Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eisuke Kato | -- | 1693 | 2023-12-13 08:13:56 | | | |
| 2 | Fanny Huang | Meta information modification | 1693 | 2023-12-14 04:13:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kato, E.; Oshima, S. Taste 2 Receptors in Intestine. Encyclopedia. Available online: https://encyclopedia.pub/entry/52653 (accessed on 08 February 2026).
Kato E, Oshima S. Taste 2 Receptors in Intestine. Encyclopedia. Available at: https://encyclopedia.pub/entry/52653. Accessed February 08, 2026.
Kato, Eisuke, Shota Oshima. "Taste 2 Receptors in Intestine" Encyclopedia, https://encyclopedia.pub/entry/52653 (accessed February 08, 2026).
Kato, E., & Oshima, S. (2023, December 13). Taste 2 Receptors in Intestine. In Encyclopedia. https://encyclopedia.pub/entry/52653
Kato, Eisuke and Shota Oshima. "Taste 2 Receptors in Intestine." Encyclopedia. Web. 13 December, 2023.
Copy Citation
Taste 2 receptors (T2Rs) are G-protein-coupled receptors responsible for sensing bitter tastes. Many studies have shown the expression of T2Rs in extraoral tissues and the unique role of T2Rs in each tissue. Single-nucleotide polymorphisms of T2Rs are associated with the risk of obesity and diabetes, and the organs/tissues associated with the development of these metabolic diseases, including the intestine, adipose, muscle, liver, and pancreas, are reported to express T2R genes. This result suggests that T2Rs in extraoral tissues contribute to the development of obesity and diabetes.
taste 2 receptor
bitter taste receptor
obesity
diabetes
intestine
1. Introduction
Vertebrates sense bitterness through bitter taste receptors named taste 2 receptors (T2Rs). T2Rs are characterized by their genetic diversity compared with other taste receptors. For example, humans and rodents have approximately 25 and 36 functional T2R genes, respectively [1][2]. Because bitterness is an unpleasant taste and bitter compounds are generally considered to have undesirable effects on health, this diversity is thought to allow for the perception of a variety of bitter compounds and avoidance of their intake.
T2Rs are expressed in taste receptor cells located in taste buds on the tongue and soft palate. However, T2R expression is not limited to the oral cavity; many studies have shown that T2R genes are expressed in a variety of organs/tissues/cells, including the respiratory tract, vascular system, brain, stomach, testis, muscle, and fat cells [3]. These extraoral T2Rs that are not involved in bitter taste perception have roles that vary from tissue to tissue.
In many tissues, extraoral T2Rs are involved in protecting the body from harmful agents. This has been well studied for tissues that come into contact with viruses, microorganisms, and parasites. In the upper respiratory tract, T2Rs are expressed on solitary chemosensory and ciliated cells, where they sense pathogens and induce a defense response [4]. Meanwhile, some studies have suggested that the roles of T2Rs are not limited to biological defense; one such suggested role is the regulation of lipid/glucose metabolism, as reports have shown correlations of T2Rs with obesity and diabetes [5][6][7][8][9].
2. Relationship of T2R, Obesity, and Diabetes
Several studies have suggested that T2Rs are involved in the regulation of body fat mass in humans. TAS2R38 is a bitter taste receptor that has commonly been the focus of studies of genetic variation that distinguishes those able and unable to taste 6-propyl-2-thiouracil (PROP). The genotyping of women with anorexia nervosa, healthy controls, and morbidly obese patients, or of three ethnically diverse groups (European Americans, African Americans, and Asians) has suggested an association between single-nucleotide polymorphism in TAS2R38 and the development of obesity [5][6]. Single-nucleotide polymorphisms at three positions in TAS2R38 alter amino acids and produce two haplotypes: PAV (proline-alanine-valine) and AVI (alanine-valine-isoleucine). Of these two haplotypes, obesity was found to be more common in those with AVI (those unable to taste PROP) than in those with PAV (those able to taste PROP). In another study, minor alleles of polymorphisms in TAS2R4 and TAS2R5, the receptors that detect the dietary polyphenol epicatechin [10], were found to be associated with lower BMI [7].
The manipulation of signal transduction pathways typically linked to T2R activation influences the progression of obesity. Knockout of α-gustducin in C57BL/6 mice was found to increase thermogenesis and protect against high-fat-diet-induced obesity despite increased energy intake [11]. Loss of α-gustducin diminishes the signal from T2Rs (see Figure 2 for the signaling pathway). It is thus indicated that T2R signaling leads to increased body fat mass upon consumption of a high-fat diet. The increased heat production was explained by the increased expression of UCP-1, which was confirmed in white adipose tissue (WAT) at the mRNA expression level and brown adipose tissue (BAT) at the protein level, suggesting a role of T2Rs in these tissues [11]. However, α-gustducin participates in the signaling of taste 1 receptors (T1Rs), which sense sweet and umami tastes, and loss of α-gustducin also reduces signaling from the T1Rs. Adipose tissue has also been shown to express T1Rs [12], and the differences observed in α-gustducin-knockout mice should be considered to include effects owing to the loss of signaling through T1Rs.
