Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xueqin Zhang | -- | 3291 | 2023-12-11 14:14:01 | | | |
| 2 | Rita Xu | Meta information modification | 3291 | 2023-12-12 02:34:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, X.; Zhang, X.; Li, Y.; Zhang, Y. Light-Based 3D Bioprinting. Encyclopedia. Available online: https://encyclopedia.pub/entry/52585 (accessed on 07 March 2026).
Zhang X, Zhang X, Li Y, Zhang Y. Light-Based 3D Bioprinting. Encyclopedia. Available at: https://encyclopedia.pub/entry/52585. Accessed March 07, 2026.
Zhang, Xueqin, Xin Zhang, Ying Li, Yuxuan Zhang. "Light-Based 3D Bioprinting" Encyclopedia, https://encyclopedia.pub/entry/52585 (accessed March 07, 2026).
Zhang, X., Zhang, X., Li, Y., & Zhang, Y. (2023, December 11). Light-Based 3D Bioprinting. In Encyclopedia. https://encyclopedia.pub/entry/52585
Zhang, Xueqin, et al. "Light-Based 3D Bioprinting." Encyclopedia. Web. 11 December, 2023.
Copy Citation
The emergence of additive manufacturing, commonly referred to as 3D printing, has led to a revolution in the field of biofabrication. Numerous types of 3D bioprinting, including extrusion bioprinting, inkjet bioprinting, and lithography-based bioprinting, have been developed and have played pivotal roles in driving a multitude of pioneering breakthroughs in the fields of tissue engineering and regenerative medicine.
light-based 3D bioprinting
photopolymerization
hydrogel
1. Introduction
The incidence of vital human organ failure has significantly increased with the extended human lifespan. The emergence and development of tissue engineering provide a promising solution to these challenges and are considered to have provided an effective method for eventually achieving the regeneration of human tissues and organs in the future [1][2][3]. Hydrogels are ideal materials for tissue engineering, as they can be tailored to a variety of mechanical, chemical, and biological characteristics for cell adhesion, proliferation, and migration. The conventional tissue-engineering strategy entails seeding cells onto a porous hydrogel scaffold first. With subsequent in vivo culturing, these cells undergo proliferation and differentiation, ultimately leading to the construction of a biological substitute [4][5][6]. Dynamic reciprocity within a 3D microenvironment, which can simulate the extracellular matrix (ECM), is crucial for cell growth [7]. Thus, it is very important to control the biomaterials in 3D space precisely to fabricate scaffolds with adjustable mechanical, physical, and rheological characteristics that perfectly mimic ECM. Traditional techniques such as freeze-drying [8], electrospinning [9], and thermally induced phase separation [10] make fabricating hydrogel scaffolds with precisely controlled microstructures difficult. The advent of 3D bioprinting made it possible to construct complex organ and tissue-like structures accurately (Figure 1A) [11][12][13][14][15]. Using computer-aided design (CAD), 3D printing can build desired structures in a precise and reproducible way [16][17]. Three-dimensional bioprinting based on traditional 3D printing can integrate cells, biomaterials, and bioactive factors into user-set geometries, which makes it a powerful tool in the fabrication of complex biomimetic tissue [18][19], drug-testing models [20], disease models [21], surgical implants [22], and smart sensors [23].
Within decades, a variety of 3D bioprinting techniques have been developed, including extrusion bioprinting [24][25], inkjet bioprinting [26], stereolithography (SLA)-based bioprinting [27], digital-light-processing-based (DLP-based) bioprinting [28], and computed-axial-lithography-based (CAL-based) bioprinting [29]. Hydrogels employed in 3D printing are crosslinked using various strategies, including chemical and physical crosslinking, to achieve the required strength and stability for maintaining the fidelity and resolution of the printed structures [30]. Additionally, the crosslinking of hydrogels can provide adequate support for cell growth within the printed structures. Chemical crosslinking methods, such as azide–alkyne cycloaddition, hydrazide–aldehyde coupling, thiol-ene coupling, enzymatic crosslinking, and photocrosslinking, offer significant advantages in 3D bioprinting of tissues [30]. Covalent bonds formed through these methods tend to provide greater tunability and higher stability for the printed structures, making them ideal for creating bioprinted tissues. Physical crosslinking methods, including hydrogen bonds, hydrophobic interactions, and ionic interactions, are also employed for crosslinking bioprinted hydrogels [31]. However, hydrogels crosslinked through these non-covalent bonds tend to be less stable, which makes them unsuitable for long-term in vitro cultivation. Currently, many of the 3D bioprinting methods employ light to crosslink or solidify photoreactive bio-inks. Extrusion-based bioprinting can use light to crosslink bio-inks before, after, or during extrusion, while lithography bioprinting can use light to directly solidify bio-inks (Figure 1B). Using light in 3D bioprinting offers several advantages, including rapid reaction rates, minimal heat production, and spatiotemporal control of the reaction [32]. Light-based bioprinting can be realized through the photopolymerization of photosensitive materials. In contrast to conventional bio-inks, the bio-inks utilized in light-based 3D bioprinting need to be integrated with photoreactive moieties to enable fast and selective photopolymerization of the bio-inks (Figure 1C) [33]. Normally, UV light and visible light can both be used as light sources in photopolymerization. However, as UV light may induce genetic mutations and even lead to cell death, visible light sources are commonly employed in light-based bioprinting to ensure cell viability and avoid potential harm to cells [34]. Photo-initiators (PIs) are a key component of photosensitive bio-inks. When irradiated by light, PIs can be excited to generate active species and subsequently initiate the polymerization of biomaterials. Within the last two decades, PIs and biomaterials with excellent biocompatibility, bioactivity, and biodegradability, which are suitable to be used in light-based 3D bioprinting, have been developed [7]. Another advantage of light-based bioprinting is that photopolymerization reactions can occur within aqueous solutions under physiological conditions, which can significantly reduce the use of harsh and cytotoxic reagents. Thus, light-based bioprinting is exceptionally well-suited for applications involving cells.
