Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergio Nogales | -- | 7341 | 2023-12-08 13:14:53 | | | |
| 2 | Mona Zou | Meta information modification | 7341 | 2023-12-11 09:49:21 | | | | |
| 3 | Mona Zou | Meta information modification | 7341 | 2023-12-11 09:49:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nogales-Delgado, S.; Álvez-Medina, C.M.; Montes, V.; González, J.F. Catalysis for Biogas Steam Reforming. Encyclopedia. Available online: https://encyclopedia.pub/entry/52527 (accessed on 08 February 2026).
Nogales-Delgado S, Álvez-Medina CM, Montes V, González JF. Catalysis for Biogas Steam Reforming. Encyclopedia. Available at: https://encyclopedia.pub/entry/52527. Accessed February 08, 2026.
Nogales-Delgado, Sergio, Carmen María Álvez-Medina, Vicente Montes, Juan Félix González. "Catalysis for Biogas Steam Reforming" Encyclopedia, https://encyclopedia.pub/entry/52527 (accessed February 08, 2026).
Nogales-Delgado, S., Álvez-Medina, C.M., Montes, V., & González, J.F. (2023, December 08). Catalysis for Biogas Steam Reforming. In Encyclopedia. https://encyclopedia.pub/entry/52527
Nogales-Delgado, Sergio, et al. "Catalysis for Biogas Steam Reforming." Encyclopedia. Web. 08 December, 2023.
Copy Citation
Hydrogen production from natural gas or biogas, at different purity levels, has emerged as an important technology with continuous development and improvement in order to stand for sustainable and clean energy. Regarding biogas, which can be obtained from multiple sources, hydrogen production through the steam reforming of methane is one of the most important methods for its energy use. In that sense, the role of catalysts to make the process more efficient is crucial, normally contributing to a higher hydrogen yield under milder reaction conditions in the final product.
methane
Ni-based catalysts
sintering
coking
poisoning
promoters
hydrogen
syngas
catalyst support
1. Use of Catalysts in Biogas Steam Reforming
1.1. Main Considerations
In general, the catalyst is located inside a tubular reactor, which can be arranged as a fixed or fluidized bed. The most studied/used configuration is the fixed bed, due to its simplicity and reproducibility, However, it is possible that the deactivation in this configuration is higher than that of the fluidized bed [1], because carbon depositions (between catalyst particles/pellets) are less prevented as the catalyst is immobile. To highlight the recent appearance of new types of reactors in this type of reactions, the photo-thermal ones [2], which can reach H2 production velocities of 17.4 μmol s−1 with an STH efficiency of 22.5% and CO selectivity of 1% in the optimal design under concentrated light irradiance of 16 kW·m−2 in the lab, these reactors are positioned as an alternative to be developed to solve the great disadvantage of energy input.
When studying SMR, it is important to keep in mind that the diffusion of feed biogas and water is homogeneous. For this, the water feed can be performed in liquid form and vaporize inside the reactor, a fact that can lead to a gradient in the concentration of water along the catalytic bed. Another way to introduce water is in the vapor phase, a fact that implies having a vaporizer and a steam flow controller prior to entering the reforming reactor, in addition to avoiding condensation in the steam conduction.
What is perhaps one of the most influential parameters in the effectiveness of the reforming process is the activity of the catalyst. The order of catalytic activities on active metals for SRM has been reported: Rh > Ru > Ni > Ir = Pd = Pt > Co > Fe. In addition, other studies have calculated the TOF in DRM for various metals and showed that the order differs for Al2O3 support and SiO2 support. The order of TOF of the methane reaction rate on each support is presented below [3]: Ni > Ru > Rh, Ir (SiO2 support); Rh > Ni > Ir > Pt, Ru > Co (Al2O3 support). Recent studies have assessed the activity of several composite structured catalysts, showing the following decreasing activity: Rh > Ru > Pt > Ni [4]. In any case, for SRM and DRM, it is common to use Ni catalysts, which are active and inexpensive, supported on metal oxides such as Al2O3, which have a high heat resistance.
Generally, the catalyst consists of an active phase dispersed on a support. The active phase in catalysts for biogas reforming is commonly composed of nickel, whereas the support is usually an aluminosilicate. These materials are normally used due to their catalytic activity, resistance to operating conditions, commercial availability, and versatility. Within aluminosilicates, many types can be found commercially, and in different forms, like powder, spheres of various sizes, and pellets, etc. This variety allows for a great adaptability to the type of reactor, since it is possible to adjust some parameters such as the contact time, charge loss within the reactor, and deactivation, etc. In the following sections, these aspects will be covered.
1.2. Kinds of Catalysts and Their Preparation
Different catalysts can be used in biogas steam reforming, like the following [5]:
-
Monometallic catalysts: they are mainly Ni-based catalysts, which are very popular in the literature due to their great catalytic activity and relatively low cost compared to other equivalent catalysts. However, they have some negative effects (which will be explained in detail in following sections) during steam reforming, such as deactivation due to coke deposition or poisoning.
-
Catalysts with promoters: the abovementioned catalysts can be considerably improved by adding promoters (such as B, Ir, La, or Mg) that can help to improve the global performance during SRM thanks to the improvement of metal–support interaction or the ability to promote a higher dispersion compared to traditional catalysts. Recent works point out the relevance of adding some promoters (La and Mg) to typical Ni/Al2O3 catalysts, in order to improve their catalytic performance. Thus, these additives improved the stability and dispersion of the active phase, with a better deactivation resistance [6].
-
Bimetallic or polymetallic catalysts: to avoid deactivation derived from sintering or coke deposition, the use of combined metallic catalysts could present a positive effect. Bimetallic catalysts are mainly based on Ni or Co combined with noble metals, non-noble metals, or metalloids, whereas polymetallic catalysts are combinations of different metals, like: Ni, Cu, and Zn; Ni, Co, and Ce; and Ni, Ru, and Mg. These combinations can present not only additive, but synergistic effects [7][8].
The main characteristics of these catalysts will be explained in further detail in the following sections, paying attention to different factors such as the catalyst support, active phase, and the interaction between them, which will determine the catalytic performance during methane or biogas steam reforming. For instance, the activity of the resulting catalyst and its resistance to sintering will vary depending on the use of different promoters. In that sense, the preparation of a certain catalyst is vital to understanding some of the final properties of this product. There are different ways to prepare catalysts for this purpose, such as impregnation, co-precipitation, and the sol–gel method.
In the impregnation method (Figure 1 and Figure 2a), a precursor solution is combined with an active solid support phase, and then the solvent is removed by drying. In the application of this method, the solid and the solution are contacted in two ways: wet impregnation (WI) and incipient wet impregnation (IWI) (see Figure 1). In addition, there are dual mechanisms depending on the impregnation method used. WI involves a diffusion process, whereas IWI uses a capillary action method that allows the solution to penetrate pores in the support.
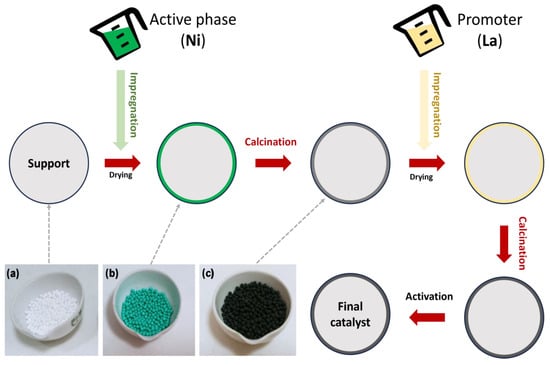
Figure 1. Different stages for catalyst preparation, including images of the most representative ones: (a) catalyst support; (b) wet impregnation with a Ni solution; and (c) calcination.
