Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wellington Fernandes Silva | -- | 2643 | 2023-12-13 01:42:33 | | | |
| 2 | Lindsay Dong | + 4 word(s) | 2647 | 2023-12-18 02:04:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, W.; Rego, E. Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. Encyclopedia. Available online: https://encyclopedia.pub/entry/52635 (accessed on 07 February 2026).
Silva W, Rego E. Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. Encyclopedia. Available at: https://encyclopedia.pub/entry/52635. Accessed February 07, 2026.
Silva, Wellington, Eduardo Rego. "Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings" Encyclopedia, https://encyclopedia.pub/entry/52635 (accessed February 07, 2026).
Silva, W., & Rego, E. (2023, December 13). Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. In Encyclopedia. https://encyclopedia.pub/entry/52635
Silva, Wellington and Eduardo Rego. "Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings." Encyclopedia. Web. 13 December, 2023.
Copy Citation
Remarkable strides have been performed in the treatment of adults diagnosed with Philadelphia-positive lymphoblastic leukemia (Ph+ ALL) through the integration of newer-generation tyrosine-kinase inhibitors and monoclonal antibodies. However, it is crucial to acknowledge that most medical centers worldwide lack access to these therapies. As a result, primary strategies employed for curing this disease continue to rely on a combination of chemotherapy and allogeneic stem-cell transplantation.
acute lymphoblastic leukemia
Philadelphia chromosome
tyrosine-kinase inhibitor
blinatumomab
1. Introduction
The integration of tyrosine-kinase inhibitors (TKI) into frontline treatment was a breakthrough in Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) [1]. Previously, Ph+ ALL was an almost incurable disease, with few patients able to sustain their response, even with allogeneic stem-cell transplantation (alloSCT) consolidation [2]. The combination of TKIs and chemotherapy was able to increase complete molecular remission (CMR) rates and allowed more patients to proceed to alloSCT. Furthermore, long-term remission could be achieved without alloSCT in a smaller subset [3][4][5].
Overall, Ph+ ALL is treated with TKI plus chemotherapy in most countries, followed by standard consolidation with alloSCT [1]. Several protocols have been tested over the last decades, with a wide range of intensities of cytotoxic chemotherapy combined with imatinib, dasatinib, nilotinib, and, more recently, ponatinib. Doses of TKIs have also varied across protocols, as well as the monitoring of minimal residual disease (MRD) [6]. Traditionally, in Ph+ ALL, the quantification of BCR-ABL1 transcript by real-time polymerase chain reaction (RT-PCR) has been the standard for MRD monitoring, mirroring the follow-up of chronic myeloid leukemia [6].
More recently, the medical community has witnessed even greater achievements in Ph+ ALL, with the emergence of newer-generation TKIs, such as ponatinib, and the integration of blinatumomab, a bispecific CD3-CD19 antibody [7]. These innovative approaches have achieved unprecedented survival rates, with an estimated 2-year event-free survival (EFS) of 90% for 54 patients with newly diagnosed Ph+ ALL treated with blinatumomab and ponatinib [8][9].
2. Diagnostic Barriers in Low- to Middle-Income Countries (LMIC)
Patients diagnosed with B-cell phenotype lymphoblastic leukemia should undergo prompt screening for BCR-ABL1 fusions. Ideally, this screening should be conducted using PCR or fluorescence in situ hybridization (FISH) methodologies, as they allow for a timely diagnosis of the fusion. It is essential to seek both BCR-ABL1 transcripts, namely p190 and p210 [10]. At the diagnosis, these methodologies have similar sensitivities, being complimentary in a minority of cases where PCR can be negative or with low leukemic burden, where FISH can be suboptimal [11]. Summarily, PCR seems to be a more comprehensive method as it is more sensitive, less expensive, and allows the determination of which fusion must be tracked along the treatment [11].
Typically, treatment protocols for ALL commence with a week-long pre-phase. This pre-phase usually includes corticosteroids, either with or without cyclophosphamide. The primary goal of this pre-phase is to reduce the disease burden in the peripheral blood, as well as to address any electrolyte and coagulation abnormalities present at the time of leukemia presentation [10][12]. During this initial period, it is crucial to determine the Philadelphia chromosome status of the patients, which will guide the selection of specific treatment regimens for those who are Philadelphia chromosome-positive. Unfortunately, there are limited data available regarding the turnaround time for BCR-ABL1 testing in LMICs. Performing routine genetic testing for acute leukemia can pose challenges in certain healthcare centers [13][14][15]. A delayed detection of the Philadelphia chromosome can result in patients receiving general induction remission protocols without TKIs, and, regrettably, more intensive treatments than necessary for this patient population. Therefore, it is strongly recommended that all patients receive their Philadelphia chromosome status results within a week to avoid unnecessary, intensive induction treatments (see ‘frontline induction’).