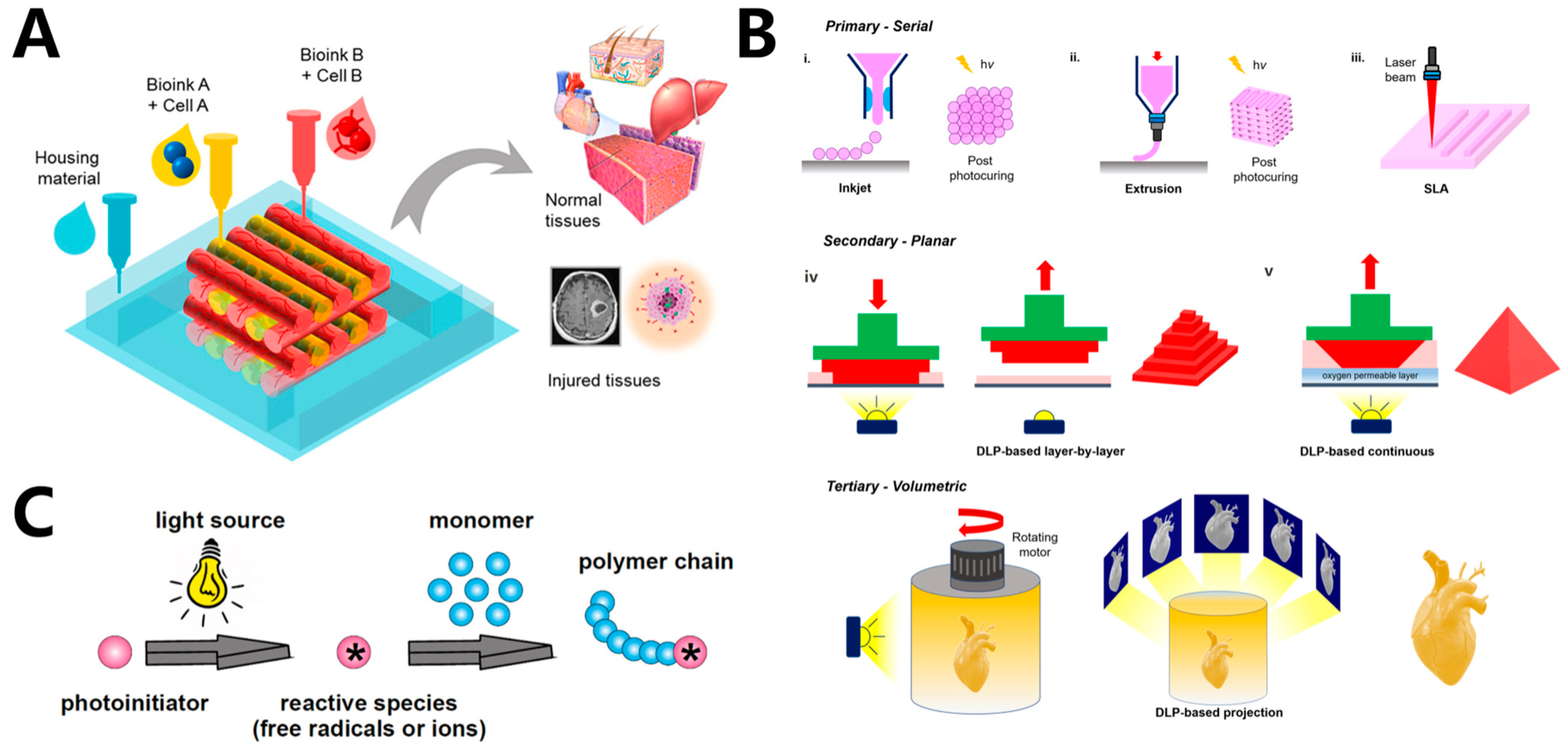
Figure 1. (A) 3D bioprinting structures for tissue engineering. (B) Schematic illustration of light-based bioprinting technology, including inkjet bioprinting, extrusion bioprinting, and lithography-based bioprinting. i–iii: in primary configuration, structures are printed either dot by dot or line by line. iv–v: in secondary configuration, structures are printed layer by layer via DLP-based projection of patterns into a vat containing bio-ink. -In tertiary configuration, 3D structures are created volumetrically by projecting patterns into a rotating vat containing bio-inks. (C) General mechanism of photopolymerization.
2. Light-Based 3D Bioprinting Methods and Applications
Light-based bioprinting has advantages, such as high printing resolution, the capability to print various materials, a controllable microstructure, minimal damage to cells, strong controllability, and fast processing speed. Among various techniques, extrusion bioprinting, SLA, and DLP have reaped the greatest benefits from the practicality of photoactivated biomaterials so far. The ultimate objective of tissue engineering and regenerative medicine is to successfully construct human organs and tissues. However, the current state of technology falls short of fully achieving the regeneration of organ tissues. Light-based bioprinting uses light as a source of energy for manufacturing, which allows the precise manipulation of photocurable materials, growth factors, and cells, in terms of both space and time, to create intricate structures. Researchers are harnessing bioprinting techniques to emulate complex human tissues in controlled laboratory environments, thus offering promising potential for organ and tissue regeneration. Presently, there is a wealth of research focused on employing light-based 3D bioprinting for the regeneration of diverse tissues, including bone [35], skin [36], liver [37], heart [37][38], blood vessels [39], and so on.
2.1. Light-Based Inkjet 3D Bioprinting
Inkjet bioprinting is derived from the commonly used 2D inkjet printing. Ink droplets are propelled out of a microscopic orifice via thermal or piezoelectric actuation and deposited drop by drop on the platform to fabricate 3D structures. The generated droplets are on the micrometer scale (10–50 μm in diameter) to ensure the printing resolution [40]. After being deposited on the printing platform, the droplet can be gelled simultaneously by physical and chemical (irradiation) processes, thus ensuring printing fidelity [41]. Inkjet bioprinting offers several advantages, including fast printing speed, high cell viability, and low cost. Additionally, the cell viability in the droplet remains high after printing. Furthermore, by incorporating multiple inkjet heads, the fabrication of 3D multimaterial or multicolor structures can be achieved, a feat that is quite challenging with SLA or DLP [42]. Therefore, inkjet printing has gained widespread attention in the field of tissue engineering [26][43][44]. Mugnaini et al. [45] synthesized photocrosslinkable methacrylic pullulan. Aqueous dispersions of methacrylated pullulan were used as the bio-ink. Inkjet printing demonstrated shorter printing times and higher flexibility in printable architectures. Nevertheless, inkjet printing also presents some limitations. Its printing process is often accompanied by the generation of satellite droplets, which negatively impacts the printing.