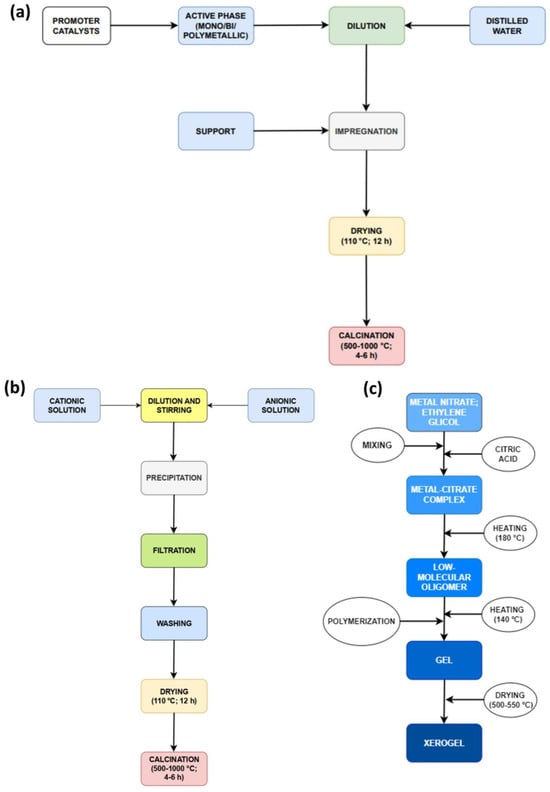
Figure 2. Main steps for different catalyst developments: (a) impregnation; (b) precipitation; and (c) sol–gel.
In wet impregnation, an excess solution is used which is separated from the solid by drying for a certain period of time. During the diffusion process, the composition of the solution changes, forming a residue of impurities and releasing heat due to adsorption in a short time interval. The wet impregnation method is effective for the preparation of the metal catalysts used in methane reforming processes, achieving a high yield and catalyst microstructure. This is the reason why this is the majority catalyst preparation according to the literature. However, a drawback of this method is sintering, especially on catalysts with high oxide loading. In wet impregnation, the metals are dispersed on the surface because the precursor is distributed on the support. This results in high use rates but a low dosage of active metal on the surface, which can result in a non-uniform dispersion of the catalysts [9].
Impregnation by incipient wetting, also known as dry or capillary impregnation, is a method in which the pore volume of the active phase/support is approximately equal to or slightly larger than the volume of the solution. To explain the mechanism of the capillary action of the incipient wetting impregnation method, several reactions occur at different rates. The selective adsorption of charged or uncharged species occurs via H-bonds, van der Waals, or Coulomb forces. Ions are then exchanged between the electrolyte and the charged surfaces, resulting in the polymerization/depolymerization of the ions deposited on the surface. This is followed by the partial dilution of the solid on the surface. After the impregnation of the catalyst into the solid support/active phase, drying and calcination (at different temperatures according to the nature of the active phase) are performed to obtain the desired catalyst material. The products of the impregnation processes are highly dependent on the precursors used, and the parameters that can influence the final mixture include the pH of the solution, the type of solvent, the concentration, and the nature of the dissolved solids [10].
Co-precipitation (Figure 2b) is a versatile method that can be applied to the synthesis of simple, mixed, or supported catalysts [9][11]. Coprecipitation, often referred to as the “one-pot method”, is a conventional approach for synthesizing catalysts in the context of methane steam reforming. The formation of metallic precipitates occurs from oversaturated solutions of their salts. Consequently, all precipitation methods share common components, specifically a soluble source of divalent or trivalent cations and a strong base, such as sodium hydroxide (NaOH), which promotes the precipitation of ions. This process involves mixing metal salts in an aqueous phase with alkaline solutions, resulting in the formation of insoluble metal hydroxides and/or carbonates. During the combination stage, reaction parameters such as temperature, evaporation, salt concentration, and pH stimulate the precipitation process.
These parameters can modify the growth and size of crystallites. The precipitation method consists of five steps: dissolution, precipitation, filtration, drying, and calcination. During the dissolution stage, the active-phase precursors (in salt form) dissolve or hydrolyze in a medium (normally water) to obtain hydroxides in a homogeneous solution. Subsequently, filtration and drying steps are needed, allowing the solids to filter and dry at the boiling temperature of the medium. The dried sample is then crushed, and a binder is added. The appropriate binder is selected to promote easy conversion into vapor and CO2 during calcination and the subsequent activation. The calcination stage uses air (at an optimal temperature) to convert the material from its hydroxide or salt form into oxides. Several publications have resorted to the precipitation method to synthesize the catalyst support, and the catalyst for methane reforming processes [12][13][14][15].
The sol–gel method (Figure 2c) presents a different approach to prepare new materials. Conventional sol preparation involves the hydrolysis and condensation of metal precursors, resulting in a colloid suspension comprising various systems. Colloids are generated when one phase disperses into another, and the dispersed molecules have a dimension between 1 nm and 1 µm [9]. Depending on the kind of solvent, there are two pathways for using the sol–gel method: the aqueous sol–gel method, which refers to the use of water in the reaction, and the non-aqueous sol–gel method, which refers to the use of an organic solvent. In the aqueous method, O2 from water decomposition is necessary for metallic oxide formation.
This method is advantageous due to the high affinity of most precursors for water. However, the main reactions (hydrolysis, condensation, and drying) occur simultaneously, making it difficult to control the particle morphology and process reproducibility. However, this disadvantage is insignificant when preparing metal oxides in bulk.
Thus, the aqueous method can be utilized for preparing bulk metal oxides as opposed to small-scale preparation [16]. In the non-aqueous method, also known as the non-hydrolytic method, the required O2 is provided by solvents (like ketones and alcohols) or metal precursors. The organic solvent also contributes to modifying the process to refine the final properties of the material, such as the morphology, particle size, temperature, and humidity.
Most sol–gel processes use tetraethoxysilane (TEOS) in an aqueous solution, which forms SiO2. This hydrolytic medium is required for hydrolysis and condensation reactions to occur. Hydrolysis is a chemical reaction where silanol (Si-OH) is generated from the reaction between water and an alkoxide (Si-OR), such as TEOS. Si-OH and Si-OR are responsible for the subsequent condensation reactions in the process, resulting in the formation of siloxane in a complex system of competition between hydrolysis and condensation during the intermediate steps of the sol–gel process [17].
Furthermore, the influence of acidic and basic conditions should be considered, as they compete and have their respective peculiarities. The acidic route allows for the syntheses of more branched compounds, whereas the basic route allows for the production of more spherical and compact materials. These parameters are defined around the point of zero charge (PZC), which is determined by the material’s structure and porosity. The pH range of silica is between 1.5 and 4.5, and the condensation of silica species has a limited influence.
Pechini [18] patented a preparation method that adopted the principles used in the sol–gel method with modifications, which employs small molecules and chelating ligands. Initially, a homogeneous solution of metal/citrate complexes is formed in the method and the mixture is then converted into a covalently bonded polymeric matrix, thereby trapping the metal ions. The principle of the Pechini method is to slow down the thermal decomposition of the organic structure to control the resulting material. The primary reaction in this method is the transesterification that occurs between ethylene glycol and citric acid [19]. The Pechini method offers some benefits, including its simplicity, independence from process conditions due to the resulting material’s ion positivity, and the use of a low temperature for precursor treatment, resulting in complete sintering elimination. However, its drawbacks involve the use of toxic ethylene glycol and a significant amount of organic reagents per mass unit [9].
There are other preparation methods that provide interesting catalysts, such as ion exchange, plasma synthesis, or the combination of solution combustion synthesis (SCS) with the wetness impregnation (WI) technique, offering high activities in Rh-based catalysts under typical SR operating conditions [20].
It should be noted that catalytic performance is highly influenced by the preparation method, as the catalyst dispersion and interaction with the support depend on the corresponding procedure, as observed in dry reforming [21]. Consequently, these methods aim to obtain a catalyst with specific characteristics, as explained in the following subsection.
Firstly, the selection of a suitable catalyst support is essential due to its surface characteristics, but also on account of its thermal or mechanical resistance. Also, strong interactions with the active phase are desired to delay deactivation processes.