3. Genetic Evaluation of Ph+ ALL
Complimentary genetic evaluation through conventional karyotyping is typically available at most medical centers upon diagnosis of Ph+ ALL and can provide valuable insights. Additional cytogenetic alterations (ACAs) beyond the t(9;22)(q34;q11.2) translocation are detected in 40–60% of Ph+ ALL cases, and their prognostic significance remains a topic of debate [16]. The most commonly reported ACAs in Ph+ ALL include the gain of an extra Philadelphia chromosome or the “+der(22)t(9;22),” gain of the X chromosome, trisomy 8 or 21, high hyperdiploidy (greater than 50 chromosomes), monosomy 7, or deletions of chromosomes 7p and 9p, among others [17][18].
Whereas studies conducted in the pre-TKI era consistently demonstrated poorer survival rates in cases with ACAs, the same cannot be said for patients treated with modern regimens [18]. The prognostic impact of ACAs in Ph+ ALL largely depends on the type of alteration and the treatment protocol used. For instance, Aldoss et al. reported that among 78 adult patients with Ph+ ALL who underwent alloSCT and had available cytogenetic data, 53% had any ACA, with monosomy 7 being the most common (29%). In this cohort, primarily treated with imatinib-based regimens, a significant difference in EFS was observed (79.8% versus 39.5%, p = 0.01) [19].
Despite being cost-effective and widely accessible, conventional karyotyping is now recognized as suboptimal for examining baseline genomic alterations in Ph+ ALL. Higher-resolution techniques, such as microarray or multiplex ligation-probe analysis (MLPA), have revealed that deletions in the gene encoding Ikaros (IKZF1) on the 7p chromosome are common in Ph+ ALL (70–80%) [20][21][22]. Whereas the isolated impact of IKZF1 deletions on Ph+ ALL remains debatable, the association of this alteration with other copy-number alterations (CNA)—known as the “IKZF1-plus” subgroup—consistently demonstrates poor survival in this disease [23][24].
The IKZF1-plus subgroup is present in approximately 40% of Ph+ ALL cases and comprises patients with concurrent IKZF1 deletion and CDKN2A/B and/or PAX5 deletions [25][26]. These genomic alterations are associated with a poor prognosis, independent of the TKI used in therapy (imatinib, dasatinib, or ponatinib), and appear to be resistant to the addition of blinatumomab [26][27]. Unfortunately, these lesions require screening by multiplex platforms, which are not always available in LMICs.
4. Frontline Induction in Ph+ ALL
Achieving complete remission (CR) is a prerequisite for curing Ph+ ALL. Whereas substantial efforts have been made in the past to increase the rate of MRD negativity after induction, it is now universally accepted that low-intensity induction is both safe and recommended for Ph+ ALL, especially in scenarios where early death is not negligible [28][29]. Several centers worldwide still offer conventional induction upfront, while BCR-ABL1 testing requires a wait in accordance with their practice.
Chalandon et al. reported a non-inferiority randomized trial comparing reduced-intensity chemotherapy (vincristine, dexamethasone, and imatinib) with a standard arm consisting of Hyper-CVAD plus imatinib. Due to fewer induction-related deaths, the hematologic CR rate was higher in the experimental arm (98.5% vs. 91.0%), with no differences in CMR rates [29]. Even though previous experiences with corticosteroids plus TKI have been published, this study validated this approach, even for young patients. In LMICs, this approach holds particular value as there are more reports of induction-related deaths [30][31].
During this initial phase, once again, early identification of the BCR-ABL1 fusion allows more patients to avoid intensive upfront chemotherapy, reducing early mortality [29][30]. It is worth noting that the Philadelphia chromosome is more frequent in older patients; therefore, lower-intensity protocols are often more appropriate for most patients [32]. These regimens usually combine higher doses of TKI with corticosteroids and/or vincristine. Febrile neutropenia is the most reported event, although peripheral neuropathy and liver toxicity can also occur during this phase. The use of asparaginase alongside TKI is rarely employed in adults, with few reports of increased liver toxicity associated with this combination [33].
The induction phase typically lasts for four weeks, and patients are managed through hospitalization. Blood transfusions and antibiotics for febrile neutropenia are routinely required. Anti-infective prophylaxis with trimethoprim-sulfamethoxazole and acyclovir is recommended, and the use of antifungal agents may also be considered [10]. It is important to note that azoles can potentially cause liver toxicity, especially when administered alongside TKIs [34].
5. Availability of TKIs
In parallel with the chronic myeloid leukemia (CML) scenario, the affordability of TKIs is another obstacle for improving results [35]. Although imatinib has lost patent protection for a long time now, making low-cost generic versions of the drug available in most LMICs, the same cannot be said about remaining TKIs. The cost per drug varies greatly among countries worldwide, especially in Latin America. Some countries may deal with higher prices for several medicines compared to those in European countries. This can be the result of ineffective or lack of pricing regulations in some countries, such Chile, Peru, and Mexico [36].