2.2. Light-Based Extrusion 3D Bioprinting
Due to its simplicity, versatility, reliable nature, and relatively cost-effectiveness, extrusion 3D printing stands as the most commonly used bioprinting technique for fabricating cell-laden hydrogel networks [32][46][47]. The printing process selectively deposits bio-inks, which are composed of cells, biomaterials, growth factors, and other components layer by layer on the printing platform. It can be categorized into pneumatic, piston-driven, and screw-driven dispensing (Figure 2A–C) [48]. Pneumatic dispensing uses air pressure to extrude bio-inks, while piston and screw-driven dispensings use vertical and rotational mechanical forces to extrude bio-inks, respectively. Bio-inks with viscosity ranging from 30 cP to 6 × 107 cP are suitable for extrusion 3D bioprinting. As the extrusion process involves the extrusion of bio-inks from syringes with narrow nozzles or needles, bio-inks possessing shear-thinning characteristics are more favored for the extrusion process [49][50][51]. Nonetheless, extrusion-based printing does have its limitations. The printing resolution of extrusion-based printing is relatively low (>100 μm) when compared to other light-based 3D bioprinting [52]. Moreover, the shear forces produced during the extrusion process can result in reduced cell viability, which becomes particularly evident when higher-density cells are encapsulated.
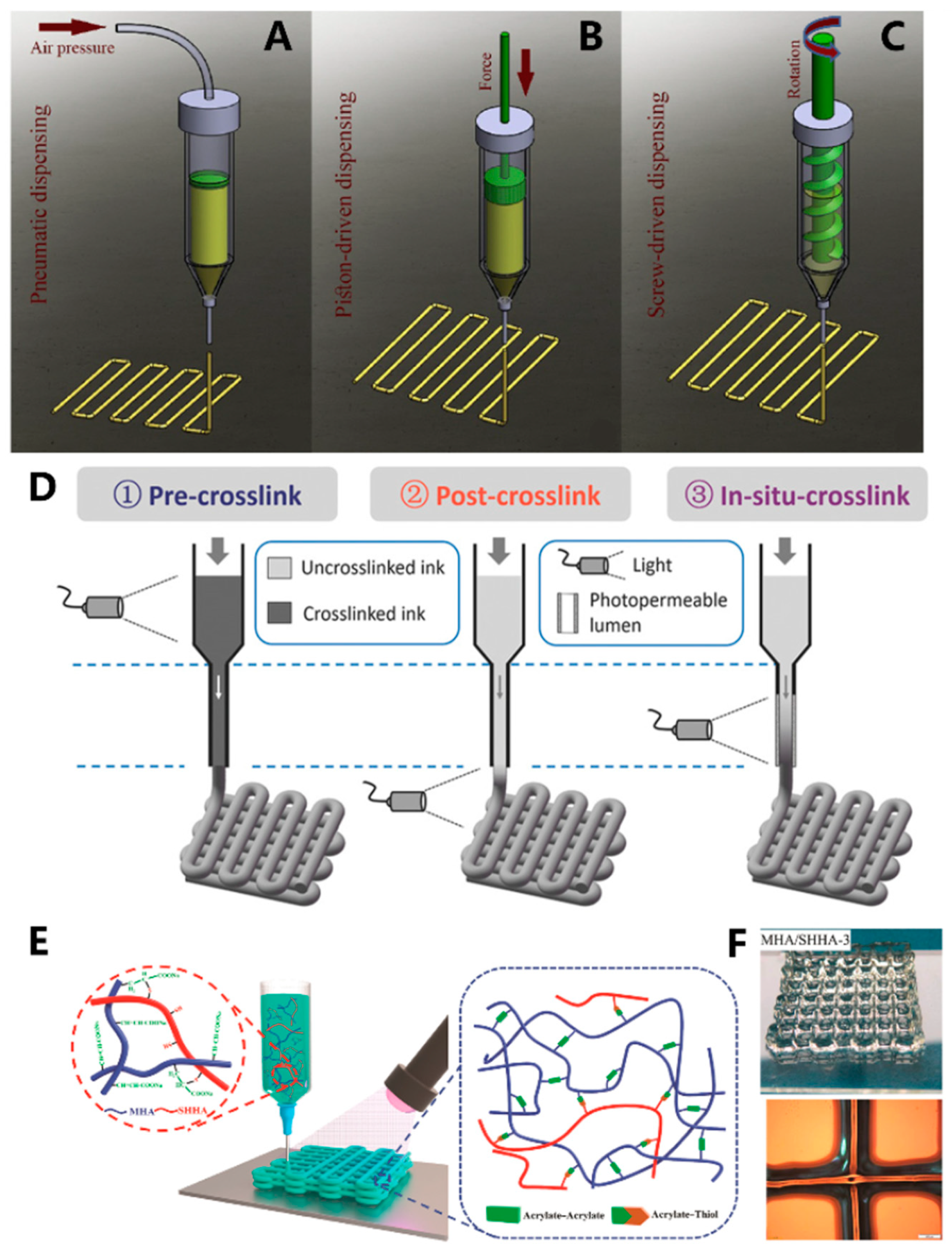
Figure 2. Schematic illustration of the three extrusion bioprinting methods: (A) pneumatic, (B) piston-driven, and (C) screw-driven dispensing method; (D) schematic of three different crosslinking strategies for bioprinting HAMA inks, where crosslinking occurs before, after, or during extrusion; (E) mechanism of the formation MHA/SHHA hydrogels; (F) top: image of the printed MHA/SHHA hydrogel network, down: microscopy image of the printed MHA/SHHA hydrogel.