Second, the active phase will play an essential role in biogas steam reforming, promoting this reaction, as explained in detail in following sections. In that sense, the distribution of this phase on the catalyst support is important, which will be determined by the surface characteristics of the support (pore size distribution) and the preparation method. For instance, in impregnation processes, the concentration of the active-phase precursor in the dilution will determine the final distribution of the active phase to a greater extent, as high concentrations could promote the agglomeration of the active phase, obtaining bigger active sites that usually imply a decrease in the surface area of the final catalyst, with a subsequent lower CH4 conversion. On the other hand, lower concentrations would imply fewer active sites on the surface, which would decrease the hydrogen production. In a sense, an intermediate solution is suitable, taking into account the pore volume of the support and the concentration of the precursor required to cover the surface, avoiding agglomeration.
Finally, the use of bimetallic, trimetallic, or polymetallic catalysts is also advisable to complement the characteristics of typical active sites such as Ni. Also, the use of promoters (who are not directly involved in catalytic activity, but contribute to a suitable performance) is necessary, in order to promote a strong interaction between the active phase and the support. In this sense, the introduction of these components could change the development of the catalyst, as observed in Figure 1 in the case of impregnation, where successive steps should be carried out to introduce the promoter.
1.3. Characteristics of Catalysts
One of the key factors concerning the catalytic steam reforming of biogas is the main characteristics of the catalyst used. As in any field where catalysts are used, their properties should be perfectly adapted to the requirement of the corresponding conversion process. In this case, concepts such as the support (including shape or geometry), active phase (including the interaction with the support, which will determine the sintering or coke deposition resistance), or surface area should be taken into account, as observed in further subsections. It should be noted that the interaction between the active phase and the support is essential for understanding the catalytic performance during biogas steam reforming, as it will determine the resistance of the final catalyst to some factors such as poisoning, carbon deposition, or sintering, among others. Also, the combination of multiple metals in the active phase could improve some properties in the resulting heterogeneous catalyst, especially concerning some factors such as a longer useful life or selectivity towards hydrogen production.
1.3.1. Catalyst Support
The support plays an important role in a suitable catalytic design, as it holds the active phase where the catalytic conversion will take place. In that sense, concerning biogas steam reforming, the nature of this support (normally alumina or silica), its porosity, mechanical resistance, and geometry will allow for a maximum interaction of the gas phase with the solid catalyst, depending on operating conditions such as flow rate and pressure, etc. Figure 3 shows the different shapes of the catalyst supports used for these purposes, with a great interest in spheres and hole catalysts, according to the literature. In any case, other shapes are equally used, proving the versatility of catalytic steam reforming.
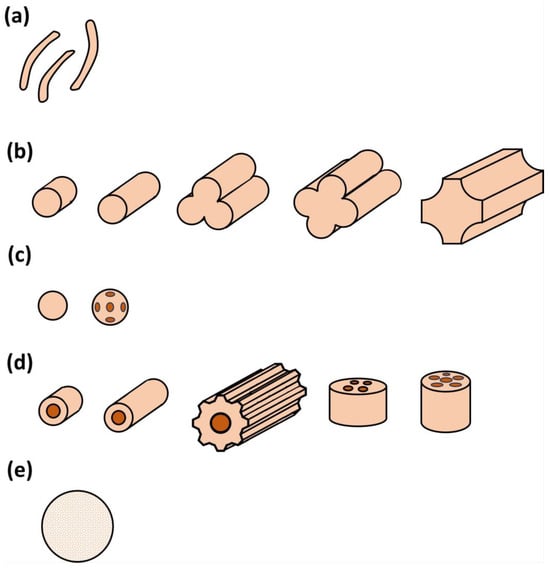
Figure 3. Different shapes of catalyst supports: (a) amorphous pellets; (b) extrudates (solid, hollow, trifolium, and quadrifolium, etc.); (c) sphere carriers, including holes; (d) hollow supports, with single hole cylinders, ribbed cylinders or with multiple holes; and (e) ceramic foam.
Also, there are other alternative supports, like CeO2, which contribute to a better catalytic activity. Recent studies have carried out the combined steam and dry reforming of biogas using a Ni/CeO2-Al2O3 catalyst with a bimodal porous structure. When the CeO2 concentration was 5%, a great catalytic activity was found, thanks to the more intimate contact with alumina and the higher metal–support interaction, preserving it from carbon deposition by 70% [22].
These differences in shapes offer a wide range of SRM conditions. In any case, the maximum interaction between biogas and catalyst is highly desired, trying to avoid the free passage of gas as much as possible. Also, another aspect to be taken into account is the surface of the supports, as it plays a vital role both in catalyst preparation and their corresponding final performance.
Thus, the pore size distribution of different supports (see Figure 4 for different examples in SEM images) can influence the impregnation of active phases during catalyst preparation, whereas the performance of the final catalysts and (including some processes such as coke deposition or sintering) is highly determined by the pore size, whose profile should be selected to favorize a long useful life of the catalyst.
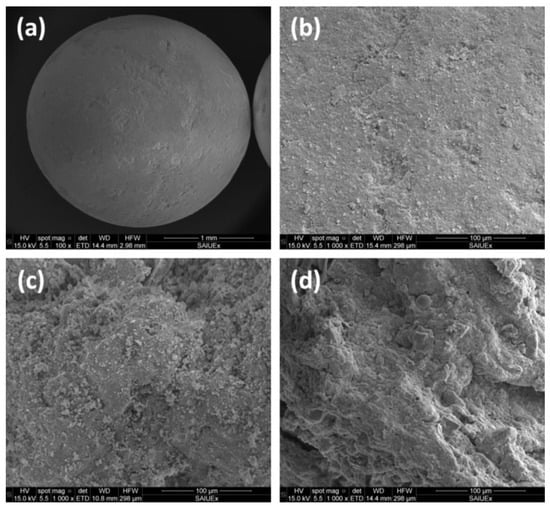
Figure 4. SEM images of a sphere catalytic support (a) and its surface (b), and comparison with other different surfaces: dried sewage sludge (c) and pyrolyzed sewage sludge (d).
Equally, there are other characteristics of the support that should be considered, like its thermal and mechanical stabilities, which are essential in biogas steam reforming for different reasons. Firstly, due to the high temperatures taking place in this process, thermal stability is an ideal prerequisite to avoid surface or even structural changes in catalysts due to thermal shocks. Also, mechanical stability is important to avoid catalyst breakup due to different factors such as friction or high pressures, reducing the amount of detritus within the reactor and subsequent blockages. In that sense, the use of resistant materials like Al2O3 or SiO2 is common, whereas other catalysts based on carbonaceous materials could present some challenges in that regard.
In this regard, innovative works have been carried out where the role of the support is essential. For example, metal-foam-coated Pd–Rh catalysts with variable CeZrO2–Al2O3 support compositions were used in biogas steam reforming, resulting in higher CH4 conversion with the extent of CeZrO2 in the catalyst, a decreasing H2/CO ratio, suppressed coke deposition due to oxygen storage, and an improvement in oxygen reducibility, with an improvement in resistance to the deterioration of surface area, pore structure, and active-phase dispersion [23][24]. Mesoporous catalysts prepared via a reverse precipitation method, Ni2xCe1−xO2 (x = 0.05, 0.13, 0.2), were compared with a commercial catalyst (R67), obtaining a higher H2/CO ratio and excellent activity [25].
1.3.2. Active Phase
Generally, catalysts consist of an active phase, usually a noble metal or acid/base site, deposited and dispersed on a porous support such as alumina, silica, or other material. The solid catalyst’s active phase has a high affinity for molecules of specific reactants. Initially, the molecules chemically attach themselves to the active surface before reacting each other. This way, the activity of catalysts is normally proportional to the number of active sites on the surface. In the case of metal-supported catalysts, the active sites are represented by the exposed metal surface.
Nickel (a transition metal) is commonly used as an active phase in SRM processes due to its availability, low cost, and high activity. However, due to sintering and coke deposition, Ni-based catalysts are subject to rapid deactivation [26].
In addition to nickel, noble metals such as rhodium (Rh), ruthenium (Ru), palladium (Pd), platinum (Pt), and iridium (Ir) show promising potential as candidates for SMR due to their exceptional catalytic abilities and resistance to carbon deposition. Several experimental and numerical studies have reported that the catalytic activity of noble metals could be ordered as Rh∼Ru > Ir > Pt∼Pd [7].