When it comes to second- and third-generation TKIs, the situation is more critical in most countries, with a high rate of unaffordability pointed in pharmacoeconomical studies [36]. Imatinib generics are routinely available for USD 300–USD 3000/year in several countries, while dasatinib will be available as a generic formulation in the next few years and can be prescribed elsewhere now. The prices of patented TKIs range from USD 150,000 to USD 250,000+/year, being unaffordable even for some developed countries [37].
6. Consolidation and Maintenance Therapy
Although CR is achieved in virtually all newly diagnosed Ph+ ALL patients, relapse rates remain high even with continued therapy, resulting in unsatisfactory long-term outcomes [6]. Previous studies in elderly populations treated with TKIs plus corticosteroids have shown that, without additional chemotherapy, leukemia progresses rapidly, indicating the need for more intensive post-remission therapy [38].
Most treatment protocols have incorporated cytotoxic chemotherapy during this phase, aiming to achieve deeper molecular remission rates and penetrate the blood–brain barrier (BBB) [5]. Retrospective data suggest that chemotherapy plays a crucial role in eradicating ABL-mutated clones, which are responsible for driving most relapses in Ph+ ALL [39][40].
The attainment of CMR is considered essential for curing Ph+ ALL [41]. As there have been few randomized trials in this disease, most current recommendations are based on the review of phase II trials and retrospective data. Outcomes obtained after these regimens indicate a complex pattern of MRD response, influenced by both the type of TKI and the associated chemotherapy.
In patients up to 18 years old, a randomized trial comparing dasatinib to imatinib showed that the former outperformed the latter with no additional toxic effects. This trial demonstrated a significant survival benefit with fewer relapses in the dasatinib arm, which can likely be extrapolated to adults [42].
Whereas integrating TKIs into chemotherapy backbones is feasible, it may lead to increased toxicity. A study by Ravandi et al. reported that 31 out of 72 patients considered eligible for the Hyper-CVAD plus dasatinib trial received fewer than the intended 8 cycles due to poor tolerance [43].
In resource-constrained settings and with the unavailability of newer drugs, adjustments in the consolidation blocks are necessary. Dose reductions, particularly in patients who achieve CMR early, should be considered. Although it is common to interrupt chemotherapy in patients with poor tolerance in clinical practice, clinicians should be aware of the increased risk of relapse in such cases.
7. MRD Monitoring in Ph+ ALL
Extensive literature has provided robust evidence for the use of MRD for prognostication and guiding therapy in ALL [44]. However, in the case of Ph+ ALL, the available evidence is more limited and often contentious. In this subgroup, patients are usually monitored by RT-PCR for BCR-ABL1, in bone marrow, in parallel with other conventional methods, such as flow cytometry or the quantitative PCR of rearranged immunoglobulin (IG) genes [45]. In LMICs, most MRD studies rely on flow cytometry due to the high costs associated with NGS. The quantification of BCR-ABL1 is variably available in many centers and often requires internal validation for use [46].
Overall, it is recommended that Ph+ ALL patients undergo monitoring using both qPCR and flow or IG methods, as they complement each other. The overall concordance between BCR-ABL1 and IG qPCR is 69%, as reported by Cazzaniga et al., who observed significantly higher positivity by BCR-ABL1 in a pediatric cohort [47]. This study highlighted an important issue: while BCR-ABL1 is a valuable prognostic and monitoring tool in Ph+ ALL, its prognostic significance in patients with no detectable abnormal B cells is uncertain. In the GRAAPH-2014 trial, 38% of enrolled patients consistently showed discordant results between IG and BCR-ABL1, suggesting the persistence of non-lymphoblastic BCR-ABL1-positive cells.
8. ABL Mutations and Management of TKI
After the establishment of TKI-based therapy in Ph+ ALL, studies on relapsed disease have revealed the frequent occurrence of point mutations in the BCR-ABL1 kinase domain (KD) [1][48]. Whereas initial evidence suggests that small clones with mutations exist at the time of diagnosis in Ph+ ALL, routine upfront ABL mutation testing is not currently recommended [48][49]. This is because the presence of these mutations does not always preclude a primary response to TKIs, as chemotherapy and newer agents, such as blinatumomab, play a role in eradicating such small clones [1][10].
However, the situation is different when it comes to assessing ABL mutations at the time of relapse or treatment failure in Ph+ ALL. In such cases, the incidence of imatinib- or dasatinib-resistant mutations increases, even when less sensitive methods like conventional Sanger sequencing are used [28]. Alternative approaches for screening ABL mutations using NGS or digital PCR are not currently recommended outside of research settings, as they do not alter treatment decisions [50]. Although there is no formal recommendation, it is widely suggested that Ph+ ALL patients undergo ABL mutation testing only when they experience a loss of molecular response or in the event of relapse. The emergence of the “gatekeeper” T315I mutation confers resistance to imatinib and dasatinib but remains susceptible to ponatinib [6].
