2. Lead (Pb2+) Toxicity and Sources
Residues of heavy metals are distributed into the water from various manufacturing facilities. Before they are discharged into the environment, they should be reduced to a level that is safe for human consumption
[8]. However, they can still harm aquatic life and animals. Due to their widespread use in the industry, lead Pb
2+ ions are considered the most toxic target heavy metals. They can affect the health and well-being of humans and animals due to their binding to various components of living organisms
[8]. The LD50 of lead acetate is around 100–200 mg/kg for oral ingestion in rats. In general, lead is a toxic compound that can cause a variety of health problems, including brain damage, neurological disorders, and reproductive problems. Even low levels of exposure to lead can be harmful, especially for children
[9][10].
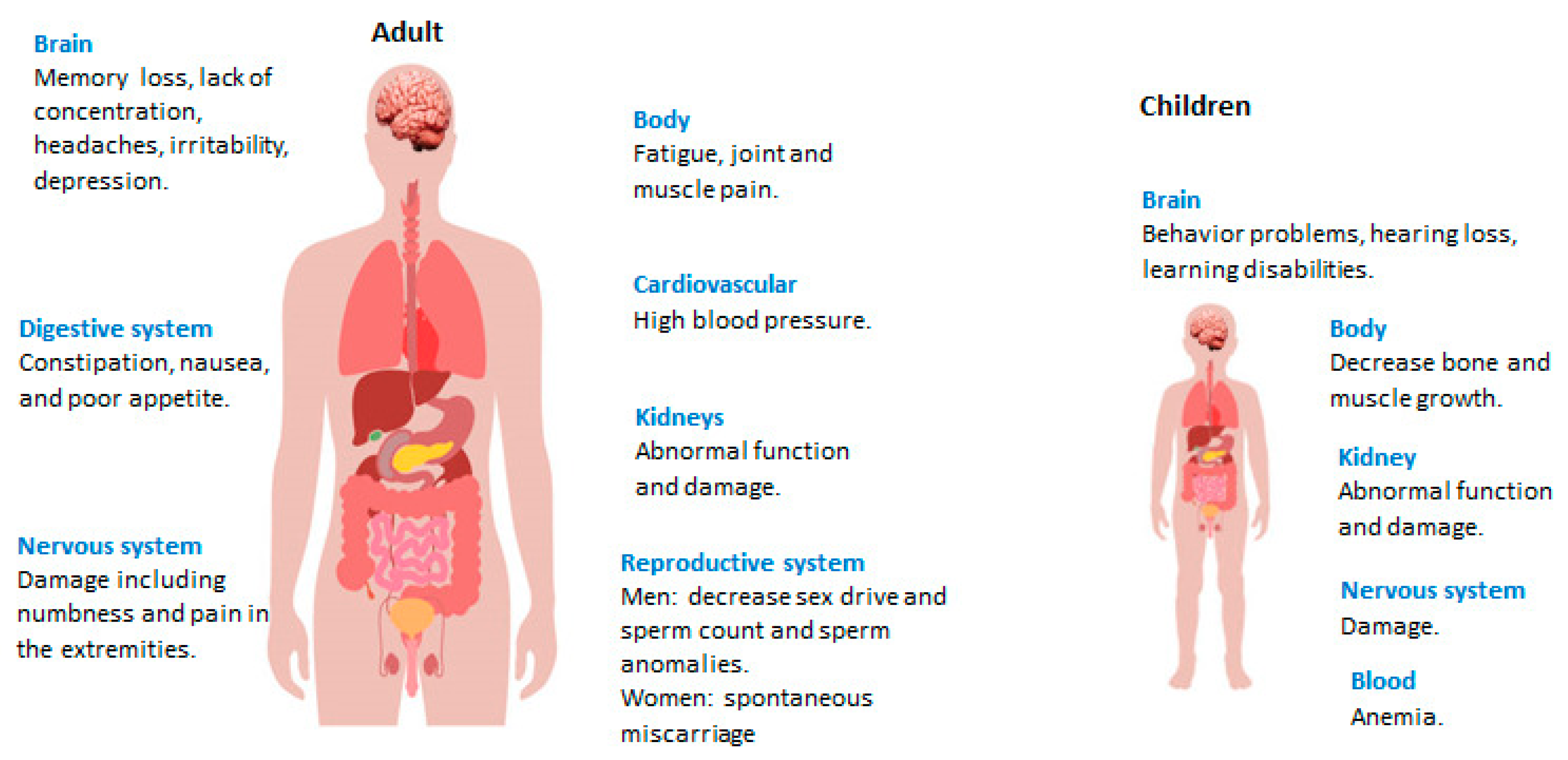
Figure 1 shows the potential health complications resulting from lead compounds for both children and adults.