It is important to consider different factors to make the addition of an active phase efficient. This way, catalysts with high activity, low concentrations of active phase, and subsequently high dispersion are desirable for carrying out high conversions in biogas upgrading into syngas. For this purpose, recent studies have proven different Ru-based catalysts (with different supports), with Ru/MgO showing an excellent catalytic performance in the bi-reforming of model biogas due to Ru dispersion with an ultra-small particle size [27]. Consequently, the use of nanoparticles seems to offer a promising outlook in this field. Also, the role of multi-metallic active phases is important for obtaining specific and interesting properties, as in the case of poisoning resistance. Thus, a catalyst (NiCeSnRh/Al2O3) was used in the bi-reforming of biogas, offering a high resistance to sulfur compounds [28].
1.3.3. Advantages and Disadvantages
There is such a wide range of catalysts that can be developed that it is difficult to cover the advantages and disadvantages related to their use. Nevertheless, there seems to be some common patterns, mainly to do with the active phase, which, in many cases, is the main limiting factor when it comes to producing an economically feasible catalyst. In that sense, the role of catalyst in techno-economic assessments in biogas steam reforming is important, as explained in the corresponding section. Essentially, it is a matter of cost–benefit analysis, paying attention to the benefits offered by a specific catalyst and its relative abundance. Regarding the active phase, Ni-based catalysts are popular for this reason, as they offer acceptable catalytic activity at a relatively low cost compared to other metals. However, there are other factors such as a higher propensity for deactivation processes, which could imply operational problems in the medium term, which could be solved with other more expensive catalysts based on Ru or the use of promoters such as La. In other words, life cycle assessments of catalysts for biogas steam reforming are, as in many other cases, essential for obtaining a cost–benefit balance.
Despite their advantages, noble-metal-based catalysts are limited due to their high prices. One way to keep the excellent performance of noble metals while maintaining a reasonable price is to combine two or more types of metals, using cheap transition metals (usually nickel or cobalt) as a base and noble metals as promoters, chemicals that are added to the catalyst in order to improve its catalytic properties. Bi/polymetallic catalysts have gained increasing attention in recent years, and the synergistic effect between commonly used metallic elements has been investigated experimentally and numerically. Numerical studies focus on the reaction pathway and the activation energies of certain reaction steps (specifically, C-H bond breaking during CH4 decomposition), as well as the adsorption energies of atomic or molecular species on the catalyst surface, which are indicators of the catalytic activity and stability of the material [7].
Cobalt is also considered to be a promising promoter in SRM due to its good activity for the WGS reaction, which helps shift the equilibrium towards H2 production. However, a problem related to the use of Co is its tendency to oxidize when the temperature and vapor partial pressure are in the range used for SMR. Alloying it with Ni is a possible solution to this problem while preserving the advantages of both elements [26].
Compared to the relatively simple mono and bimetallic systems, the application of catalysts containing three or more types of active metals in SMR has not been investigated in detail. The existing literature mainly examines Ni-based materials with the addition of two or three commonly used elements, such as Co, Cu, Pt, and Ru, etc. [7].
1.3.4. Catalytic Performance in Biogas Steam Reforming
Thus, after considering the relationship between the support and the active phase, the typical catalytic steam reforming of methane is explained in Figure 5, where the main steps that take place during the process are included.
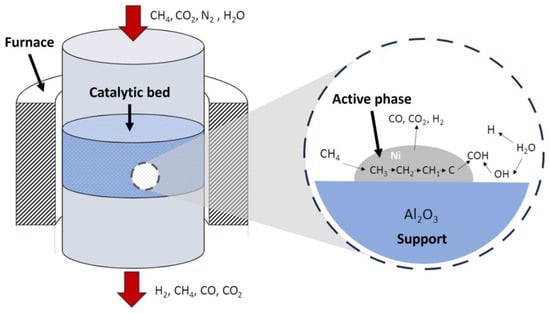
Figure 5. Stages taking place during catalytic steam reforming in a reactor. Example for a Ni-Al2O3 catalyst.
Thus, the kinetic model of methane reforming, shown in Figure 3, is based on the following steps:
-
H2O is adsorbed on the catalyst and dissociates, giving rise to adsorbed oxygen atoms and H2 in the gas phase.
-
CH4 is adsorbed on the catalyst and dissociates, generating CH2 radicals and adsorbed H atoms.
-
The adsorbed CH2 radicals and oxygen react, with bonds being formed and breaking at the same time, generating a transition state (CHO) and H2.
-
The adsorbed CHO dissociates into adsorbed CO and H or reacts with adsorbed oxygen to produce CO2 and H in parallel (controlling stage).
-
The adsorbed CO reacts with adsorbed oxygen to form CO2, or is desorbed to give gas-phase CO.
2. Catalytic Deactivation
One of the main aspects that should be taken into account for a suitable catalytic performance is the life cycle of catalysts during biogas steam reforming, especially in cases such as those with Ni-based catalysts and other heterogeneous catalysts. Indeed, deactivation can be caused by several factors due to mechanical, thermal, or chemical processes [29], that will be explained in following subsections. To a lesser extent, the durability of a catalyst can be affected by other factors, like its kind and shape, or operating conditions like steam to carbon ratio, pressure, or temperature [30].
2.1. Sintering
This is a process due to the agglomeration and growth of metal crystallites of the active phase, occurring at high temperatures. Considering that SRM usually requires high temperatures (above 600 °C), this is an event that should be considered. In that sense, Hüttig and Tamman temperatures determine the atom or crystallite migration for a certain metal, one third and one half of the melting point of the corresponding metal, respectively. Considering the typical operating conditions for biogas SR, the reaction temperatures for this process are high enough to provoke surface and bulk atom migration. Consequently, as included in Figure 6, there are three different stages in sintering on support’s surface.
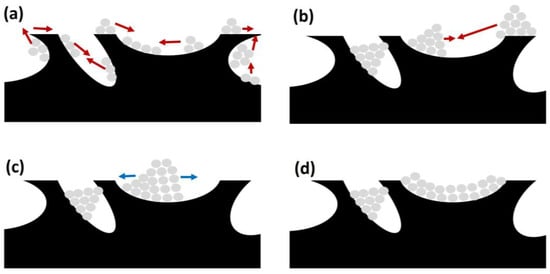
Figure 6. Different stages during sintering of active phase on catalyst support (catalyst support in black and metal particles in grey): (a) migration (red arrows); (b) collision (red arrows); (c) spreading (blue arrows); and (d) blockage (also in (b,c)).
First, atomic migration takes place, with a detachment from the crystallites and migration on the support’s surface, generating bigger metal particles (Figure 6a). Afterwards, these bigger particles or crystallites can also migrate and collide, obtaining bigger particles (see Figure 6b). The process ends with particle spreading on the catalyst surface, as observed in Figure 6c, and blocking active sites.
This way, this phenomenon is related to a decrease in catalytic activity due to two main causes. First, sintering implies a decrease in the surface area of active sites, reducing the efficiency of the catalysts. Second, as observed in Figure 6d, crystallite growth can block pores on the catalyst support, containing further active sites that otherwise would be available for SRM [29][31].
Sintering can also be influenced by other aspects such as the catalyst structure and its porosity and metal–support interactions, promoting strong metal–support interactions that could decelerate the sintering [30].
2.2. Poisoning
Poisoning is due to a strong chemisorption of chemical species on catalytic sites, blocking them and avoiding SRM. There is a wide range of chemical products that can poison catalysts in biogas steam reforming. Among them, one of the most important ones is hydrogen sulfide (H2S), whose content in biogas after biodigestion processes is not negligible, ranging from few ppm up to 1%. High H2S content, apart from a poisoning effect, could be harmful or even deadly, promoting corrosion in industrial facilities, and decreasing the heating value of fuel gas. As a consequence, H2S removal before biogas steam reforming is necessary to avoid a decrease in the global yield [32][33].