Light-assisted extrusion bioprinting offers a way to address the shortcomings of conventional extrusion-based 3D printing. Irradiation can be applied during the printing process, after the completion of printing, or after the deposition of each extrusion layer (Figure 1B) [48]. The timing of light exposure depends primarily on the nature of the bio-ink and the stability of each layer. Ouyang et al. [53] utilized a photocurable HAMA-based bio-ink containing mouse embryonic fibroblasts (NIH/3T3) as raw material for 3D bioprinting. They conducted pre-crosslinking (exposure before extrusion), post-crosslinking (exposure after extrusion), and in situ crosslinking (exposure during extrusion) strategies (Figure 2D). The results demonstrated that pre-crosslinked HAMA displayed reduced flowability through the printing nozzle due to prior crosslinking, leading to decreased cell viability (approximately 47%) caused by cell compression. Although post-extrusion light exposure improved cell viability, the low-viscosity HAMA bio-ink led to poor 3D structural formation. By replacing the printing nozzle with a transparent capillary and introducing light exposure during the extrusion process, the HAMA hydrogel can crosslink prior to deposition. The in situ crosslinking method effectively enhances the formability of the bio-ink, reduces the pressure exerted on cells during extrusion, and ultimately raises cell survival rates to above 95%. Wan et al. [54] fabricated a malleated sodium hyaluronate (MHA)/thiolated sodium hyaluronate (SHHA) hydrogel by simultaneous extrusion deposition and thiol-acrylate photopolymerization (Figure 2E). The obtained MHA/SHHA 3D structure showed good structural stability and high resolution (Figure 2F).
2.3. Suspension Bioprinting
To address the issue of poor formability of bio-inks with low viscosity, Lee et al. developed the freeform reversible embedding of suspended hydrogels (FRESH) technology (Figure 3A) [54]. In this technique, bio-ink is extruded into a shear-thinning fluid bed, which can provide adequate support for shaping the bio-ink. The support material is solid at low shear stress and exhibits fluidity at high shear stress. Notably, at low levels of shear stress, the support material maintains its solidity, displaying high viscosity and resistance to deformation. However, as the shear stress increases, the support material becomes more fluid and exhibits reduced viscosity [55]. After the completion of printing, the support material is washed away. Complex structures such as heart valves and heart structures with high precision were fabricated using FRESH (Figure 3B). The printed ventricle exhibited synchronized contraction, and the wall thickness of the ventricle showed a 14% increase in thickness during contraction. Moreover, the heart structure was capable of electrical signal propagation. Wu et al. [56] employed omnidirectional freeform fabrication with sacrifice ink within a photopolymerizable Pluronic F-127–diacrylate matrix to fabricate 3D biomimetic microvascular networks arbitrary designs (Figure 3C). Bhattacharjee et al. [55] used a non-thixotropic granular Carbopol ETD 2020 polymer soft granular gel as the support medium. The medium exhibited local shear thinning and became fluidic near the extrusion nozzle without disturbing neighboring regions. When the nozzle moved away, the gel rapidly solidified. This allows for the fabrication of structures that were difficult to print before. Using this soft gel, the team was able to create intricate large 3D structures (including thin closed shells and hierarchically branched tubular networks) using a variety of materials such as silicones, hydrogels, colloids, and living cells (Figure 3D–F). These printed structures exhibited high fidelity and a high aspect ratio, demonstrating the potential of suspension bioprinting for tissue fabrication, particularly vascular structure fabrication.
