra, G. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: Results from a 10-year registry. Eur. Heart J. 2011, 32, 1105–1113.
- Saguner, A.M.; Vecchiati, A.; Baldinger, S.H.; Rüeger, S.; Medeiros-Domingo, A.; Mueller-Burri, A.S.; Haegeli, L.M.; Biaggi, P.; Manka, R.; Lüscher, T.F.; et al. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ. Cardiovasc. Imaging 2014, 7, 230–239.
- Groeneweg, J.A.; van der Zwaag, P.A.; Jongbloed, J.D.; Cox, M.G.; Vreeker, A.; de Boer, R.A.; van der Heijden, J.F.; van Veen, T.A.; McKenna, W.J.; van Tintelen, J.P.; et al. Left-dominant arrhythmogenic cardiomyopathy in a large family: Associated desmosomal or nondesmosomal genotype? Heart Rhythm. 2013, 10, 548–559.
- Lemola, K.; Brunckhorst, C.; Helfenstein, U.; Oechslin, E.; Jenni, R.; Duru, F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: Long term experience of a tertiary care centre. Heart 2005, 91, 1167–1172.
- Malik, N.; Mukherjee, M.; Wu, K.C.; Zimmerman, S.L.; Zhan, J.; Calkins, H.; James, C.A.; Gilotra, N.A.; Sheikh, F.H.; Tandri, H.; et al. Multimodality imaging in arrhythmogenic right ventricular cardiomyopathy. Circ. Cardiovasc. Imaging 2022, 15, 106–121.
- Mast, T.P.; James, C.A.; Calkins, H.; Teske, A.J.; Tichnell, C.; Murray, B.; Loh, P.; Russell, S.D.; Velthuis, B.K.; Judge, D.P.; et al. Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiol. 2017, 2, 293–302.
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. International Experts. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2020, 41, 1414–1429.
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639.
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019, 16, e301–e372.
- Tu, B.; Wu, L.; Zheng, L.; Liu, S.; Sheng, L.; Liu, L.; Zhu, Z.; Yao, Y. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers: Anti-arrhythmic drug for arrhythmogenic right ventricular cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 769138.
- Domínguez, F.; Lalaguna, L.; López-Olañeta, M.; Villalba-Orero, M.; Padrón-Barthe, L.; Román, M.; Bello-Arroyo, E.; Briceño, A.; Gonzalez-Lopez, E.; Segovia-Cubero, J.; et al. Early preventive treatment with enalapril improves cardiac function and delays mortality in mice with arrhythmogenic right ventricular cardiomyopathy type 5. Circ. Heart Fail. 2021, 14, e007616.
- Fabritz, L.; Hoogendijk, M.G.; Scicluna, B.P.; van Amersfoorth, S.C.; Fortmueller, L.; Wolf, S.; Laakmann, S.; Kreienkamp, N.; Piccini, I.; Breithardt, G.; et al. Load-reducing therapy prevents development of arrhythmogenic right ventricular cardiomyopathy in plakoglobin-deficient mice. J. Am. Coll. Cardiol. 2011, 57, 740–750.
- Mentz, R.J.; Anstrom, K.J.; Eisenstein, E.L.; Sapp, S.; Greene, S.J.; Morgan, S.; Testani, J.M.; Harrington, A.H.; Sachdev, V.; Ketema, F.; et al. Effect of torsemide vs. furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: The TRANSFORM-HF randomized clinical trial. JAMA 2023, 329, 214–223.
- Gaine, S.P.; Calkins, H. Antiarrhythmic drug therapy in arrhythmogenic right ventricular cardiomyopathy. Biomedicines 2023, 11, 1213.
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter repair for patients with tricuspid regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842.
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461.
- Baumwol, J. “I Need Help”—A mnemonic to aid timely referral in advanced heart failure. J. Heart Lung Transplant. 2017, 36, 593–594.