Depending on the kind of catalyst, this negative effect could be more noticeable. For instance, Ni is very sensitive to poisoning (see Equation (3)), whereas other metals like Co seem to offer a lower affinity for sulfur, with possible and interesting uses in bimetallic catalysts. Equally, other metals can react with H2S, like Ag, Cu, Fe, Ru, or Pt [34].
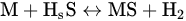
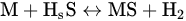
where M can be any abovementioned metal. As a consequence of the interaction of hydrogen sulfide with the active site (through sulfidation), the catalyst cannot take part in steam reforming, partially or completely reducing its activity during the process.
In that sense, adsorption and absorption seem to be suitable techniques for removing hydrogen sulfide before biogas steam reforming, requiring low concentrations for this purpose (up to 5 ppm) before biogas processing in steam reforming facilities [30]. Specifically, the use of alkanolamines (such as methyl ethanolamine or methyl diethanolamine), alkaline salts, organic solvents, deep eutectic solvents, or ionic liquids for absorption, as well as zeolites, metal oxides, or carbon-based sorbents for adsorption, could be interesting treatments for removing H2S under ambient pressure and low operating temperatures [33][35][36][37]. It must be borne in mind that, as previously explained, sewage sludge reuse as an active carbon (obtained through pyrolysis and gasification processes) to adsorb H2S could be an interesting starting point for implementing a circular economy in wastewater treatment plants. Finally, recent studies have proposed a simplified heterogeneous fixed-bed reactor model to simulate the influence of H2S poisoning on Ni-Al2O3 catalysts for methane steam reforming, with a good agreement between the simulated and experimental experiences if an order of deactivation (n = 1) is assumed [38]. These kinds of simulations are quite useful, as they can be easily adapted to different H2S concentrations in biogas and GHSV. Also, the use of bimetallic catalysts to increase poisoning resistance is another interesting aspect to be taken into consideration, as explained in previous studies where the use of a Rh-Ni/Ce-Al2O3 catalyst showed a higher resistance to poisoning, being reversible by using regeneration processes, after which, the catalyst did not show selectivity to the reverse WGS reaction, allowing for high H2 yields [39].
2.3. Carbon Deposition
Coking could represent another negative factor, related to the physical formation of carbon deposits due to gas-phase chemical reactions like methane cracking or CO disproportionation [30]. Carbon deposition can imply the deactivation or blockage of active sites, which decreases the effectiveness of the active phase over reaction time. As observed in Figure 7, successive phases take place during carbon deposition, with different effects depending on its degree of severity.
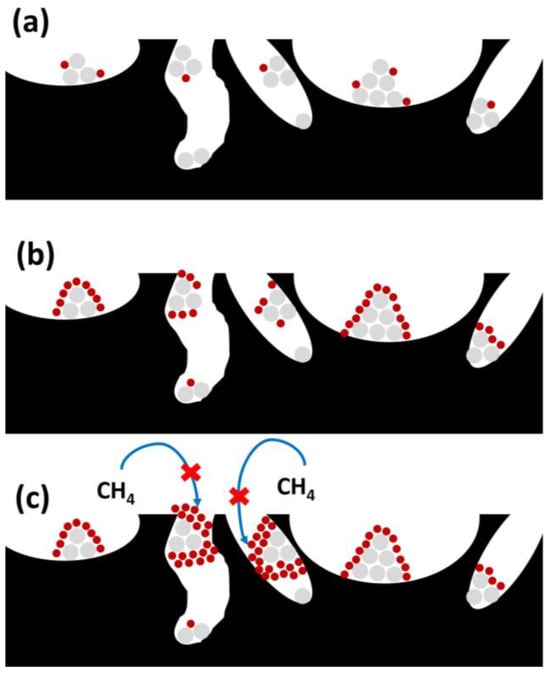
Figure 7. Different stages during coke deposition (coke in red, active phase in grey, and support in black): (a) coke production and chemisorption; (b) coke spreading with the subsequent encapsulation; and (c) active sites and pore blockage.
This way, coke chemisorption or adsorption takes place on active sites (Figure 7a), reducing their access to reactants. In further stages, coke diffusion or dispersion to generate active site encapsulation occurs (as observed in Figure 7b), completely blocking the active sites to reactants, and pore blockage takes place (Figure 7c), hampering the SRM reaction on the available active sites. This fact takes place especially when the CO and CH4 decomposition is faster than the carbon removal.
To avoid this phenomenon, the use of promoters to strengthen the metal–support interactions could present a positive effect. Also, the particle size of the active phase could play an important role in controlling coke deposition [30].
4. Key Points to Improve the Performance of Biogas Steam Reforming
Considering the previous section, it is essential to take steps to solve deactivation processes, which hinders a suitable catalytic performance (with high methane conversions) and long service life. These steps could be taken, as observed in Figure 8, before, during, or after biogas steam reforming. As mentioned earlier (as it will be discussed throughout this text), every detail counts when it comes to contributing to a better catalytic performance in this process. Each stage is explained in the following subsections. It should be noted that these steps directly affect the performances of catalysts during steam reforming, but other aspects related to this process could be equally improved, such as the membrane reactor performance (by reducing the coke deposition or hydrogen sulfide content) or the maintenance of steam reforming facilities (delaying corrosion with a decrease in H2S content). Nonetheless, other factors could be affected, implying efficiency loss and increases in costs.
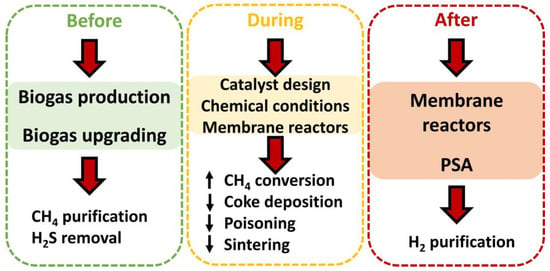
Figure 8. Steps carried out to improve the performance (through better conversion or longer useful life) of catalysts in steam reforming.
2.1. Steps before Steam Reforming (Biogas Production and Upgrading)
Regarding the previous steps, there are many processes that could improve methane production during the anaerobic digestion of biomass, as well as biogas upgrading to increase methane concentration, avoiding undesirable compounds such as hydrogen sulfide. As explained in the introduction section, there are plenty of measures for increasing the efficiency of biogas production and quality. Thus, the use of additives (such as microorganisms, enzymes, or inorganic compounds) could facilitate different steps during anaerobic digestion by removing inhibitors (such as ammonia, long-chain fatty acids, and acidification caused by VFAs, etc.) and creating suitable conditions for microorganism proliferation [40]. In this sense, the use of active carbons obtained from digestates could be interesting (such as sewage sludge, whose active carbon obtained through hydrothermal carbonization could be a promising starting point for valorizing this waste, as explained in previous works [41]), as it could be an example of an applied circular economy during biodigestion. On the other hand, biogas upgrading is essential for removing H2S (which could deactivate catalysts through poisoning) and for increasing the CH4 concentration in biogas (which is desirable for carrying out a more efficient SRM). For the former, adsorption systems are usually selected (for instance, activated carbons or nanoparticles), although there are many techniques for retaining H2S, such as biological desulfurization, membrane separation (with polymeric membranes, normally capable of retaining CO2 and H2S [42]), or absorption with inorganic solutions (many of them based on iron) [32][33][43][44]. Also, some techniques such as PSA can be used to upgrade biogas by increasing methane content [45].
2.2. Steps during Steam Reforming (Catalyst Design, Chemical Conditions, Use of Membrane Reactors)
2.2.1. Catalyst Design (Promoters and Bi-Metallic Catalysts)
Regarding the catalyst design, if a certain catalyst is selected, with the aim of achieving the maximum conversion and stability, the catalyst should present as much dispersion of the active phase as possible, as there is not a minimum particle size from which a decrease in activity is found.
Another important factor is avoiding active-phase mobility and agglomeration, promoting that the active phase is dispersed enough to reduce the possibility of collision with other particles and the subsequent agglomeration. It is achieved by reducing the active phase concentration, whereas the number of active sites decrease. The typical Ni concentration in catalysts is 20% w/w, obtaining particle sizes from 10 to 100 nm.