Figure 1. Health impact of lead exposure.
Lead Pb
2+ is a metallic element that exists as
cerussite (lead carbonate) and
galenite (lead sulphide)
[11]. Lead-acid batteries, electrical plating firms, electrical technology, steel construction companies, and explosive companies are the most significant sources of lead waste
[11]. However, because of their single-atom-metal feature, Pb
2+ ions seldom disintegrate in the ecosystem and must be controlled by a variety of means
[12]. The United States Environmental Protection Agency (USEPA) has issued Temporary Potable Water Health Advisory limits of 0.015 mg/L for Pb
2+ because they are damaging to human health
[8].
Table 1 represents the main forms of lead Pb
2+ in aqueous media, their generation source, industrial remediation, and whether they are easy or difficult to remove
[13][14][15][16][17]. Natural Pb
2+ waste has mainly been detected as a result of hill fires and volcanic explosions. Non-natural sources of Pb
2+ pollution mainly refer to industrial emissions and transportation supplies
[18]. The accumulated ions have serious consequences in humans. They have a destructive effect on the neurological system, haematology, cardiovascular disorders, and renal organs, especially in kids
[12]. The incorporation of Pb
2+ by marine organisms is reliant on various factors such as the pH value, dissolved inorganic carbon (DIC), temperature, and dead organic matter
[12]. These factors can affect the speciation of Pb
2+, which can affect its toxicity, mobility, and bioaccessibility in aquatic systems
[12]. In freshwater microalgae, a reduction in the internalization of Pb
2+ was observed when DIC was present. This finding suggests that the presence of DIC can facilitate lead ion uptake
[12]. In fact, lead can be formed into complexes with anionic compounds in the environment. Multiple anionic varieties, like biota exudate, cell walls, and humic acid, can also contain ligands that are N-, S-, and P-. These can easily be used to form soluble and stable complexes in freshwater lakes with a pH range of 5 to 9 due to their affinity for Pb
2+. The most serious side effects of lead accumulation are renal and neurological system injury, mental abnormalities, and tumours in humans
[5][19][20][21]. Many harmful metals are toxic after a short time, yet minimal amounts are critical for crucial functions
[22]. In this context, permanently and successfully eliminating such contaminants remains a difficult challenge
[3][23].
Table 1. Main common forms of lead (Pb2+) in aqueous media, chemical formula, main characteristics, generation source, industrial remediation.