The association between single-nucleotide polymorphisms in the human T2R genes and the risk of type 2 diabetes has been reported by Dotson et al. [8]. Through the genotyping of an Amish family and the oral glucose tolerance test, they found that two single-nucleotide polymorphisms in TAS2R7, one in a non-coding region and the other in a coding region, and one single-nucleotide polymorphism in TAS2R9 in a coding region, are associated with the regulation of glucose and insulin levels. They also showed that the single-nucleotide polymorphism in TAS2R9 alters the amino acid sequence (Ala187 to Val187), leading to a diminished response upon stimulation by ofloxacin, procainamide, and pirenzepine in cellular models. These results led to the conclusion that polymorphism in T2Rs alters the response to their ligands, and this altered response may have an influence on glucose and insulin homeostasis.
3. T2Rs in Intestine Associated with the Development of Obesity and Diabetes
3.1. Expression of T2R Genes in Intestine
The intestine absorbs nutrients as well as secretion of incretin hormones to participate in the development of diabetes and obesity. Several groups have reported the expression of T2Rs in gut tissue. For example, whole-mouse tissue analysis by Prandi et al. showed consistent expression of Tas2r108, 126, 135, 137, 138, and 143 throughout the gut (stomach, small, and large intestine) together with several T2R genes expressed in specific organs [13]. Researchers' group also found the expression of Tas2r108, 126, 135, 137, 138, and 143 in the small intestine of C57BL/6J mice [14]. More precisely, tuft cells [15], Paneth cells [13], goblet cells [13], and enteroendocrine cells [16][17] in the gut tissue of mice were found to express T2R genes.
Vagezzi et al. showed that the expression of Tas2r138 in the gut is regulated by diet or diet-associated changes in intraluminal conditions [18]. Specifically, they obtained the following findings: (1) fasting decreases and re-feeding restores the expression of Tas2r138 in the stomach; (2) feeding on a diet supplemented with lovastatin and ezetimibe to deplete cholesterol absorption for 1 week upregulated the expression of Tas2r138 in the duodenum, jejunum, and proximal colon; and (3) feeding on a high-fat diet for 8 weeks, but not 2 weeks, upregulated the expression of Tas2r138 in the colon. The exact mechanism by which the expression of Tas2r138 is regulated was not confirmed, but Vagezzi et al. suggested the following: (1) fasting-induced decrease in Tas2r138 in the stomach might be regulated by the taste-related molecules contained in the gastrin and ghrelin cells; (2) cholesterol depletion enhances cholesterol-sensitive SREBP-2 expression and enhances the expression of Tas2r138 [19]; and (3) the unaltered expression of Tas2r138 by short-term feeding on a high-fat diet suggests that a high-fat diet does not directly influence such expression, and long-term feeding on a high-fat diet to alter the intraluminal conditions, especially the gut microbiota, might enhance such expression to serve as a defensive mechanism against pathogenic bacteria. Although the assumed mechanisms regulating the expression of Tas2r138 differ in each condition, these results suggest an association of the diet or diet-associated changes with the expression of Tas2r138.
In humans, as shown in the Genotype-Tissue Expression (GTEx) database, the expression of TAS2R4, 5, 14, and 20 is relatively high in the small intestine and colon, whereas the expression of TAS2R3, 10, 13, 19, 30, 31, 38, 43, 46, 50, and 60 is moderate to low (Figure 1). Moreover, Pham et al. showed that human enteroendocrine L-cells isolated from ileum tissues express TAS2R38 protein [20].
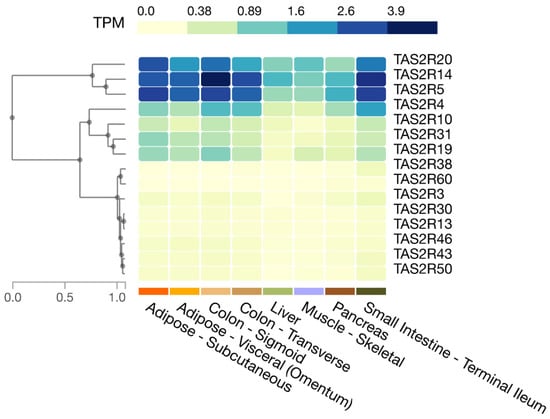
Figure 1. Expression of T2R genes in selected human tissues. Figure created in the GTEx portal (https://gtexportal.org/home/, accessed on 21 July 2023).