In addition, feeding should be considered in catalyst design. In other words, catalyst deactivation by the pollutants included in biogas should be reduced. To reduce the effects related to coke deposition, the active phase surface on the support should be maximized, so that the diffusion of gas is enough to avoid a reducing atmosphere that allows for CO and CO2 decomposition, producing carbon which is placed on the active phase surface and generating carbide and coke.
The use of promoters in the active phase has been studied and reviewed, with some common additives such as alkalis (K and Na), transition metals (La, Zr, and Zn), and non-metals (Al and B) [46][47].
An interesting issue with room for improvement is the resistance of catalysts to poisoning due to H2S. This compound is usually removed through previous adsorption before steam reforming in a reactor [48], and few scientific articles have dealt with an increase in the resistance of catalysts to this pollutant. Some studies have focused on reviewing the influence of sulfur content in feeding for several processes [49]. As a conclusion, Ni seems to be a catalyst with a difficult direct protection from poisoning, with noble metals offering better results, except for Rh. On the other hand, there is a possibility of using metals from groups 4 to 6, through bifunctional catalysts, which could be a promising alternative.
2.2.2. Operating Conditions
There are several influences on catalytic performance depending on the operating conditions, mainly related to the promotion of deactivation processes [50]. Taking into account that methane steam reforming is an endothermic reaction (see Equation (1)), the effect of temperature is clear, with higher CH4 conversions with temperature (as observed in specific cases such as the catalytic steam reforming of biogas with a Rd catalyst) [51]. However, intermediate solutions should be achieved, as extra energy costs associated with keeping the reactor temperature should be avoided, as explained in following sections.
Concerning steam addition, high S/C ratios (at least 1.5, achieving excess of feed vapor) are recommended to avoid coke deposition, among other factors like high pressure [52][53]. However, from an economic point of view, the production of large quantities of superheated vapor would imply a considerable increase in costs [54]. Additionally, some studies have pointed out the possible catalyst deactivation on time-on-stream, especially at high temperatures, observing a direct correlation between deactivation rates and high S/C, mainly due to the steam-induced metal–support interaction, resulting in an inactive spinel phase and not due to metal reoxidation [55].
Also, as previously explained, temperature presents opposite effects. On the one hand, high temperatures would imply a sintering effect, whereas low temperatures could promote coke deposition. In the case of steam and temperature, optimization and intermediate steps should be considered, because intermediate conditions to meet both low energy costs and high methane conversions should be obtained (apart from the obvious effects on catalytic performance in biogas steam reforming).
Equally, the CH4/CO2 ratio in biogas seems to present an influence on catalytic performance. In that sense, according to recent studies using two different catalysts (4% Ni/NiAl2O4/Al2O3 and 3.1% Ru/Al2O3), an increase in CO2 implied a decrease in H2/CO ratio and H2 yield, finding an optimal CH4/CO2 ratio of 1.5/1 [56]. Therefore, CH4/CO2 ratios above 1 are advisable for a suitable catalytic performance, as explained in previous studies for Ni-based catalysts, possibly due to a CO2-promoted Boudouard reaction, implying further coke deposition [57].
At this point, it is essential to consider the optimization and modelling of steam reforming for any specific case, as will be discussed in future sections.
2.2.3. Membrane Reactors
The use of membrane reactors is another interesting starting point for improving the performance of hydrogen production from biogas through steam reforming. Thus, the aim of this technology is to purify the hydrogen obtained during steam reforming using thin membranes (the selective layer is usually made of Pd and Ag on different kinds of supports, like stainless steel or alumina) where H2 permeates, to obtain a final gas with a high purity (up to 95–99%) [58][59]. Figure 9 shows the different stages that take place during biogas (or methane, in a simplified form in this case), including the reactant inlet, H2 permeation through the membrane, and, finally, the retentate and permeate outlets [60].
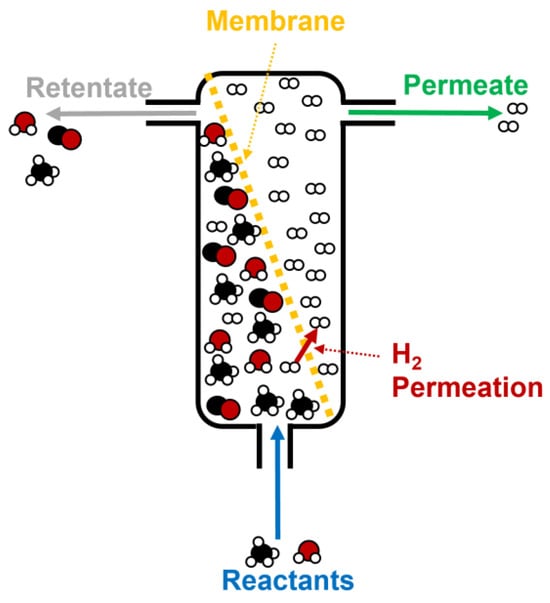
Figure 9. Scheme of a standard membrane reactor for hydrogen production during methane steam reforming.
It should be noted that the catalyst is usually put into contact with the membrane, in order to assure that the chemical reactions (and the subsequent products, like H2) are as close as possible to the membrane.
This way, the chemical balance of the reactions observed in Equations (1) and (2) could be oriented towards product generation, as hydrogen is rapidly removed from the reaction medium to be delivered in a highly pure gas stream (permeate), whereas the rest of the products (mainly CO and CO2, along with unreacted CH4 and H2O) are separated in another stream (retentate), which can be further treated to obtain higher yields in hydrogen [61]. This fact is very interesting, as some chemical conditions could be softened to obtain a higher efficiency during biogas steam reforming.
In that sense, temperature and pressure could be lowered, whereas the catalyst design can vary (for instance, the active phase in catalysts could be reduced, with the subsequent savings for this process). Regarding temperature decrease, it could imply a positive aspect for catalyst deactivation, as sintering effects could be delayed at lower temperatures, and hydrogen recovery in a membrane reactor is usually improved [62].
However, coke deposition could be promoted at low temperatures, which could present a reverse impact in methane steam reforming [63]. As in the case of many catalysts, H2S present negative effects in membrane reactors, as Pd poisoning (the most popular element used in membranes) would provoke a progressive loss in separating performance [58][59].
2.3. Steps after Biogas Steam Reforming (Hydrogen Purification)
Even though there is not a clear and direct link between purification processes once biogas is converted into synthesis gas (a mixture of H2 and CO, among other compounds) and their influence on catalyst performance, some indirect positive effects could be found.
As observed in Figure 10, there are different biogas steam reforming configurations to carry out hydrogen production with a high purity. In that sense, considering the first route (Figure 10a), the use of membrane reactors in situ could contribute, as explained in the previous subsection, to shifting the reaction balance towards product generation, with the subsequent possibility of decreasing chemical conditions such as temperature and, consequently, a delay in deactivation processes such as sintering.
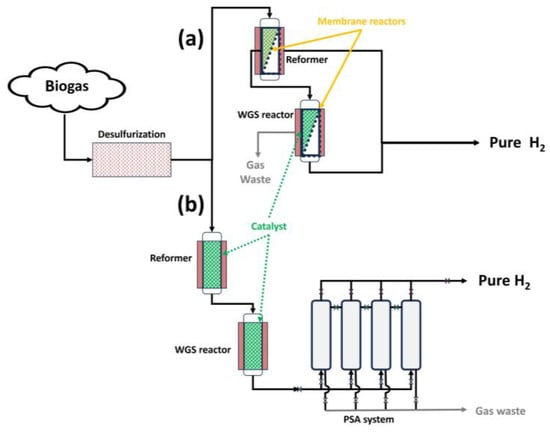
Figure 10. Different routes for biogas steam reforming to obtain high-purity hydrogen: (a) through membrane reactors; and (b) through PSA.
However, in Figure 10b, the purification process (in this case, PSA, where an adsorbent is used to bind molecules depending on the gas component, type of adsorbent material, partial pressure, and operating temperature [64][65]) takes place after the products are obtained, with no direct effect on the chemical balance.