3. Conventional and Emerging Methods for Lead treatment
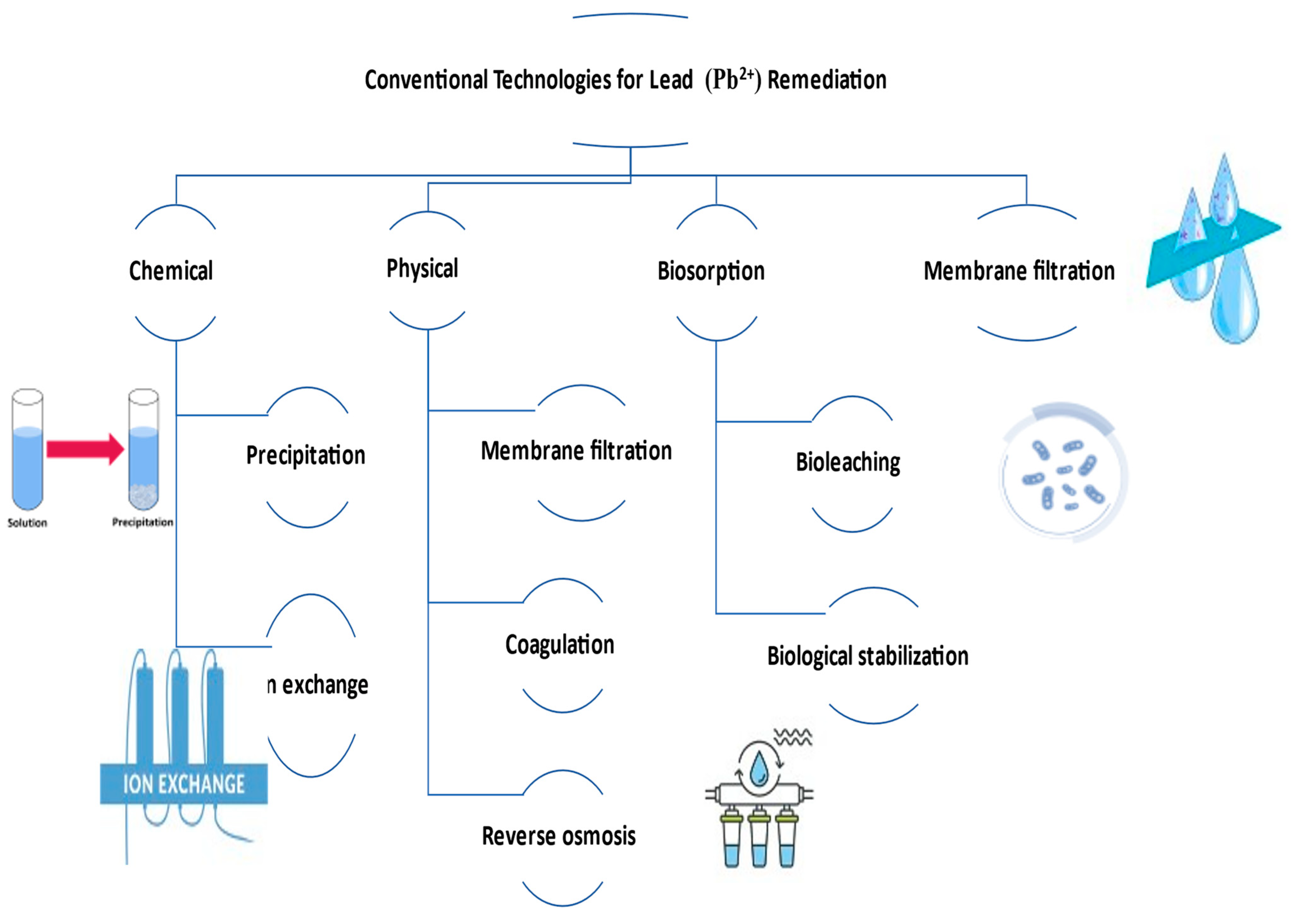
There are various treatment methods that have been proposed to remove Pb
2+ ions from aquatic media. These include chemical, physical, biosorption, and membrane filtration
[12]. Precipitation and ion exchange are examples of chemical techniques. Membrane filtration adsorption and coagulation are part of the physical separation or purification approaches while bioleaching, biological stabilization, and biosorption are among the biological ones
[5] (
Figure 2). Although the above-mentioned techniques have some advantages, they have also encountered various limitations
[5]. Chemical precipitation is widely used in industries to remove Pb
2+ ions. This process can be performed by adjusting the water phase’s pH
[5]. It can also separate the compounds from the solution by separating them into carbonate, sulphide, or hydroxide. Despite its advantages, this method has its drawbacks
[5]. Ion exchange is performed by using solid ion exchangers, which are commonly used in the recovery of Pb
2+ ions from wastewater. This method has been implemented in the past to salvage metal plating effluents
[5]. However, its practicality is often limited by the high cost of operation and secondary pollution
[5]. Other than the high cost, the most common factors that prevent solid ion exchangers from being used effectively are secondary pollution and reusability issues
[5].