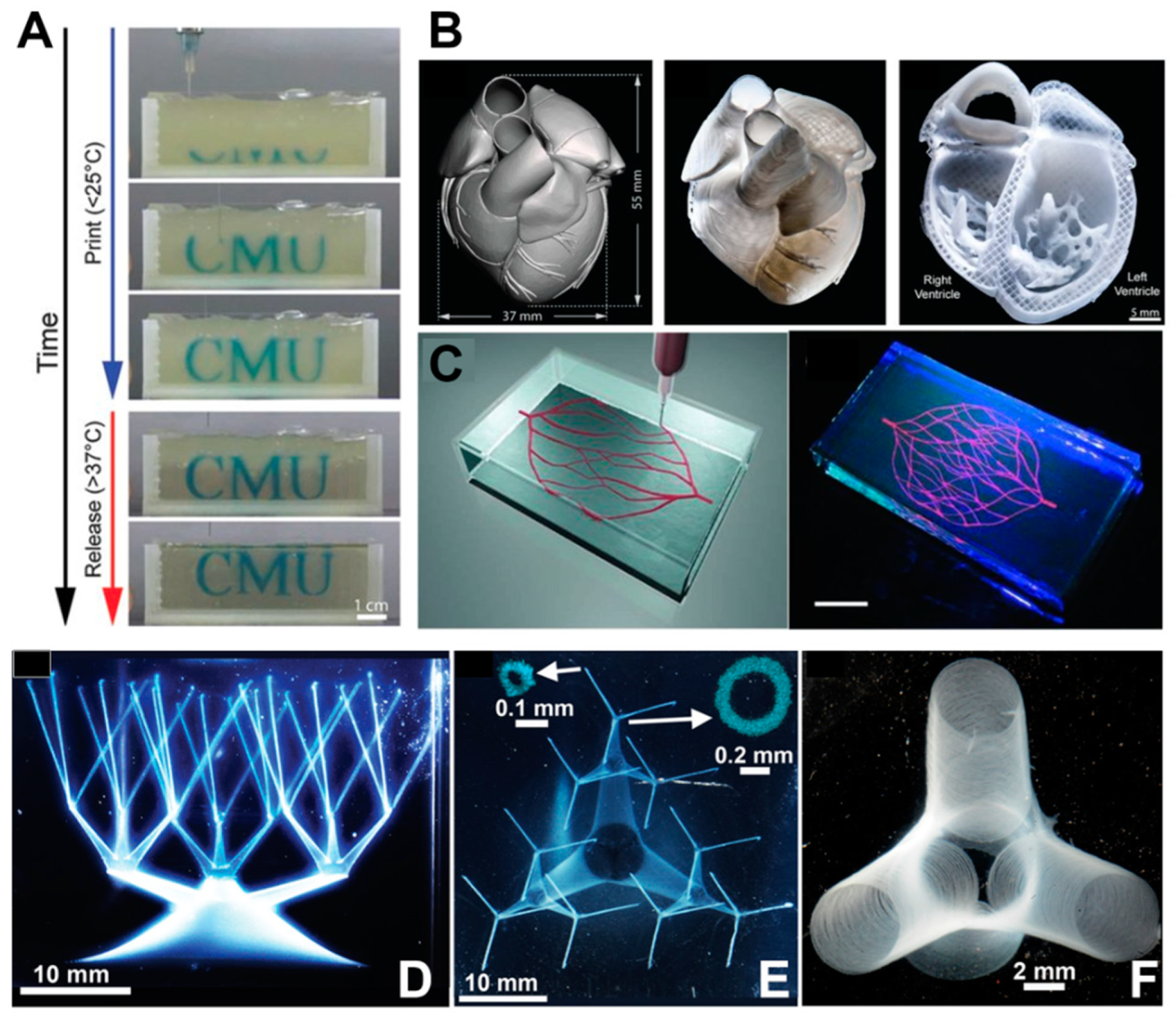
Figure 3. (A) Time-lapse sequence of 3D bioprinting of the letters “CMU” using FRESH v2.0; (B) Left: MRI-derived 3D human heart scaled to neonatal size. Middle: organ-scale FRESH 3D bioprinting of neonatal-scale human heart (middle image). Right: screw-driven dispensing method (right image); (C) Left: schematic illustration of the printing of a vascular structure using omnidirectional printing. Right: fluorescence photograph of the vascular structure printed using omnidirectional printing within a photo-polymerizable Pluronic F-127–diacrylate matrix, scale bar: 10 mm. (D,E) Structures resembling hollow vessels featuring a wide range of sizes in both diameter and aspect ratio. Scale bar: 10 mm. Insets: Confocal cross-sections with a scale of 0.1 mm. (F) An image depicting truncated vessels near a junction exhibited hollow tubes with slender walls, and the diameter of the vessel is about 100 μm, with a scale bar of 2 mm.
2.4. Stereolithography, SLA
SLA, one of the earliest commercialized 3D printing technologies, made its debut in bioprinting in 2004 when Boland’s team at Clemens University employed SLA to craft cell-encapsulated poly(ethylene glycol) diacrylate porous tissue-engineering scaffolds [57]. This marked the inception of SLA’s application in the field of bioprinting. Compared with extrusion printing, where bio-ink within ladened cells is physically extruded on a printing platform, SLA uses a focused laser to selectively solidify the bio-ink layer by layer. As a result, SLA offers advantages, including high spatial resolution (20~50 μm), multiscalability, rapid printing speed of complex structures (lattice and patterned structures), and higher cell viability. Presently, SLA has garnered widespread attention for the fabrication of tissue-engineering scaffolds. Wang et al. [58] utilized an SLA printer equipped with a 500~600 nm laser bioprinted fibroblast-laden GelMA hydrogels featuring complex structures (Figure 4A–D). Eosin Y was employed as the PI. The NIH-3T3 cell encapsulated within the hydrogel demonstrated robust viability and proliferated well to form 3D intercellular networks (Figure 4E). The results indicated that the Eosin Y/GelMA system was suitable for long-duration bioprinting and tissue regeneration. Lam et al. [59] reported a swine-derived chondrocyte-laden photopolymerized HAMA hydrogel network, which was bioprinted by SLA. After culturing for 14 days, cartilage-specific collagen Type II was detected, and cartilage-like tissue was formed. This demonstrated that bioprinted HAMA cartilage may find clinical application in repairing cartilage defects.
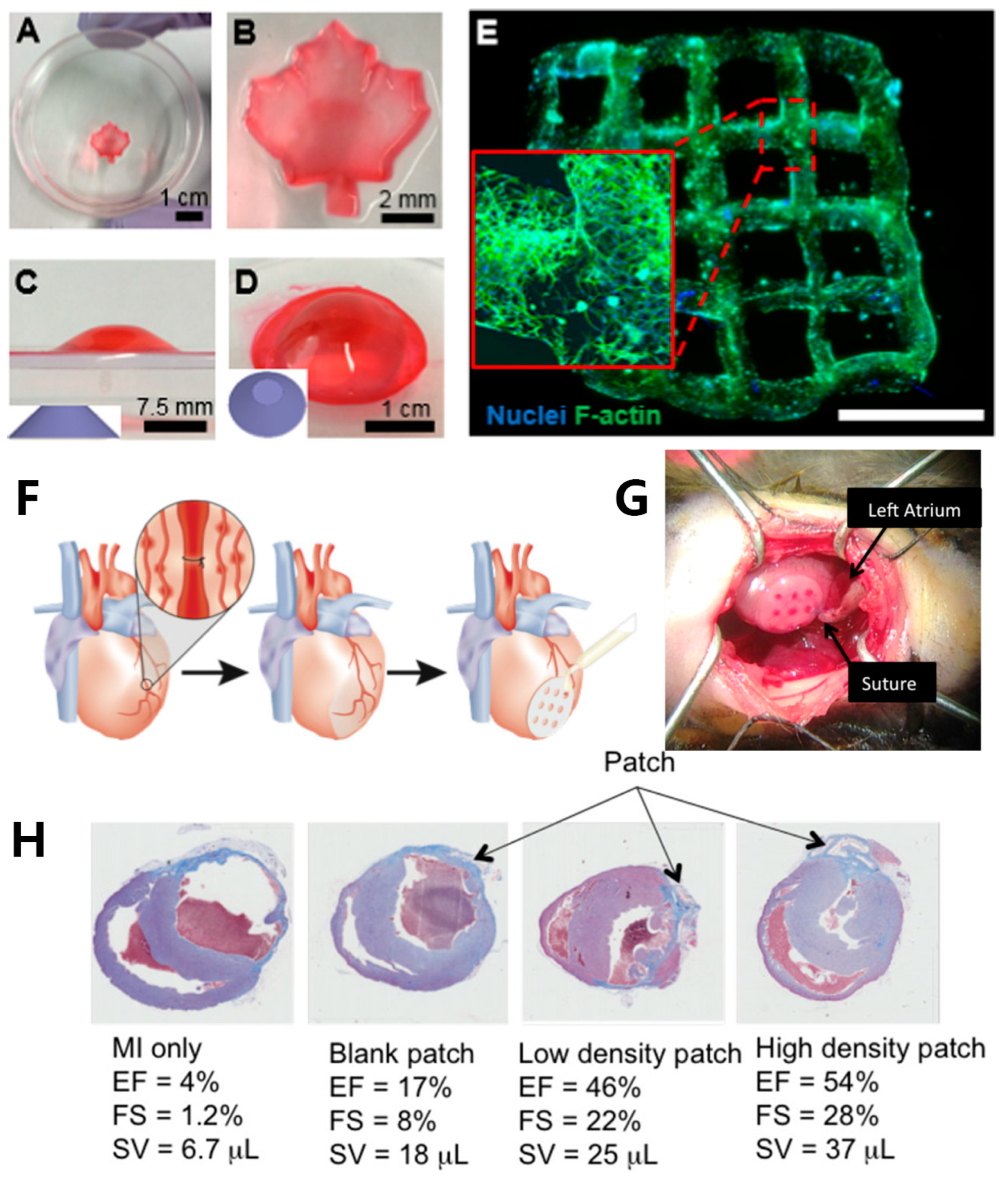
Figure 4. Maple-leaf pattern hydrogel networks fabricated by SLA: (A) top view of the hydrogel; (B) magnified image of the printed hydrogel; Images depicting the truncated cone structure from a lateral perspective (C) and an overhead view (D); (E) bioprinted NIH-3T3 cell-laden hydrogel sample cultured for 5 days, DAPI was used to stain nuclei (blue) and phalloidin 488 was used to stain F-actin (green), scale bar: 2 mm; (F) schematic illustration of the in vivo murine myocardial infarction model and the placement of gel patch; (G) image depicting the positioning of the patch onto the outer surface of the heart (epicardium); (H) histological pictures illustrating the state of each heart condition with different gel patches after 8 weeks.