- Dalal, D.; Nasir, K.; Bomma, C.; Prakasa, K.; Tandri, H.; Piccini, J.; Roguin, A.; Tichnell, C.; James, C.; Russell, S.D.; et al. Arrhythmogenic right ventricular dysplasia: A united states experience. Circulation 2005, 112, 3823–3832.
- Scheel, P.J.; Giuliano, K.; Tichnell, C.; James, C.; Murray, B.; Tandri, H.; Carter, D.; Fehr, T.; Umapathi, P.; Vaishnav, J.; et al. Heart transplantation strategies in arrhythmogenic right ventricular cardiomyopathy: A tertiary ARVC centre experience. ESC Heart Fail. 2021, 9, 1008–1017.
- Giuliano, K.; Scheel, P.; Etchill, E.; Fraser, C.D.; Suarez-Pierre, A.; Hsu, S.; Wittstein, I.S.; Kasper, E.K.; Florido, R.; Tandri, H.; et al. Heart transplantation outcomes in arrhythmogenic right ventricular cardiomyopathy: A contemporary national analysis. ESC Heart Fail. 2022, 9, 988–997.
- Tedford, R.J.; James, C.; Judge, D.P.; Tichnell, C.; Murray, B.; Bhonsale, A.; Philips, B.; Abraham, T.; Dalal, D.; Halushka, M.K.; et al. Cardiac transplantation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2012, 59, 289–290.
- Lewsey, S.C.; Sharma, K.; Kilic, A.; Halushka, M.K.; Vaishnav, J.; Wittstein, I.S.; Tandri, H.; Calkins, H.; James, C.A.; Gilotra, N.A. Dual-organ transplantation in a Woman with right ventricular failure secondary to arrhythmogenic Right Ventricular cardiomyopathy. JACC Case Rep. 2020, 2, 59–63.
- Engel, M.; Shiel, E.; Chelko, S.P. Basic and translational mechanisms in inflammatory arrhythmogenic cardiomyopathy. Int. J. Cardiol. 2023, 131602.
- Chelko, S.P.; Asimaki, A.; Andersen, P.; Bedja, D.; Amat-Alarcon, N.; DeMazumder, D.; Jasti, R.; MacRae, C.A.; Leber, R.; Kleber, A.G.; et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. J. Clin. Investig. 2016, 1, e85923.
- Chelko, S.; Asimaki, A.; Lowenthal, J.; Bueno-Beti, C.; Bedja, D.; Scalco, A.; Amat-Alarcon, N.; Andersen, P.; Judge, D.P.; Tung, L.; et al. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 2019, 140, 1491–1505.
- Reisqs, J.; Moreau, A.; Charrabi, A.; Sleiman, Y.; Meli, A.C.; Millat, G.; Briand, V.; Beauverger, P.; Richard, S.; Chevalier, P. The PPARγ pathway determines electrophysiological remodelling and arrhythmia risks in DSC2 arrhythmogenic cardiomyopathy. Clin. Transl. Med. 2022, 12, e748.
- Qiu, Z.; Zhao, Y.; Tao, T.; Guo, W.; Liu, R.; Huang, J.; Xu, G. Activation of PPARα Ameliorates Cardiac Fibrosis in Dsg2-Deficient arrhythmogenic cardiomyopathy. Cells 2022, 11, 3184.
- Shiba, M.; Higo, S.; Kondo, T.; Li, J.; Liu, L.; Ikeda, Y.; Kohama, Y.; Kameda, S.; Tabata, T.; Inoue, H.; et al. Phenotypic recapitulation and correction of desmoglein-2-deficient cardiomyopathy using human-induced pluripotent stem cell-derived cardiomyocytes. Hum. Mol. Genet. 2021, 30, 1384–1397.
- Romeo, F.J.; Mavropoulos, S.A.; Ishikawa, K. Progress in clinical gene therapy for cardiac disorders. Mol. Diagn. Ther. 2023, 27, 179–191.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
635
Revisions:
2 times
(View History)
Update Date:
14 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