Figure 2. Conventional technologies for lead remediation.
Physical approaches in the removal of contaminants offer numerous advantages such as low cost, minimal bi-product regeneration and high removal capacity
[5]. Over the past two decades, the development of new technologies for the removal of heavy metals from aqueous discharge has been greatly supported by the use of economical materials such as agricultural, industrial (
Figure 3), and urban residue
[5]. Numerous studies have been carried out on various types of adsorbents such as chitosan, clay, lignin, activated carbons, and synthetic polymeric materials. The widely used biopolymer known as chitosan is commonly used as an effective adsorbent for the removal of various heavy metals and hazardous substances such as proteins and dyes
[24]. Chitosan is the only polycation found in nature, and its charge density varies according to the degree of acetylation and the pH of the medium
[24]. The polymer’s solubility is determined by its acetylation degree and molecular weight. Chitosan oligomers are soluble at all pH levels, from acidic to basic (physiological pH 7.4). It has good biodegradability and mechanical characteristics
[24]. However, sometimes the dissolved sorbent can still be difficult to separate from the solution after the removal of the contaminants.
Figure 3. Advanced technologies for lead remediation.
The various kinds of adsorbent materials used to remove inorganic/heavy metal contaminants from effluent can be categorized into three main categories expressed by organic, inorganic, and by-products released from industry
[25]. Others have classified them as natural, synthetic, and modified. The low price and great productivity of these materials are the main factors that determine their effectiveness in real-world wastewater systems
[12]. The capacity of the adsorbent to remove contaminants depends on various factors, such as type of material, the size of the pore, and the structural characteristics of the adsorbent
[25]. Large-sized surface areas of adsorbents provide many sites for the physical and chemical entrapment of contaminants in wastewater
[25].
In terms of their industrial applications, various features such as mechanical integrity, recyclability, and adsorbent stability need to be addressed
[25]. The flexibility of the adsorbent to be modified also can be an important factor in improving the adsorption performance. Hence, several adsorbent materials from carbon material are ideal choices. Carbonaceous adsorbents are the first choice for researchers who are looking to remove harmful metals and chemicals from water. These are made from various materials such as graphite, activated carbon, charcoal, and activated sludge
[26]. Their porous structure allows them to adsorb non-organic and organic chemicals in either gaseous or liquid phases. The primary source of activated carbon is usually coal, coconut shells, peat, and lignite. It can also be produced from various other precursor materials such as walnut wood, palm shell, coconut husk, wheat bran, and rice husk
[26]. The physical and chemical characteristics of activated carbon can be affected by their preparation process and the precursor biomass
[26]. Most of the known carbonaceous materials used to remove chemicals are either activated carbon or carbon nanotubes (CNTs). Carbon nanotubes can be categorized into multi-walled (MWCNT) and single-walled (SWCNT) types
[26]. Activated carbon is usually less efficient due to its low porosity, while carbon nanotubes are usually used for comparison
[26]. The high adsorption capacity of activated carbons can be attributed to their pore structures and porous nature. Compared to the average pore diameter of SWCNT and MWCNT, the average size of activated carbons was five times lower
[27].