SLA is well-suited for fabricating microchannel structures, which is challenging to accomplish with extrusion bioprinting. Microchannels play a crucial role in facilitating the efficient transportation of oxygen and nutrients, therefore enhancing cell viability, migration, and proliferation [60]. Melhem et al. [60] utilized SLA to print a cardiac repair patch incorporated microchannels of controlled diameters (500 and 1000 μm) (Figure 4F,G). A bio-ink consisting of a PEGDA solution suspended with mesenchymal stem cells (MSCs) was employed. Ladened with MSCs, the cardiac repair patch consistently released a variety of therapeutic cytokines and exosomes, promoting the effective repair of injured cardiac muscle. Additionally, using a patch with an optimized channel diameter proved to be effective in the reduction of MSC cell loss. In vivo, murine myocardial infarction models were employed to evaluate the efficiency of MSC-laden gel patches containing microchannels. After 8 weeks, untreated mice and those with patches lacking microchannels exhibited left ventricular dilation and wall thinning. In contrast, mice hearts treated with a micro-channeled patch loaded with a higher density of MSCs demonstrated minimal necrosis at the injury site. These treated hearts experienced significantly reduced or negligible ventricular dilation and wall thinning (Figure 4H).
2.5. Digital Light Processing, DLP
DLP is a 3D-printing method that employs a projector based on the digital micromirror device (DMD) or liquid crystal display (LCD) to solidify photoactive bio-inks layer by layer in a pre-designed form (Figure 1B) [32][61]. The fabrication principle endows DLP with several advantages, including high printing speed, excellent accuracy, and improved cell viability. Unlike SLA, DLP utilizes an area light source instead of a laser point, leading to significantly faster printing speeds. Furthermore, DLP employs LED or LCD as a cost-effective alternative to a laser light source. These features position DLP as a highly competitive tissue fabrication method when compared to other printing methods [62][63][64]. Ma and coworkers [65] fabricated a hexagonal GelMA/MeHA hydrogel hepatic model with a stiffness similar to the liver using DLP technology. The model incorporated human-induced pluripotent stem cells (hiPSCs) in conjunction with support human umbilical vein endothelial cells (HUVECs) and adipose-derived stem cells (ADSCs). After 7 days of culture, a more pronounced development of spheroids was observed compared to the model consisting solely of hiPSC-HPCs in the tri-culture 3D model (Figure 5B). In another study, Ma et al. [66] utilized DLP technology to fabricate an in vitro liver model with customizable mechanical properties, serving as a platform to investigate the growth and invasion of hepatocellular carcinoma (HCC) (Figure 5A). The bio-ink, comprising GelMA and liver dECM, employed LAP as the PI. By adjusting the irradiation time (10 s, 20 s, and 40 s), they successfully achieved hexagonal hydrogels with stiffness levels of approximately 0.5 kPa, 5 kPa, and 15 kPa, respectively. These stiffness levels corresponded to different stages of liver cirrhosis (Figure 5C). After 7 days, an increased number of HepG2 cells were observed in the rigid hexagonal scaffold, while fewer HepG2 cells with lower stiffness were observed in the scaffolds (Figure 5C). These results demonstrated the significant potential of the liver model platform for pathophysiological learning and drug screening.