9. CNS Prophylaxis
Recent protocols have shown that enhancing intrathecal prophylaxis can reduce the occurrence of central nervous system (CNS) relapses [51]. In most modern protocols, prophylactic irradiation (RT) is no longer employed. However, CNS-directed therapy can still be considered in cases of CNS-3 disease, particularly if cranial nerve palsy or parenchymal involvement is present [52]. In Ph+ ALL, dasatinib has demonstrated efficacy in the CNS and is recommended for patients with CNS disease [52][53].
10. Allogeneic Stem-Cell Transplantation and Alternative Approaches
Traditionally, alloSCT has been considered the standard consolidation therapy for Ph+ ALL in first CR [10]. However, clinical trials focused on deferring this procedure in patients who achieve CMR are lacking. Retrospective reports suggest that there is no survival benefit of alloSCT in patients who attain this level of response [29][54][55].
The decision of whether to proceed with alloSCT after achieving CR in Ph+ ALL should consider several factors: (i) clinical eligibility for the procedure, considering that many patients with this disease may be older or have comorbidities that increase toxicity; (ii) the achievement of CMR; (iii) the presence of higher-risk genetic features, such as IKZF1plus; (iv) the availability of newer-generation TKIs, like ponatinib or monoclonal antibodies (blinatumomab or inotuzumab), in the case of disease progression or loss of CMR; (v) access to the monitoring of the BCR-ABL1 transcript and MRD; And (vi) local toxicity and mortality rates associated with alloSCT, especially in LMICs [6][56][57].
Patients in CMR who are candidates to defer alloSCT should ideally undergo consolidation with intensive chemotherapy while maintaining continuous TKI exposure. These patients should be kept on TKI maintenance indefinitely [7]. Initial monitoring by bone marrow aspirate is recommended every 3 months for 1–2 years, followed by BCR-ABL1 monitoring using qPCR in peripheral blood indefinitely [10][58]. In Figure 1, a suggested management flowchart for Ph+ ALL is provided.
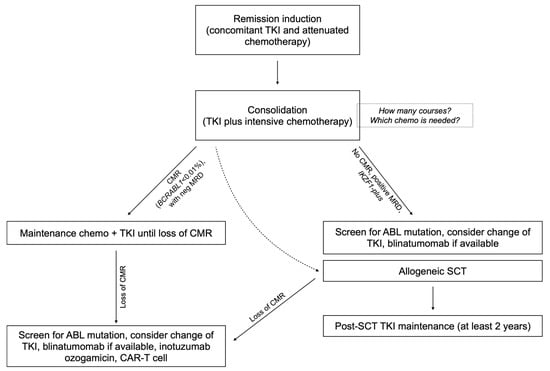
Figure 1. Flowchart of the suggested algorithm for the treatment of Ph+ ALL with chemo plus TKI combinations.
11. Conclusions
In conclusion, effectively managing Ph+ ALL in LMIC presents a multifaceted challenge that demands innovative approaches and collaborative efforts. Whereas the advanced treatments and targeted therapies available in well-resourced settings have significantly improved survival rates for Ph+ ALL patients, the disparities in access to these therapies in resource-limited regions cannot be overlooked. It is crucial for healthcare providers, policymakers, and organizations to work together to develop cost-effective strategies, improve diagnostic capabilities, and expand access to essential treatments. Overall, especially in resource-constrained scenarios, upfront low-intensity regimens are recommended, followed by intensive chemotherapy as for consolidation, always associated with TKI. Imatinib is the most cost-saving TKI, while dasatinib seems to offer deeper responses and arguably increased survival rates in children. In eligible patients, alloSCT should be carefully implemented, considering local mortality and the availability of newer lines of treatment. Optimizing existing resources and fostering international partnerships will be instrumental in bridging the gap and ensuring that Ph+ ALL patients everywhere receive the best possible care, regardless of their geographic location or socioeconomic status.
References
- Foà, R.; Chiaretti, S. Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2022, 386, 2399–2411.
- Gleibetaner, B.; Gleißner, B.; Gökbuget, N.; Bartram, C.R.; Janssen, B.; Rieder, H.; Janssen, J.W.G.; Fonatsch, C.; Heyll, A.; Voliotis, D.; et al. Leading Prognostic Relevance of the BCR-ABL Translocation in Adult Acute B-Lineage Lymphoblastic Leukemia: A Prospective Study of the German Multicenter Trial Group and Confirmed Polymerase Chain Reaction Analysis. Blood 2002, 99, 1536–1543.