Different materials have been used to adsorb lead ions from aqueous systems. Carboxymethyl lignin nanoparticles have the highest adsorption capacity while Andean Sacha inchi shell biomass has the lowest capacity. This is justified by the nature of the adsorbed material. Nanoparticles have the highest adsorption capacity among all adsorbents. They have nanosized particles with a high surface-to-volume ratio and thus enhance their adsorption capacity. There are a few aspects in the determination of high-quality adsorbent material such as equilibrium/adsorption time, regeneration capacity, bi-products, recyclability, dose of adsorbent, and many more. Equilibrium time is an important criterion to determine the efficiency of the adsorbents and their applicability. Based on this, Jujube pit biochar showed the least equilibrium time (30 min) compared to Poly Ethelene imine-grafted cellulose with 4800 min. Logically speaking, adsorption time is a crucial aspect when evaluating the activity of an adsorbent. This means adsorbents with the lowest equilibrium time are the best choice as a practical and applicable adsorbent. Additionally, the regeneration capacity is an important factor that determines adsorbent feasibility and reproducibility. In other words, regeneration or recyclability can decide the number of cycles that could be used by the same material with a good adsorption capacity. The regeneration cycle also determines the durability of the adsorbent, which can affect the cost of adsorbent maintenance. This limits the accumulation of such a material and allows for metal separation. Only two studies out of seven studied the regeneration of the adsorbents as in JPB and cellulose/PEI. On the other hand, a limited number discussed the time of equilibrium in spite of its importance in nominating the best-adsorbed material. Wood manufacturing units incorporate bark as a by-product. Tannin is an organic substance that can be used to remove heavy metal ions. It contains polyhydroxy compounds that can adsorb metal ions
[5]. Masri et al.
[28], for instance, were the first to report that Douglas fir and black oak bark can remove mercury, lead, and cadmium
[5]. The most commonly used in-process adsorbents for wastewater treatment are composed of metal oxides, layered double hydroxides (LDHs)
[29], silica
[30], clays
[31], magnesium oxide (MgO), and zeolite
[32]. They also adsorb metal—organic frameworks MOFs
[33]. There are also various types of substances that can be utilized for water treatment. For instance, pre-treatment can be performed to improve the efficiency of the process
[34]. In addition, chemically modified adsorbents made of metal/metal oxide are commonly used for wastewater treatment both in bulk and nanostructured
[35]. They have various advantages such as their high mechanical stability, flexibility, and capacity to adsorb metal ions. Referring to adding porosity and adjusting morphology for these materials, nanostructured material with higher surface area can be created to improve the efficiency of the treatment process. Since nanoparticles are small, they can be prone to aggregation. This can be prevented by using porous supports such as silica
[36], carbon
[37], clays, or biochar
[38]. For the removal of pollutants from wastewater, various kinds of metal oxides are commonly used, including iron oxide (Fe
2O
3 and Fe
3O
4), aluminium oxide (Al
2O
3), manganese oxide (MnO
2), titanium oxide (TiO
2), zinc oxide (ZnO), magnesium oxide (MgO), and zirconium oxide (ZrO
2)
[3][39]. On the other hand, since heavy metal has a polarity, surface modification or chemically modified metal oxides (CMMOs) are ideal to be applied as adsorbents. Additionally, a superior surface must maximize the process’s effectiveness to ensure high adsorption capacity. Numerous studies claim that the removal of heavy metals with sufficient surface resulted in better capacity. In a study by Saleh et al., a multi-wall carbon nanotube (MWCNT) composite coated with manganese dioxide (MnO
2) was used as an adsorbent for the removal of lead
[40]. The researchers noted that the optimal removal rate was achieved between pH 6 and 7. They noted that the increase in the layer thickness of the nanocomposite led to the higher removal of Pb (II). They also found needles with a diameter of up to 30 nm and a length of up to 300 nm
[3]. They could remove Pb (II) ions with 97% efficiency, and the optimal condition was found at pH-5.2 and C
o = 5 mg L
−1. The properties of lead ions have been fitted by Sips isotherm and Langmuir. The unique physical properties of iron and metal oxides, which enhance the removal capacity, are also responsible for this. The metal removal capacity of a superparamagnetic nanosphere was enhanced by its core–shell structure and the functionalization of the amino group. The improved capacity was observed when the structure was grafted with an amino group. The metal removal efficiency of various ions, such as cadmium (II), copper (II), and lead (II), were 99.96%, 199.9 mg g
−1, 88.05%, and 177.8 mg g
−1, 90.79% and 181.6 mg g
−1, respectively. The process was carried out according to Langmuir’s model, and the kinetics exhibited pseudo-second-order characteristics
[3].