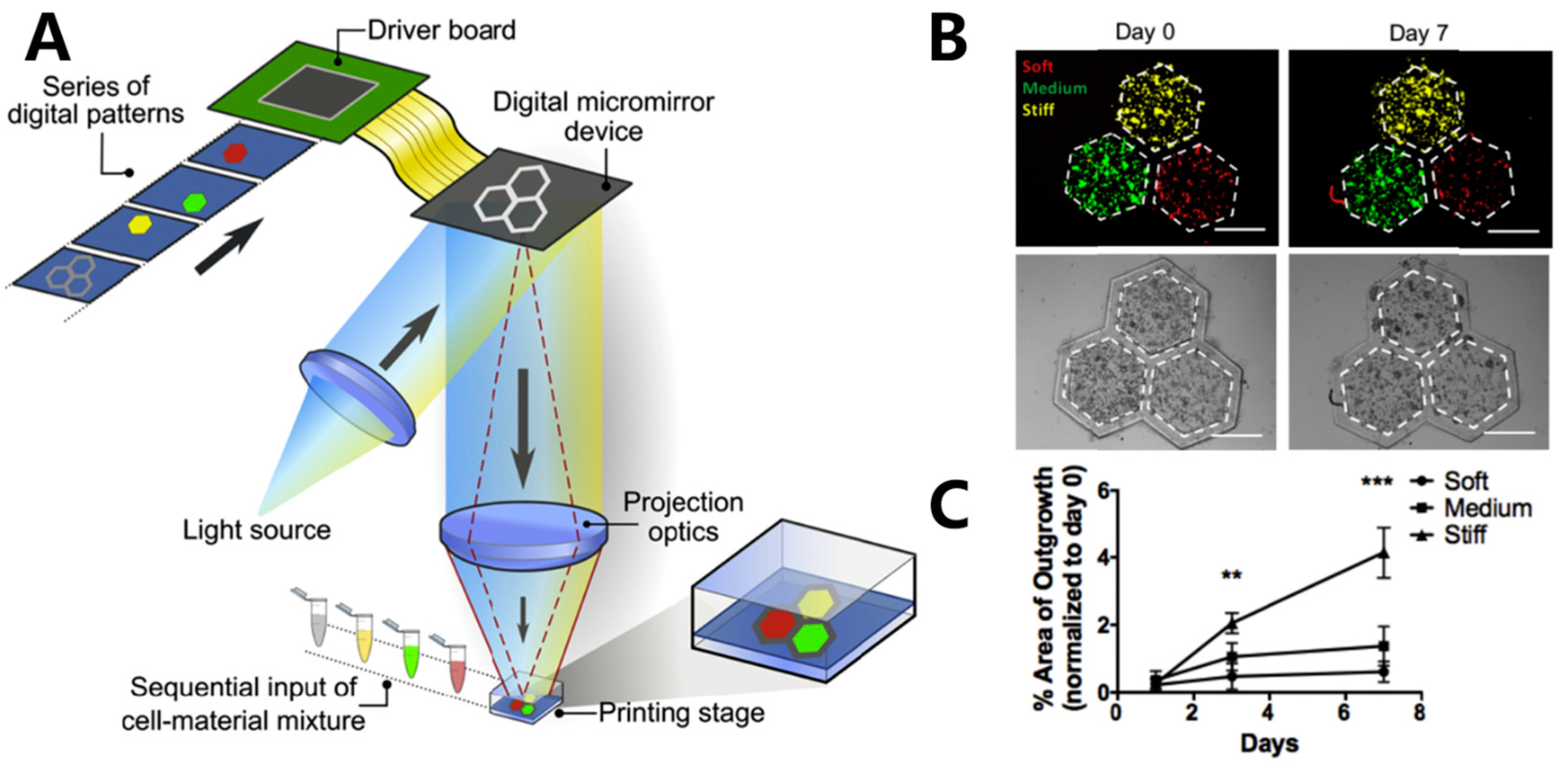
Figure 5. (A) Schematic illustration of the bioprinting of hexagonal cell-laden hydrogel scaffolds; (B) merged fluorescence images (top) and bright-field images (down) Displaying the recorded positions of HepG2 cells in location to the hexagonal areas over 7 days. Red: soft, green: medium, yellow: stiff condition, scale bar: 500 μm; (C) graphical representation illustrating the changing percentage of cell invasion over a period of time, categorized by the three different scaffolds ** p ≤ 0.01, *** p ≤ 0.001.
2.6. Computed Axial Lithography (CAL)
Although DLP is known for its fast printing speed and high printing resolution, it remains constrained by a two-dimensional accumulation process when constructing 3D structures. This manufacturing approach highlights a constraint in enhancing the printing speed of DLP. To address this issue, Taylor’s team drew inspiration from computed tomography (CT) imaging and developed a layerless technique termed computed axial lithography (CAL), or volumetric printing. This technique facilitates the single-step fabrication of complex 3D structures, as depicted in Figure 1B [32][67]. In this technique, a pre-designed sequence of light patterns is projected onto a printing reservoir containing bio-ink. The reservoir rotates around an axis. The light source simultaneously projects various patterns into the bio-ink. The planar light beam selectively cures the photosensitive bio-ink in the printing reservoir. Consequently, through the accumulation of light exposure, specific regions of the photosensitive bio-ink undergo solidification, enabling volumetric fabrication of 3D objects. The CAL technique successfully addressed the constraints associated with SLA and DLP, particularly their inability to print certain types of bio-inks, especially those characterized by high molecular weight and viscosity. CAL is capable of printing bio-inks with viscosities as high as 9 × 104 cP, therefore effectively broadening the spectrum of printable bio-inks in 3D bioprinting. Furthermore, CAL allows for the volumetric printing of large-sized structures, offering the potential for a significant improvement in printing speed.
Moreover, in the conventional layer-by-layer printing process of traditional SLA and DLP, hydrogel materials with lower moduli are prone to deformation or collapse, and the prolonged printing time can result in cell death within the encapsulated constructs. Furthermore, CAL can also avoid stress caused by layer accumulation, therefore enhancing the viability of encapsulated cells. Thus, CAL demonstrated great potential in the rapid fabrication of intricate hydrogel structures.
References
- Langer, R. Biomaterials in drug delivery and tissue engineering: One laboratory’s experience. Acc. Chem. Res. 2000, 33, 94–101.
- Sahara, M. Recent Advances in Generation of In Vitro Cardiac Organoids. Int. J. Mol. Sci. 2023, 24, 6244.
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705.