- Ribera, J.-M.; Oriol, A.; González, M.; Vidriales, B.; Brunet, S.; Esteve, J.; del Potro, E.; Rivas, C.; Moreno, M.-J.; Tormo, M.; et al. Concurrent Intensive Chemotherapy and Imatinib before and after Stem Cell Transplantation in Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Final Results of the CSTIBES02 Trial. Haematologica 2010, 95, 87–95.
- Bleckmann, K.; Schrappe, M. Advances in Therapy for Philadelphia-Positive Acute Lymphoblastic Leukaemia of Childhood and Adolescence. Br. J. Haematol. 2016, 172, 855–869.
- Daver, N.; Thomas, D.; Ravandi, F.; Cortes, J.; Garris, R.; Jabbour, E.; Garcia-Manero, G.; Borthakur, G.; Kadia, T.; Rytting, M.; et al. Final Report of a Phase II Study of Imatinib Mesylate with Hyper-CVAD for the Front-Line Treatment of Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Haematologica 2015, 100, 653–661.
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults With Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia—From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens. JAMA Oncol. 2022, 8, 1340–1348.
- Ribera, J.-M.; Chiaretti, S. Modern Management Options for Ph+ ALL. Cancers 2022, 14, 4554.
- Short, N.J.; Jabbour, E.; Jain, N.; Macaron, W.; Huang, X.; Montalban-Bravo, G.; Kadia, T.M.; Daver, N.G.; Haddad, F.; Zoghbi, M.; et al. A Phase II Trial of Ponatinib and Blinatumomab in Adults with Newly Diagnosed Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia (Ph+ ALL). J. Clin. Oncol. 2023, 41, e19013.
- Jabbour, E.; Short, N.J.; Jain, N.; Huang, X.; Montalban-Bravo, G.; Banerjee, P.; Rezvani, K.; Jiang, X.; Kim, K.H.; Kanagal-Shamanna, R.; et al. Ponatinib and Blinatumomab for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia: A US, Single-Centre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2023, 10, e24–e34.
- Hoelzer, D.; Bassan, R.; Dombret, H.; Fielding, A.; Ribera, J.-M.; Buske, C. Acute Lymphoblastic Leukaemia in Adult Patients: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v69–v82.
- Cox, M.C.; Maffei, L.; Buffolino, S.; Del Poeta, G.; Venditti, A.; Cantonetti, M.; Aronica, G.; Aquilina, P.; Masi, M.; Amadori, S. A Comparative Analysis of FISH, RT-PCR, and Cytogenetics for the Diagnosis of Bcr-Abl Positive Leukemias. Am. J. Clin. Pathol. 1998, 109, 24–31.
- Brown, P.A.; Shah, B.; Advani, A.; Aoun, P.; Boyer, M.W.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1079–1109.
- Silva, W.F.; Amano, M.T.; Perruso, L.L.; Cordeiro, M.G.; Kishimoto, R.K.; de Medeiros Leal, A.; Nardinelli, L.; Bendit, I.; Velloso, E.D.; Rego, E.M.; et al. Adult Acute Lymphoblastic Leukemia in a Resource-Constrained Setting: Outcomes after Expansion of Genetic Evaluation. Hematology 2022, 27, 396–403.
- Aguiar, T.F.; da Conceição Barbosa, T.; Maciel, A.L.T.; Blunck, C.B.; Sellos-Laclette, J.; de Melo, A.C.; Mansur, M.B.; Emerenciano, M. Outcome of Adolescents and Young Adults with Acute Lymphoblastic Leukemia in a Single Center in Brazil. Hematol. Transfus. Cell Ther. 2023, 45, S108–S112.
- Crespo-Solis, E.; Espinosa-Bautista, K.; Alvarado-Ibarra, M.; Rozen-Fuller, E.; Pérez-Rocha, F.; Nava-Gómez, C.; Ortiz-Zepeda, M.; Álvarez-Vera, J.L.; Ramos-Peñafiel, C.O.; Meillón-García, L.A.; et al. Survival Analysis of Adult Patients with ALL in Mexico City: First Report from the Acute Leukemia Workgroup (ALWG) (GTLA). Cancer Med. 2018, 7, 2423–2433.
- Jain, P.; Gu, J.; Kanagal-Shamanna, R.; Tang, Z.; Patel, K.P.; Yao, H.; Fang, L.; Bao, H.-Y.; Liu, C.-H.; Lin, P.; et al. Clinical Implications of Cytogenetic Heterogeneity in Philadelphia Chromosome Positive (Ph+) Adult B Cell Acute Lymphoblastic Leukemia Following Tyrosine Kinase Inhibitors and Chemotherapy Regimens. Leuk. Res. 2019, 84, 106176.