Another study by Ignatius et al. 2014 investigated the rhizo-filtration of lead-containing wastewater using Plectranthus amboinicus, an aromatic medicinal plant
[41]. This study was conducted due to the motivation of heavy metal contamination in water bodies and groundwater which leads to many aquatic and terrestrial plants accumulating heavy metals when grown hydroponically. The study suggests that the plant can be considered for the clean-up of contaminated wastewater along with biomass disposal alternatives
[41]. However, mass production of such a phytoremediation method necessitates a deeper knowledge of plant–metal interactions, particularly the capacity of various plant species to accumulate metals in different regions of the plants, and that is essential for efficient remediation.
4. Multiple Modification Strategies for Conventional and Emerging Adsorbents
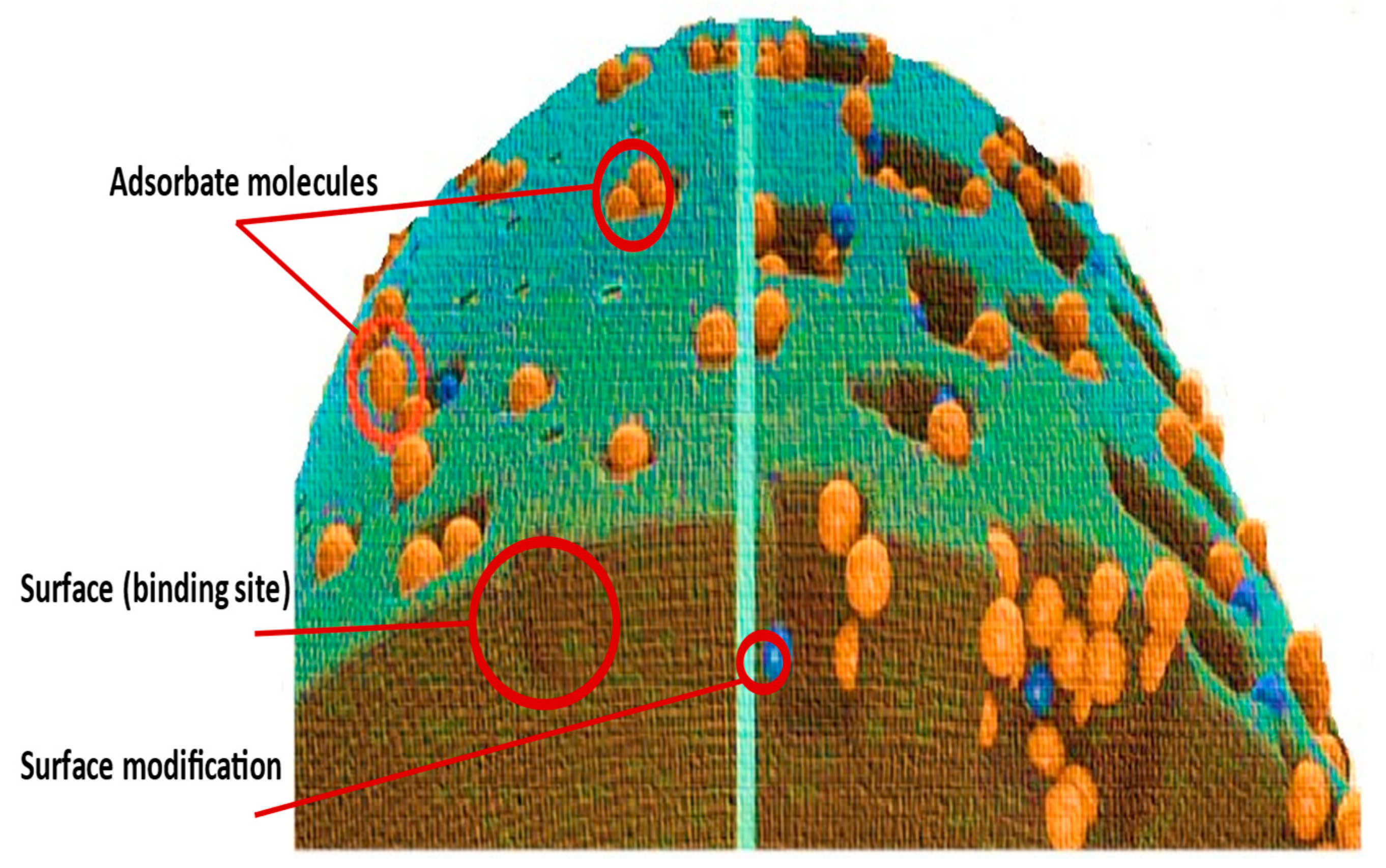
Adsorption is always considered a surface phenomenon; therefore, its efficacy is highly reliant mostly on the surface chemistry of both modified and natural materials. Surface functions are determined by the nature of the precursor and the mechanism applied for its modification. Abegunde et al. conducted a review discussing the influence of chemical changes on adsorbent performance
[42]. Physicochemical alteration of the adsorbents seems like customizing a material’s surface by manipulating its physical, chemical, or even biological properties as illustrated in
Figure 2. These features distinguish the material from the original and make it suitable for the targeted function. Adsorbents are commonly employed by scientists due to their high surface area, high adsorption capacity, and quick kinetics
[3]. Moreover, a good adsorbent should be non-hazardous and not attach to pollution particles. It should be highly selective for contaminants even with trace amounts and easily regenerate
[3]. Based on the previous features, as well as other different structural and morphological traits, scientists proved that nanomaterials (NMs) could perform as active adsorbents
[3]. When converting from micrometre (m) to nanoscale (nm), the energy of the surface rises rapidly, ending with poor particle stability. In addition, higher agglomeration is caused by increased van der Waals bonding forces
[3]. Therefore, surface modification would enhance stability in addition to increasing dispersity and thus will control agglomeration (
Figure 4).
Figure 4. Surface modification of adsorbents.