3.2. Role of T2Rs in Intestine
T2Rs in intestinal tuft cells activate immune responses in response to parasites and their secretory products in mice [15]. The role of T2Rs in Paneth cells and goblet cells is unclear, but it has been suggested that they are involved in protection against pathogens through the regulation of antimicrobial responses and the production of mucins [13]. Therefore, T2Rs in these types of cells are unlikely to be involved in the development of obesity or diabetes.
Some T2Rs are expressed in enteroendocrine cells, and therefore, they are likely to be involved in obesity and diabetes. T2Rs in enteroendocrine cells regulate the secretion of the hormone incretin, which in turn promotes the secretion of insulin from the pancreas. The human enteroendocrine cells NCI-H716 secrete glucagon-like peptide-1 (GLP-1) in response to stimulation with denatonium benzoate and quinine, but knockdown of TAS2R3, 44, and 46 results in decreased secretion [16]. That study also showed that the mouse proximal duodenum secretes GLP-1 in response to denatonium benzoate. Cells of the human duodenum adenocarcinoma HuTu-80 have been shown to secrete GLP-1 in response to PROP and Z7 (the ligand of TAS2R38), but such secretion is reduced when TAS2R38 is knocked down [20]. In that study, Pham et al. also showed that the oral administration of TAS2R38 ligand increased serum GLP-1 levels in BALB/c mice. In other studies, Yu et al. and Yue et al. showed that berberine upregulates the secretion of GLP-1 from NCI-H716 and STC-1 enteroendocrine cells [21][22]. Upregulation of GLP-1 secretion was inhibited by the knockdown of TAS2R38 in both studies, indicating the contribution of T2Rs to this response.
In a mouse study, Kok et al. examined the effect of KDT501, a derivative of the bitter compound isohumulone contained in hops, in mice with diet-induced obesity [23]. The oral administration of a single dose of KDT501 to fasted mice increased plasma GLP-1 levels, which also improved the glucose clearance in the oral glucose tolerance test. The intragastric administration of KDT501 for 28 days improved glucose homeostasis parameters and led to reduced body weight due to a reduction in fat mass. Meanwhile, a human study by Rose et al. showed that the intraduodenal administration of quinine increased plasma GLP-1 levels, while the consumption of a mixed-nutrient drink led to a reduction in the elevation of plasma glucose level [24].
These reports show that T2R activation has the potential to stimulate GLP-1 secretion from intestinal enteroendocrine cells and contribute to glycemic control.
References
- Behrens, M.; Foerster, S.; Staehler, F.; Raguse, J.-D.; Meyerhof, W. Gustatory Expression Pattern of the Human TAS2R Bitter Receptor Gene Family Reveals a Heterogenous Population of Bitter Responsive Taste Receptor Cells. J. Neurosci. 2007, 27, 12630–12640.
- Wu, S.V.; Chen, M.C.; Rozengurt, E. Genomic Organization, Expression, and Function of Bitter Taste Receptors (T2R) in Mouse and Rat. Physiol. Genom. 2005, 22, 139–149.
- Freund, J.R.; Lee, R.J. Taste Receptors in the Upper Airway. World J. Otorhinolaryngol.-Head Neck Surg. 2018, 4, 67–76.
- Carey, R.M.; Lee, R.J. Taste Receptors in Upper Airway Innate Immunity. Nutrients 2019, 11, 2017.
- Ortega, F.J.; Agüera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jimenez-Murcia, S.; Fernández-García, J.C.; et al. Genetic Variations of the Bitter Taste Receptor TAS2R38 Are Associated with Obesity and Impact on Single Immune Traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683.
- Chupeerach, C.; Tapanee, P.; On-Nom, N.; Temviriyanukul, P.; Chantong, B.; Reeder, N.; Adegoye, G.A.; Tolar-Peterson, T. The Influence of TAS2R38 Bitter Taste Gene Polymorphisms on Obesity Risk in Three Racially Diverse Groups. BioMedicine 2021, 11, 43–49.
- Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.J.; Lucock, M.; Beckett, E.L. Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort. Nutrients 2021, 13, 571.
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.-K.; Vigues, S.; Ott, S.H.; Elson, A.E.T.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter Taste Receptors Influence Glucose Homeostasis. PLoS ONE 2008, 3, e3974.
- Chou, W.-L. Therapeutic Potential of Targeting Intestinal Bitter Taste Receptors in Diabetes Associated with Dyslipidemia. Pharmacol. Res. 2021, 170, 105693.
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533.