- Heerema, N.A.; Harbott, J.; Galimberti, S.; Camitta, B.M.; Gaynon, P.S.; Janka-Schaub, G.; Kamps, W.; Basso, G.; Pui, C.-H.; Schrappe, M.; et al. Secondary Cytogenetic Aberrations in Childhood Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Are Nonrandom and May Be Associated with Outcome. Leukemia 2004, 18, 693–702.
- Wetzler, M.; Dodge, R.K.; Mrózek, K.; Stewart, C.C.; Carroll, A.J.; Tantravahi, R.; Vardiman, J.W.; Larson, R.A.; Bloomfield, C.D. Additional Cytogenetic Abnormalities in Adults with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia: A Study of the Cancer and Leukaemia Group B. Br. J. Haematol. 2004, 124, 275–288.
- Aldoss, I.; Stiller, T.; Cao, T.M.; Palmer, J.M.; Thomas, S.H.; Forman, S.J.; Pullarkat, V. Impact of Additional Cytogenetic Abnormalities in Adults with Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1326–1329.
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.-H.; et al. BCR–ABL1 Lymphoblastic Leukaemia Is Characterized by the Deletion of Ikaros. Nature 2008, 453, 110–114.
- van der Veer, A.; Zaliova, M.; Mottadelli, F.; De Lorenzo, P.; te Kronnie, G.; Harrison, C.J.; Cavé, H.; Trka, J.; Saha, V.; Schrappe, M.; et al. IKZF1 Status as a Prognostic Feature in BCR-ABL1–Positive Childhood ALL. Blood 2014, 123, 1691–1698.
- Deboer, R.; Koval, G.; Mulkey, F.; Wetzler, M.; Devine, S.; Marcucci, G.; Stone, R.M.; Larson, R.A.; Bloomfield, C.D.; Geyer, S.; et al. Clinical Impact of ABL1 Kinase Domain Mutations and IKZF1 Deletion in Adults under Age 60 with Philadelphia Chromosome-Positive (Ph+) Acute Lymphoblastic Leukemia (ALL): Molecular Analysis of CALGB (Alliance) 10001 and 9665. Leuk. Lymphoma 2016, 57, 2298–2306.
- Fedullo, A.L.; Messina, M.; Elia, L.; Piciocchi, A.; Gianfelici, V.; Lauretti, A.; Soddu, S.; Puzzolo, M.C.; Minotti, C.; Ferrara, F.; et al. Prognostic Implications of Additional Genomic Lesions in Adult Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Haematologica 2019, 104, 312–318.
- Pfeifer, H.; Raum, K.; Markovic, S.; Nowak, V.; Fey, S.; Obländer, J.; Pressler, J.; Böhm, V.; Brüggemann, M.; Wunderle, L.; et al. Genomic CDKN2A/2B Deletions in Adult Ph+ ALL Are Adverse despite Allogeneic Stem Cell Transplantation. Blood 2018, 131, 1464–1475.
- Chiaretti, S.; Ansuinelli, M.; Vitale, A.; Elia, L.; Matarazzo, M.; Piciocchi, A.; Fazi, P.; Di Raimondo, F.; Santoro, L.; Fabbiano, F.; et al. A Multicenter Total Therapy Strategy for de Novo Adult Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Patients: Final Results of the GIMEMA LAL1509 Protocol. Haematologica 2021, 106, 1828–1838.
- Sasaki, Y.; Kantarjian, H.M.; Short, N.J.; Wang, F.; Furudate, K.; Uryu, H.; Garris, R.; Jain, N.; Sasaki, K.; Ravandi, F.; et al. Genetic Correlates in Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Hyper-CVAD plus Dasatinib or Ponatinib. Leukemia 2022, 36, 1253–1260.
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623.
- Ravandi, F. How I Treat Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. Blood 2019, 133, 130–136.
- Chalandon, Y.; Thomas, X.; Hayette, S.; Cayuela, J.-M.; Abbal, C.; Huguet, F.; Raffoux, E.; Leguay, T.; Rousselot, P.; Lepretre, S.; et al. Randomized Study of Reduced-Intensity Chemotherapy Combined with Imatinib in Adults with Ph-Positive Acute Lymphoblastic Leukemia. Blood 2015, 125, 3711–3719.
- Silva, W.F.; Silverio, A.; Duarte, B.K.L.; Aguiar, T.F.; Bendlin, R.M.; Massaut, I.H.B.; Pagnano, K.B.B.; Velloso, E.D.R.P.; Rocha, V.; Rego, E.M. Philadelphia-Positive B-Lymphoblastic Leukemia in a Middle-Income Country—A Real-World Multicenter Cohort. Leuk. Res. 2021, 110, 106666.
- Fernandes da Silva Junior, W.; Medina, A.B.; Yamakawa, P.E.; Buccheri, V.; Velloso, E.D.R.P.; Rocha, V. Treating Adult Acute Lymphoblastic Leukemia in Brazil—Increased Early Mortality Using a German Multicenter Acute Lymphoblastic Leukemia-Based Regimen. Clin. Lymphoma Myeloma Leuk. 2018, 18, e255–e259.