As stated in the previous table, the comparison between the modified and unmodified form of the adsorbents shows clearly that the modified form of modified adsorbents is superior in adsorption capacity to the unmodified one. This shows that adsorption is a surface phenomenon, and once the surface of the adsorbent can be improved by choosing the ideal modification strategy, adsorption stability can be achieved perfectly. Among all the previous examples demonstrated in the table, cellulose has the highest adsorption capacity, which reached 976 mg/g for the modified one compared to 138 mg/g for the unmodified one. Cellulose was used for the adsorption of copper ions. It was the best applicable material with 85% regeneration. On the opposite side, chitosan had the lowest adsorption capacity with its modified state, which was around 60 mg/g, and it was used for the adsorption of lead ions. Both studies number three and seven investigate cellulose as the adsorbed material with different modification strategies. Additionally, the adsorbate in study three was for Cu while in the seventh study, it was used for the adsorption of metal ions Pb
2+ and Cu. Both studies almost have the same percent of regeneration, 85 and 86, respectively. This obviously indicates that HPFC has maximized the surface area with multiple functional groups that guaranteed better adsorption capacity. Among the previous adsorbents, different materials are used for remediating lead ions including lignin, chitosan, biochar, and cellulose. Their adsorption capacity for Pb2
+ was in the following order: biochar (594.17 mg/g), cellulose (184 mg/g), lignin (126 mg/g), and chitosan (59.85 mg/g). Through a microwave heating process, the nanoparticles of carboxymethyl-modified lignin were prepared. The two-stage antisolvent processes were utilized to decrease the adsorbent’s solubility and improve its extraction ability. The adsorbent was able to remove lead ions with a maximum sorption value of 333.26 mg/g. The effect of pH value on the adsorbent’s performance was studied in a range of 2.04 to 7.06 for lignin nanoparticle LNPs. It was shown that the former exhibited a higher adsorption capacity when the pH level increased to 6.03. On the other hand, the latter exhibited a lower sorption rate when the pH level was lowered to 2.04, which is related to more H
+ ions that combine with the -COO- group in the solution and form -COOH. The results of the study revealed that the carboxymethyl lignin particles exhibited better adsorption characteristics when compared to the lignin nanoparticles when it was at pH 6.03. They also showed better regeneration abilities
[5].