Also, other configurations (due to economic adaptations of previous steam reforming facilities) could couple membranes after steam reforming to obtain pure hydrogen, presenting a similar situation compared to the use of PSA.
In these situations, an improvement in chemical conditions can be equally found, considering the high-quality product obtained (normally >98% hydrogen, which is highly valuable in the energy market). In this context, lower reaction temperatures or catalyst concentrations could be used, offsetting the lower conversion with a valuable final product and with the possibility of recirculating gas waste in the same process or other processes. Also, biogas can be converted to synthesis gas, which can be used in other processes such as Fischer–Tropsch, where different H2/CO ratios (even at lower temperatures compared with normal steam reforming conditions to optimize hydrogen production) can be used to obtain, for instance, liquid fuels [66]. Under these circumstances, the catalyst could be less deactivated, especially if these steps are combined with the use of a suitable catalyst support or the use of promoters, which could equally improve the final conversion of methane in biogas.
References
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35, 1160–1190.
- Li, D.; Sun, J.; Zhang, Z.; Ma, R.; Wei, J. High-Efficient Sunlight-Driven Hydrogen Production from Methanol Steam Reforming on a Novel Photo-Thermo-Catalysis and Thermo-Catalysis Dual-Bed Reactor. Fuel 2024, 357, 129895.
- Ferreira-Aparicio, P.; Rodríguez-Ramos, I.; Anderson, J.A.; Guerrero-Ruiz, A. Mechanistic Aspects of the Dry Reforming of Methane over Ruthenium Catalysts. Appl. Catal. A Gen. 2000, 202, 183–196.
- Ruban, N.V.; Rogozhnikov, V.N.; Stonkus, O.A.; Emelyanov, V.A.; Pakharukova, V.P.; Svintsitskiy, D.A.; Zazhigalov, S.V.; Zagoruiko, A.N.; Snytnikov, P.V.; Sobyanin, V.A.; et al. A Comparative Investigation of Equimolar Ni-, Ru-, Rh- and Pt-Based Composite Structured Catalysts for Energy-Efficient Methane Reforming. Fuel 2023, 352, 128973.
- Schiaroli, N.; Battisti, M.; Benito, P.; Fornasari, G.; Di Gisi, A.G.; Lucarelli, C.; Vaccari, A. Catalytic Upgrading of Clean Biogas to Synthesis Gas. Catalysts 2022, 12, 109.
- Dan, M.; Mihet, M.; Borodi, G.; Lazar, M.D. Combined Steam and Dry Reforming of Methane for Syngas Production from Biogas Using Bimodal Pore Catalysts. Catal. Today 2021, 366, 87–96.
- Wang, S.; Nabavi, S.A.; Clough, P.T. A Review on Bi/Polymetallic Catalysts for Steam Methane Reforming. Int. J. Hydrogen Energy 2023, 48, 15879–15893.
- Saeidi, S.; Sápi, A.; Khoja, A.H.; Najari, S.; Ayesha, M.; Kónya, Z.; Asare-Bediako, B.B.; Tatarczuk, A.; Hessel, V.; Keil, F.J.; et al. Evolution Paths from Gray to Turquoise Hydrogen via Catalytic Steam Methane Reforming: Current Challenges and Future Developments. Renew. Sustain. Energy Rev. 2023, 183, 113392.
- Osazuwa, O.U.; Abidin, S.Z.; Fan, X.; Amenaghawon, A.N.; Azizan, M.T. An Insight into the Effects of Synthesis Methods on Catalysts Properties for Methane Reforming. J. Environ. Chem. Eng. 2021, 9, 105052.
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082.
- Perego, C.; Villa, P. Catalyst Preparation Methods. Catal. Today 1997, 34, 281–305.
- Rotaru, C.G.; Postole, G.; Florea, M.; Matei-Rutkovska, F.; Pârvulescu, V.I.; Gelin, P. Dry Reforming of Methane on Ceria Prepared by Modified Precipitation Route. Appl. Catal. A Gen. 2015, 494, 29–40.
- Świrk Da Costa, K.; Zhang, H.; Li, S.; Chen, Y.; Rønning, M.; Motak, M.; Grzybek, T.; Da Costa, P. Carbon-Resistant NiO-Y2O3-Nanostructured Catalysts Derived from Double-Layered Hydroxides for Dry Reforming of Methane. Catal. Today 2021, 366, 103–113.
- Mallikarjun, G.; Sagar, T.V.; Swapna, S.; Raju, N.; Chandrashekar, P.; Lingaiah, N. Hydrogen Rich Syngas Production by Bi-Reforming of Methane with CO2 over Ni Supported on CeO2-SrO Mixed Oxide Catalysts. Catal. Today 2020, 356, 597–603.
- Florea, M.; Matei-Rutkovska, F.; Postole, G.; Urda, A.; Neatu, F.; Pârvulescu, V.I.; Gelin, P. Doped Ceria Prepared by Precipitation Route for Steam Reforming of Methane. Catal Today 2018, 306, 166–171.
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800.
- Rex, A.; dos Santos, J.H.Z. The Use of Sol–Gel Processes in the Development of Supported Catalysts. J. Sol-Gel Sci. Technol. 2023, 105, 30–49.
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3,330,697, 11 July 1967.
- Lin, J.; Yu, M.; Lin, C.; Liu, X. Multiform Oxide Optical Materials via the Versatile Pechini-Type Sol−Gel Process: Synthesis and Characteristics. J. Phys. Chem. C 2007, 111, 5835–5845.
- Vita, A.; Italiano, C.; Ashraf, M.A.; Pino, L.; Specchia, S. Syngas Production by Steam and Oxy-Steam Reforming of Biogas on Monolith-Supported CeO2-Based Catalysts. Int. J. Hydrogen Energy 2018, 43, 11731–11744.
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.H.; Ali Khan, M.; Park, Y.K. Influence of Catalyst Synthesis Methods on Anti-Coking Strength of Perovskites Derived Catalysts in Biogas Dry Reforming for Syngas Production. Chem. Eng. J. 2022, 437, 135348.
- Dan, M.; Mihet, M.; Barbu-Tudoran, L.; Lazar, M.D. Biogas Upgrading to Syngas by Combined Reforming Using Ni/CeO2–Al2O3 with Bimodal Pore Structure. Microporous Mesoporous Mater. 2022, 341, 112082.
- Roy, P.S.; Kang, M.S.; Kim, K. Effects of Pd-Rh Composition and CeZrO2-Modification of Al2O3 on Performance of Metal-Foam-Coated Pd-Rh/Al2O3 Catalyst for Steam Reforming of Model Biogas. Catal. Lett. 2014, 144, 2021–2032.
- Roy, P.S.; Song, J.; Kim, K.; Kim, J.M.; Park, C.S.; Raju, A.S.K. Effects of CeZrO2–Al2O3 Support Composition of Metal-Foam-Coated Pd–Rh Catalysts for the Steam-Biogas Reforming Reaction. J. Ind. Eng. Chem. 2018, 62, 120–129.
- Lin, K.H.; Chang, H.F.; Chang, A.C.C. Biogas Reforming for Hydrogen Production over Mesoporous Ni2xCe1-XO2 Catalysts. Int. J. Hydrogen Energy 2012, 37, 15696–15703.
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G.; Dogu, T. Enhancement of Catalytic Performance of Ni Based Mesoporous Alumina by Co Incorporation in Conversion of Biogas to Synthesis Gas. Appl. Catal. B 2016, 198, 254–265.
- Zhong, J.; Han, B.; Zhang, Z.; Bi, G.; Xie, J. Bi-Reforming of Model Biogas to Syngas over Ru Nano-Catalysts Supported on Mg-Al Oxide Derived from Layered Double Hydroxides. Fuel 2023, 343, 127941.
- Poggio-Fraccari, E.; Mariño, F.; Herrera, C.; Larrubia-Vargas, M.Á.; Alemany, L. Bi-Reforming of Biogas for Hydrogen Production with Sulfur-Resistant Multimetallic Catalyst. Chem. Eng. Technol. 2023, 46, 1176–1184.
- Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Olazar, M.; Bilbao, J. Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review. Energy Fuels 2021, 35, 17051–17084.
- Farooqi, A.S.; Yusuf, M.; Mohd Zabidi, N.A.; Saidur, R.; Sanaullah, K.; Farooqi, A.S.; Khan, A.; Abdullah, B. A Comprehensive Review on Improving the Production of Rich-Hydrogen via Combined Steam and CO2 Reforming of Methane over Ni-Based Catalysts. Int. J. Hydrogen Energy 2021, 46, 31024–31040.
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam Reforming of Methane: Current States of Catalyst Design and Process Upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330.
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219.
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448.
- Capa, A.; González-Vázquez, M.P.; Chen, D.; Rubiera, F.; Pevida, C.; Gil, M.V. Effect of H2S on Biogas Sorption Enhanced Steam Reforming Using a Pd/Ni-Co Catalyst and Dolomite as a Sorbent. Chem. Eng. J. 2023, 476, 146803.
- Rahim, D.A.; Fang, W.; Wibowo, H.; Hantoko, D.; Susanto, H.; Yoshikawa, K.; Zhong, Y.; Yan, M. Review of High Temperature H2S Removal from Syngas: Perspectives on Downstream Process Integration. Chem. Eng. Process.-Process Intensif. 2023, 183, 109258.
- Ozekmekci, M.; Salkic, G.; Fellah, M.F. Use of Zeolites for the Removal of H2S: A Mini-Review. Fuel Process. Technol. 2015, 139, 49–60.
- Secco, C.; Fuziki, M.E.K.; Tusset, A.M.; Lenzi, G.G. Reactive Processes for H2S Removal. Energies 2023, 16, 1759.
- Fabrik, M.; Salama, A.; Ibrahim, H. Modeling of Catalyst Poisoning during Hydrogen Production via Methane Steam and Dry Reforming. Fuel 2023, 347, 128429.
- Izquierdo, U.; García-García, I.; Gutierrez, Á.M.; Arraibi, J.R.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Catalyst Deactivation and Regeneration Processes in Biogas Tri-Reforming Process. The Effect of Hydrogen Sulfide Addition. Catalysts 2018, 8, 12.
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of Additives to Improve Collective Biogas Plant Performances: A Comprehensive Review. Biotechnol. Adv. 2023, 65, 108129.
- González, J.F.; Álvez-Medina, C.M.; Nogales-Delgado, S. Biogas Steam Reforming in Wastewater Treatment Plants: Opportunities and Challenges. Energies 2023, 16, 6343.
- Roozitalab, A.; Hamidavi, F.; Kargari, A. A Review of Membrane Material for Biogas and Natural Gas Upgrading. Gas Sci. Eng. 2023, 114, 204969.
- Cattaneo, C.R.; Muñoz, R.; Korshin, G.V.; Naddeo, V.; Belgiorno, V.; Zarra, T. Biological Desulfurization of Biogas: A Comprehensive Review on Sulfide Microbial Metabolism and Treatment Biotechnologies. Sci. Total Environ. 2023, 893, 164689.
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A. Pietro Hydrogen Sulfide Adsorption by Iron Oxides and Their Polymer Composites: A Case-Study Application to Biogas Purification. Materials 2020, 13, 4725.
- Abd, A.A.; Othman, M.R.; Majdi, H.S.; Helwani, Z. Green Route for Biomethane and Hydrogen Production via Integration of Biogas Upgrading Using Pressure Swing Adsorption and Steam-Methane Reforming Process. Renew. Energy 2023, 210, 64–78.
- Wang, X.; Su, X.; Zhang, Q.; Hu, H. Effect of Additives on Ni-Based Catalysts for Hydrogen-Enriched Production from Steam Reforming of Biomass. Energy Technol. 2020, 8, 2000136.
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent Studies. Catal. Sci. Technol. 2022, 12, 3387–3411.
- Becker, C.M.; Marder, M.; Junges, E.; Konrad, O. Technologies for Biogas Desulfurization—An Overview of Recent Studies. Renew. Sustain. Energy Rev. 2022, 159, 112205.
- Hulteberg, C. Sulphur-Tolerant Catalysts in Small-Scale Hydrogen Production, a Review. Int. J. Hydrogen Energy 2012, 37, 3978–3992.
- Kumar, R.; Kumar, A.; Pal, A. Overview of Hydrogen Production from Biogas Reforming: Technological Advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855.
- Ahmed, S.; Lee, S.H.D.; Ferrandon, M.S. Catalytic Steam Reforming of Biogas—Effects of Feed Composition and Operating Conditions. Int. J. Hydrogen Energy 2015, 40, 1005–1015.
- Park, M.-J.; Kim, H.-M.; Gu, Y.-J.; Jeong, D.-W. Optimization of Biogas-Reforming Conditions Considering Carbon Formation, Hydrogen Production, and Energy Efficiencies. Energy 2023, 265, 126273.
- Pashchenko, D.; Makarov, I. Carbon Deposition in Steam Methane Reforming over a Ni-Based Catalyst: Experimental and Thermodynamic Analysis. Energy 2021, 222, 119993.
- Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375.
- Ponugoti, P.V.; Pathmanathan, P.; Rapolu, J.; Gomathi, A.; Janardhanan, V.M. On the Stability of Ni/γ-Al2O3 Catalyst and the Effect of H2O and O2 during Biogas Reforming. Appl. Catal. A Gen. 2023, 651, 119033.
- Tuna, C.E.; Silveira, J.L.; da Silva, M.E.; Boloy, R.M.; Braga, L.B.; Pérez, N.P. Biogas Steam Reformer for Hydrogen Production: Evaluation of the Reformer Prototype and Catalysts. Int. J. Hydrogen Energy 2018, 43, 2108–2120.
- Papurello, D.; Chiodo, V.; Maisano, S.; Lanzini, A.; Santarelli, M. Catalytic Stability of a Ni-Catalyst towards Biogas Reforming in the Presence of Deactivating Trace Compounds. Renew. Energy 2018, 127, 481–494.
- Iulianelli, A.; Manisco, M.; Bion, N.; Le Valant, A.; Epron, F.; Colpan, C.O.; Esposito, E.; Jansen, J.C.; Gensini, M.; Caravella, A. Sustainable H2 Generation via Steam Reforming of Biogas in Membrane Reactors: H2S Effects on Membrane Performance and Catalytic Activity. Int. J. Hydrogen Energy 2021, 46, 29183–29197.
- Amiri, T.Y.; Ghasemzageh, K.; Iulianelli, A. Membrane Reactors for Sustainable Hydrogen Production through Steam Reforming of Hydrocarbons: A Review. Chem. Eng. Process. Process Intensif. 2020, 157, 108148.
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51.
- Iulianelli, A.; Alavi, M.; Bagnato, G.; Liguori, S.; Wilcox, J.; Rahimpour, M.R.; Eslamlouyan, R.; Anzelmo, B.; Basile, A. Supported Pd-Au Membrane Reactor for Hydrogen Production: Membrane Preparation, Characterization and Testing. Molecules 2016, 21, 581.
- Ben-Mansour, R.; Haque, M.A.; Habib, M.A.; Paglieri, S.; Harale, A.; Mokheimer, E.M.A. Effect of Temperature and Heat Flux Boundary Conditions on Hydrogen Production in Membrane-Integrated Steam-Methane Reformer. Appl. Energy 2023, 346, 121407.
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The Recent Areas of Applicability of Palladium Based Membrane Technologies for Hydrogen Production from Methane and Natural Gas: A Review. Int. J. Hydrogen Energy 2023, 48, 6451–6476.
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen. Energy 2021, 46, 33756–33781.
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19.
- Al-Zuhairi, F.K.; Azeez, R.A.; Mahdi, S.A. Synthesis of Long-Chain Hydrocarbons from Syngas over Promoted Co/SiO2 Catalysts Using Fischer–Tropsch Reaction. AIP Conf. Proc. 2022, 2443, 030028.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
11 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