- Frisch, A.; Ofran, Y. How I Diagnose and Manage Philadelphia Chromosome-like Acute Lymphoblastic Leukemia. Haematologica 2019, 104, 2135–2143.
- Fielding, A.K.; Rowe, J.M.; Buck, G.; Foroni, L.; Gerrard, G.; Litzow, M.R.; Lazarus, H.; Luger, S.M.; Marks, D.I.; McMillan, A.K.; et al. UKALLXII/ECOG2993: Addition of Imatinib to a Standard Treatment Regimen Enhances Long-Term Outcomes in Philadelphia Positive Acute Lymphoblastic Leukemia. Blood 2014, 123, 843–850.
- Pajares, B.; Torres, E.; Trigo, J.M.; Sáez, M.I.; Ribelles, N.; Jiménez, B.; Alba, E. Tyrosine Kinase Inhibitors and Drug Interactions: A Review with Practical Recommendations. Clin. Transl. Oncol. 2012, 14, 94–101.
- Malhotra, H.; Radich, J.; Garcia-Gonzalez, P. Meeting the Needs of CML Patients in Resource-Poor Countries. Hematology 2019, 2019, 433–442.
- Moye-Holz, D.; Vogler, S. Comparison of Prices and Affordability of Cancer Medicines in 16 Countries in Europe and Latin America. Appl. Health Econ. Health Policy 2022, 20, 67–77.
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of Chronic Myeloid Leukemia in 2023—Common Ground and Common Sense. Blood Cancer J. 2023, 13, 58.
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as First-Line Treatment for Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Blood 2011, 118, 6521–6528.
- Bassan, R.; Rohatiner, A.Z.; Lerede, T.; Di Bona, E.; Rambaldi, A.; Pogliani, E.; Rossi, G.; Fabris, P.; Morandi, S.; Casula, P.; et al. Role of Early Anthracycline Dose-Intensity According to Expression of Philadelphia Chromosome/BCR–ABL Rearrangements in B-Precursor Adult Acute Lymphoblastic Leukemia. Hematol. J. 2000, 1, 226–234.
- Soverini, S.; Bassan, R.; Lion, T. Treatment and Monitoring of Philadelphia Chromosome-Positive Leukemia Patients: Recent Advances and Remaining Challenges. J. Hematol. Oncol. 2019, 12, 39.
- Short, N.J.; Jabbour, E.; Macaron, W.; Ravandi, F.; Jain, N.; Kanagal-Shamanna, R.; Patel, K.P.; Loghavi, S.; Haddad, F.G.; Yilmaz, M.; et al. Ultrasensitive NGS MRD Assessment in Ph+ ALL: Prognostic Impact and Correlation with RT-PCR for BCR::ABL1. Am. J. Hematol. 2023, 98, 1196–1203.
- Shen, S.; Chen, X.; Cai, J.; Yu, J.; Gao, J.; Hu, S.; Zhai, X.; Liang, C.; Ju, X.; Jiang, H.; et al. Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 358–366.
- Ravandi, F.; O’Brien, S.M.; Cortes, J.E.; Thomas, D.M.; Garris, R.; Faderl, S.; Burger, J.A.; Rytting, M.E.; Ferrajoli, A.; Wierda, W.G.; et al. Long-Term Follow-up of a Phase 2 Study of Chemotherapy plus Dasatinib for the Initial Treatment of Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Cancer 2015, 121, 4158–4164.
- Berry, D.A.; Zhou, S.; Higley, H.; Mukundan, L.; Fu, S.; Reaman, G.H.; Wood, B.L.; Kelloff, G.J.; Jessup, J.M.; Radich, J.P. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia. JAMA Oncol. 2017, 3, e170580.
- Van Dongen, J.J.M.; Van Der Velden, V.H.J.; Brüggemann, M.; Orfao, A. Minimal Residual Disease Diagnostics in Acute Lymphoblastic Leukemia: Need for Sensitive, Fast, and Standardized Technologies. Blood 2015, 125, 3996–4009.
- Pfeifer, H.; Cazzaniga, G.; van der Velden, V.H.J.; Cayuela, J.M.; Schäfer, B.; Spinelli, O.; Akiki, S.; Avigad, S.; Bendit, I.; Borg, K.; et al. Standardisation and Consensus Guidelines for Minimal Residual Disease Assessment in Philadelphia-Positive Acute Lymphoblastic Leukemia (Ph + ALL) by Real-Time Quantitative Reverse Transcriptase PCR of E1a2 BCR-ABL1. Leukemia 2019, 33, 1910–1922.