Various investigations have been conducted on the modification of cellulose nanomaterials to improve their sorption properties. These modifications can be used to remove inorganic contaminants like copper ions for example
[43]. The incorporation of both inorganic and organic groups can be achieved by placing titanium dioxide NPs on the surfaces of cellulose nanogels
[44]. This allows for the creation of a low-energy surface that can act as a hydrophobic and oleophilic material. They can also absorb diverse organic solvents and oil from the water’s surface, which has a capacity of up to 90% vol/vol
[44].
A study conducted by Loganathan and colleagues analysed the various surface modification techniques that can be used to improve the sorption capacity (from 125 mg g
−1 for amination to 363 mg g
−1 for protonation) of adsorbent materials
[45]. They found that the most effective modifications were the protonation and amine-grafted techniques
[45]. However, the properties of metal oxides, such as titanium dioxide, manganese oxide, and iron oxide, are highly advantageous when it comes to reducing environmental risks. They can be used as sorbents that can eliminate various water pollutants, such as heavy metals and industrial chemicals. Due to their various characteristics, such as their size, crystal structure, surface area, and morphology, they have been studied extensively
[45].
One paper shows how sewage sludge, which is waste from a polluted water treatment medium, can be reused as a solid support and then modified to form a bio-adsorption biochar. The modified biochar exhibited improved stability in water and was more beneficial for the environment. The bio-adsorption process was performed by reducing the pH level of the mixture. It exhibited better performance than that of conventional adsorbents, with a capacity of 594.17 mg g
−1 for Hg (II) and Pb (II). Also, it unveils good reusability and stability. The results of the study demonstrate how waste valorization can be achieved through a simple and green approach. It involves turning the sewage sludge into a biochar adsorbent that can be used to remove heavy metal ions from the waste water
[3].
5. Adsorption Mechanism and Alternative Remediator
The adsorption process is regarded as the most effective way to treat toxic compounds in wastewater
[5]. However, the advantages of the adsorption process are numerous, such as reusability, besides its potential to remove toxic substances from wastewater successfully and effectively at a low cost. Furthermore, it is also more eco-friendly than traditional methods. A wide range of natural adsorbents were produced for the removal of metal ions from effluent
[5]. Some of the characteristics that are required for the selection of an effective adsorbent include their cost efficiency, surface area, size, and distribution of pores in addition to their availability of functional moiety and polar properties for those sorbents
[5].
Understanding the adsorption process is also important to ensure better performance. The process of adsorbing involves transferring a substance known as solutes onto a solid surface. The two kinds of forces that interact with adsorbate and are present in the solution are physical and chemical
[5]. Physical ones are weak forces with no specificity in nature. Since the former is weak, adsorbed molecules can be easily attached to any surface. On the other hand, the chemical process is specific and involves the use of electrostatic or covalent bonds to bind adsorbents while physical adsorption relies on van der Waals, hydrogen bonding, and dispersion interactions
[5].
Due to the presence of different types of pollutants in wastewater, studies on the selective removal of these pollutants have become more important. One of the most important factors that can be considered when it comes to optimizing the performance of an adsorbent is the mechanism by which it interacts with the substance. The various parameters that affect the capacity of a particular adsorbent are also considered to ensure that they are effective. These include the pH level of the solution, the concentration of the adsorbates, the contact time, and the coexistence of other components
[25][46]. The rate at which a particular adsorbent can remove a substance depends on its chemical and physical properties. It starts fast and slows down steadily as it reaches equilibrium. This can be influenced by the various factors that affect the concentration of adsorbates and the solid phase’s equilibrium (solution parameters)
[5]. In addition, the importance of material design and engineering in achieving high performance and selectivity for environmental purification and energy conversion applications is evident
[47][48].