- Avau, B.; Bauters, D.; Steensels, S.; Vancleef, L.; Laermans, J.; Lesuisse, J.; Buyse, J.; Lijnen, H.R.; Tack, J.; Depoortere, I. The Gustatory Signaling Pathway and Bitter Taste Receptors Affect the Development of Obesity and Adipocyte Metabolism in Mice. PLoS ONE 2015, 10, e0145538.
- Simon, B.R.; Parlee, S.D.; Learman, B.S.; Mori, H.; Scheller, E.L.; Cawthorn, W.P.; Ning, X.; Gallagher, K.; Tyrberg, B.; Assadi-Porter, F.M.; et al. Artificial Sweeteners Stimulate Adipogenesis and Suppress Lipolysis Independently of Sweet Taste Receptors. J. Biol. Chem. 2013, 288, 32475–32489.
- Prandi, S.; Voigt, A.; Meyerhof, W.; Behrens, M. Expression Profiling of Tas2r Genes Reveals a Complex Pattern along the Mouse GI Tract and the Presence of Tas2r131 in a Subset of Intestinal Paneth Cells. Cell. Mol. Life Sci. 2018, 75, 49–65.
- Kimura, S.; Kato, E. TAS2R Expression Profile in Brown Adipose, White Adipose, Skeletal Muscle, Small Intestine, Liver and Common Cell Lines Derived from Mice. Gene Rep. 2020, 20, 100763.
- Luo, X.-C.; Chen, Z.-H.; Xue, J.-B.; Zhao, D.-X.; Lu, C.; Li, Y.-H.; Li, S.-M.; Du, Y.-W.; Liu, Q.; Wang, P.; et al. Infection by the Parasitic Helminth Trichinella Spiralis Activates a Tas2r-Mediated Signaling Pathway in Intestinal Tuft Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 5564–5569.
- Kim, K.-S.; Egan, J.M.; Jang, H.-J. Denatonium Induces Secretion of Glucagon-like Peptide-1 through Activation of Bitter Taste Receptor Pathways. Diabetologia 2014, 57, 2117–2125.
- Wu, S.V.; Rozengurt, N.; Yang, M.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Expression of Bitter Taste Receptors of the T2R Family in the Gastrointestinal Tract and Enteroendocrine STC-1 Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2392–2397.
- Vegezzi, G.; Anselmi, L.; Huynh, J.; Barocelli, E.; Rozengurt, E.; Raybould, H.; Sternini, C. Diet-Induced Regulation of Bitter Taste Receptor Subtypes in the Mouse Gastrointestinal Tract. PLoS ONE 2014, 9, e107732.
- Jeon, T.-I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 Regulates Gut Peptide Secretion through Intestinal Bitter Taste Receptor Signaling in Mice. J. Clin. Investig. 2008, 118, 3693–3700.
- Pham, H.; Hui, H.; Morvaridi, S.; Cai, J.; Zhang, S.; Tan, J.; Wu, V.; Levin, N.; Knudsen, B.; Goddard, W.A.; et al. A Bitter Pill for Type 2 Diabetes? The Activation of Bitter Taste Receptor TAS2R38 Can Stimulate GLP-1 Release from Enteroendocrine L-Cells. Biochem. Biophys. Res. Commun. 2016, 475, 295–300.
- Yue, X.; Liang, J.; Gu, F.; Du, D.; Chen, F. Berberine Activates Bitter Taste Responses of Enteroendocrine STC-1 Cells. Mol. Cell. Biochem. 2018, 447, 21–32.
- Yu, Y.; Hao, G.; Zhang, Q.; Hua, W.; Wang, M.; Zhou, W.; Zong, S.; Huang, M.; Wen, X. Berberine Induces GLP-1 Secretion through Activation of Bitter Taste Receptor Pathways. Biochem. Pharmacol. 2015, 97, 173–177.
- Kok, B.P.; Galmozzi, A.; Littlejohn, N.K.; Albert, V.; Godio, C.; Kim, W.; Kim, S.M.; Bland, J.S.; Grayson, N.; Fang, M.; et al. Intestinal Bitter Taste Receptor Activation Alters Hormone Secretion and Imparts Metabolic Benefits. Mol. Metab. 2018, 16, 76–87.
- Rose, B.D.; Bitarafan, V.; Rezaie, P.; Fitzgerald, P.C.E.; Horowitz, M.; Feinle-Bisset, C. Comparative Effects of Intragastric and Intraduodenal Administration of Quinine on the Plasma Glucose Response to a Mixed-Nutrient Drink in Healthy Men: Relations with Glucoregulatory Hormones and Gastric Emptying. J. Nutr. 2021, 151, 1453–1461.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
970
Revisions:
2 times
(View History)
Update Date:
14 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