- Bayes-Genis, A.; Galvez-Monton, C.; Roura, S. Cardiac Tissue Engineering: Lost in Translation or Ready for Translation? J. Am. Coll. Cardiol. 2016, 68, 724–736.
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Healthc. Mater. 2019, 8, 1801236.
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640.
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743.
- Zhang, X.; Zhang, Y.; Ma, G.; Yang, D.; Nie, J. The effect of the prefrozen process on properties of a chitosan/hydroxyapatite/poly(methyl methacrylate) composite prepared by freeze drying method used for bone tissue engineering. RSC Adv. 2015, 5, 79679–79686.
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735.
- Onder, O.C.; Batool, S.R.; Nazeer, M.A. Self-assembled silk fibroin hydrogels: From preparation to biomedical applications. Mater. Adv. 2022, 3, 6920–6949.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Jang, J.; Yi, H.G.; Cho, D.W. 3D Printed Tissue Models: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731.
- Ali, N.B.; Khlif, M.; Hammami, D.; Bradai, C. Optimization of structural parameters on hollow spherical cells manufactured by Fused Deposition Modeling (FDM) using Taguchi method. Cell. Polym. 2021, 41, 3–20.
- Wang, Z.; Song, Q.; Wu, H.; Feng, B.; Li, Y.; Bu, L. Synchronized 3D Printing and Corona Charging for One-Step Prototyping of Polarized Polylactic Acid Electrets. Polymers 2023, 15, 2520.
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199.
- Najihi, I.; Ennawaoui, C.; Hajjaji, A.; Boughaleb, Y. Exploring the piezoelectric porous polymers for energy harvesting: A review. Energy Harvest. Syst. 2023.
- Palaniyappan, S.; Veeman, D.; Sivakumar, N.K.; Natrayan, L. Development and optimization of lattice structure on the walnut shell reinforced PLA composite for the tensile strength and dimensional error properties. Structures 2022, 45, 163–178.
- Jorgensen, A.M.; Yoo, J.J.; Atala, A. Solid Organ Bioprinting: Strategies to Achieve Organ Function. Chem. Rev. 2020, 120, 11093–11127.
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yuksekkaya, M.; Wan, K.T.; et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689.
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168.
- Sun, Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2020, 10, 1577.
- Oh, K.C.; Park, J.M.; Shim, J.S.; Kim, J.H.; Kim, J.E.; Kim, J.H. Assessment of metal sleeve-free 3D-printed implant surgical guides. Dent. Mater. 2019, 35, 468–476.
- Najihi, I.; Ennawaoui, C.; Hajjaji, A.; Boughaleb, Y. 3D printed cellular piezoelectric polymers for smart sensors/autonomous energy harvesters. Mater. Today Proc. 2022, 66, 437–440.
- Mueller, E.; Poulin, I.; Bodnaryk, W.J.; Hoare, T. Click Chemistry Hydrogels for Extrusion Bioprinting: Progress, Challenges, and Opportunities. Biomacromolecules 2022, 23, 619–640.
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab. Chip 2019, 19, 2019–2037.
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833.
- Piironen, K.; Haapala, M.; Talman, V.; Jarvinen, P.; Sikanen, T. Cell adhesion and proliferation on common 3D printing materials used in stereolithography of microfluidic devices. Lab. Chip 2020, 20, 2372–2382.
- Fang, Z.; Shi, Y.; Zhang, Y.; Zhao, Q.; Wu, J. Reconfigurable Polymer Networks for Digital Light Processing 3D Printing. ACS Appl. Mater. Interfaces 2021, 13, 15584–15590.
- Huang, Z.; Chi-Pong Tsui, G.; Deng, Y.; Tang, C.-Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136.
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, e2002931.
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D bioprinting and photocrosslinking: Emerging strategies & future perspectives. Biomater. Adv. 2022, 134, 112576.
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694.
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611.
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762.
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111.
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423.
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13.
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672.
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464.
- Zheng, F.; Derby, B.; Wong, J. Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 2021, 13, 035006.
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978.
- Zaupa, A.; Terraza, C.; Abarzua-Illanes, P.N.; Byres, N.; Zavala, G.; Cuenca, J.; Hidalgo, C.; Viafara-Garcia, S.M.; Wolf, B.; Pino-Lagos, K.; et al. A Psychrophilic GelMA: Breaking Technical and Immunological Barriers for Multimaterial High-Resolution 3D Bioprinting. Biomacromolecules 2023, 24, 150–165.
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816.
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715.
- Mugnaini, G.; Resta, C.; Poggi, G.; Bonini, M. Photopolymerizable pullulan: Synthesis, self-assembly and inkjet printing. J. Colloid. Interface Sci. 2021, 592, 430–439.
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463.
- Compaan, A.M.; Song, K.; Huang, Y. Gellan Fluid Gel as a Versatile Support Bath Material for Fluid Extrusion Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726.
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156.
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264.
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028.
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, e1906423.
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-dimensional Printing for Medical Applications. Int. J. Bioprint 2020, 6, 12–27.
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2016, 29, 1604983.
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487.
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655.
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183.
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322.
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869.
- Lam, T.; Dehne, T.; Kruger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657.
- Melhem, M.R.; Park, J.; Knapp, L.; Reinkensmeyer, L.; Cvetkovic, C.; Flewellyn, J.; Lee, M.K.; Jensen, T.W.; Bashir, R.; Kong, H.; et al. 3D Printed Stem-Cell-Laden, Microchanneled Hydrogel Patch for the Enhanced Release of Cell-Secreting Factors and Treatment of Myocardial Infarctions. ACS Biomater. Sci. Eng. 2017, 3, 1980–1987.
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv. Healthc. Mater. 2020, 9, e2000156.
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395.
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039.
- Gong, J.; Qian, Y.; Lu, K.; Zhu, Z.; Siow, L.; Zhang, C.; Zhou, S.; Gu, T.; Yin, J.; Yu, M.; et al. Digital light processing (DLP) in tissue engineering: From promise to reality, and perspectives. Biomed. Mater. 2022, 17, 062004.
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211.
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321.
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