- Cazzaniga, G.; De Lorenzo, P.; Alten, J.; Röttgers, S.; Hancock, J.; Saha, V.; Castor, A.; Madsen, H.O.; Gandemer, V.; Cavé, H.; et al. Predictive Value of Minimal Residual Disease in Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Imatinib in the European Intergroup Study of Post-Induction Treatment of Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia, Based on Immunoglobulin/T-Cell Receptor and BCR/ABL1 Methodologies. Haematologica 2018, 103, 107–115.
- Pfeifer, H.; Wassmann, B.; Pavlova, A.; Wunderle, L.; Oldenburg, J.; Binckebanck, A.; Lange, T.; Hochhaus, A.; Wystub, S.; Brück, P.; et al. Kinase Domain Mutations of BCR-ABL Frequently Precede Imatinib-Based Therapy and Give Rise to Relapse in Patients with de Novo Philadelphia-Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood 2007, 110, 727–734.
- Soverini, S.; Vitale, A.; Poerio, A.; Gnani, A.; Colarossi, S.; Iacobucci, I.; Cimino, G.; Elia, L.; Lonetti, A.; Vignetti, M.; et al. Philadelphia-Positive Acute Lymphoblastic Leukemia Patients Already Harbor BCR-ABL Kinase Domain Mutations at Low Levels at the Time of Diagnosis. Haematologica 2011, 96, 552–557.
- Soverini, S.; Hochhaus, A.; Nicolini, F.E.; Gruber, F.; Lange, T.; Saglio, G.; Pane, F.; Müller, M.C.; Ernst, T.; Rosti, G.; et al. BCR-ABL Kinase Domain Mutation Analysis in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors: Recommendations from an Expert Panel on Behalf of European LeukemiaNet. Blood 2011, 118, 1208–1215.
- Paul, S.; Kantarjian, H.; Sasaki, K.; Marx, K.; Jain, N.; Savoy, J.M.; DiPippo, A.; Jammal, N.; Bravo, G.M.; Kadia, T.; et al. Intrathecal Prophylaxis with 12 versus 8 Administrations Reduces the Incidence of Central Nervous System Relapse in Patients with Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Am. J. Hematol. 2023, 98, E11–E14.
- Kopmar, N.E.; Cassaday, R.D. How I Prevent and Treat Central Nervous System Disease in Adults with Acute Lymphoblastic Leukemia. Blood 2023, 141, 1379–1388.
- Porkka, K.; Koskenvesa, P.; Lundán, T.; Rimpiläinen, J.; Mustjoki, S.; Smykla, R.; Wild, R.; Luo, R.; Arnan, M.; Brethon, B.; et al. Dasatinib Crosses the Blood-Brain Barrier and Is an Efficient Therapy for Central Nervous System Philadelphia Chromosome–Positive Leukemia. Blood 2008, 112, 1005–1012.
- Ghobadi, A.; Slade, M.; Kantarjian, H.; Alvarenga, J.; Aldoss, I.; Mohammed, K.A.; Jabbour, E.; Faramand, R.; Shah, B.; Locke, F.; et al. The Role of Allogeneic Transplant for Adult Ph+ ALL in CR1 with Complete Molecular Remission: A Retrospective Analysis. Blood 2022, 140, 2101–2112.
- Kim, D.-Y.; Joo, Y.-D.; Lim, S.-N.; Kim, S.-D.; Lee, J.-H.; Lee, J.-H.; Kim, D.H.; Kim, K.; Jung, C.W.; Kim, I.; et al. Nilotinib Combined with Multiagent Chemotherapy for Newly Diagnosed Philadelphia-Positive Acute Lymphoblastic Leukemia. Blood 2015, 126, 746–756.
- Silva, W.F.; Cysne, D.N.; Kerbauy, M.N.; Colturato, I.; Maia, A.C.A.; Tucunduva, L.; Barros, G.M.N.; Colturato, V.A.R.; Hamerschlak, N.; Rocha, V. Predictive Factors and Outcomes after Allogeneic Stem Cell Transplantation for Adults with Acute Lymphoblastic Leukemia in Brazil. Transplant. Cell. Ther. 2022, 28, 763.e1–763.e7.
- Ribera, J.-M.; Ribera, J.; Genescà, E. The Role of Stem Cell Transplantation in the Management of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Ther. Adv. Hematol. 2018, 9, 357–368.
- Giebel, S.; Czyz, A.; Ottmann, O.; Baron, F.; Brissot, E.; Ciceri, F.; Cornelissen, J.J.; Esteve, J.; Gorin, N.-C.; Savani, B.; et al. Use of Tyrosine Kinase Inhibitors to Prevent Relapse after Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Position Statement of the Acute Leukemia Working Party of The European Society for Blood and Marrow Transplantation. Cancer 2016, 122, 2941–2951.
More
Information
Subjects:
Hematology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
445
Revisions:
2 times
(View History)
Update Date:
18 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




