
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eleonóra Spekker | -- | 2847 | 2023-12-05 12:35:17 | | | |
| 2 | Peter Tang | + 2 word(s) | 2849 | 2023-12-06 01:55:42 | | |
Video Upload Options
Migraine is a primary headache disorder, which is an enormous burden to the healthcare system. While some aspects of the pathomechanism of migraines remain unknown, the most accepted theory is that activation and sensitization of the trigeminovascular system are essential during migraine attacks. It has been suggested that ion channels may be important participants in the pathogenesis of migraine. Numerous ion channels are expressed in the peripheral and central nervous systems, including the trigeminovascular system, affecting neuron excitability, synaptic energy homeostasis, inflammatory signaling, and pain sensation. Dysfunction of ion channels could result in neuronal excitability and peripheral or central sensitization.
1. Introduction
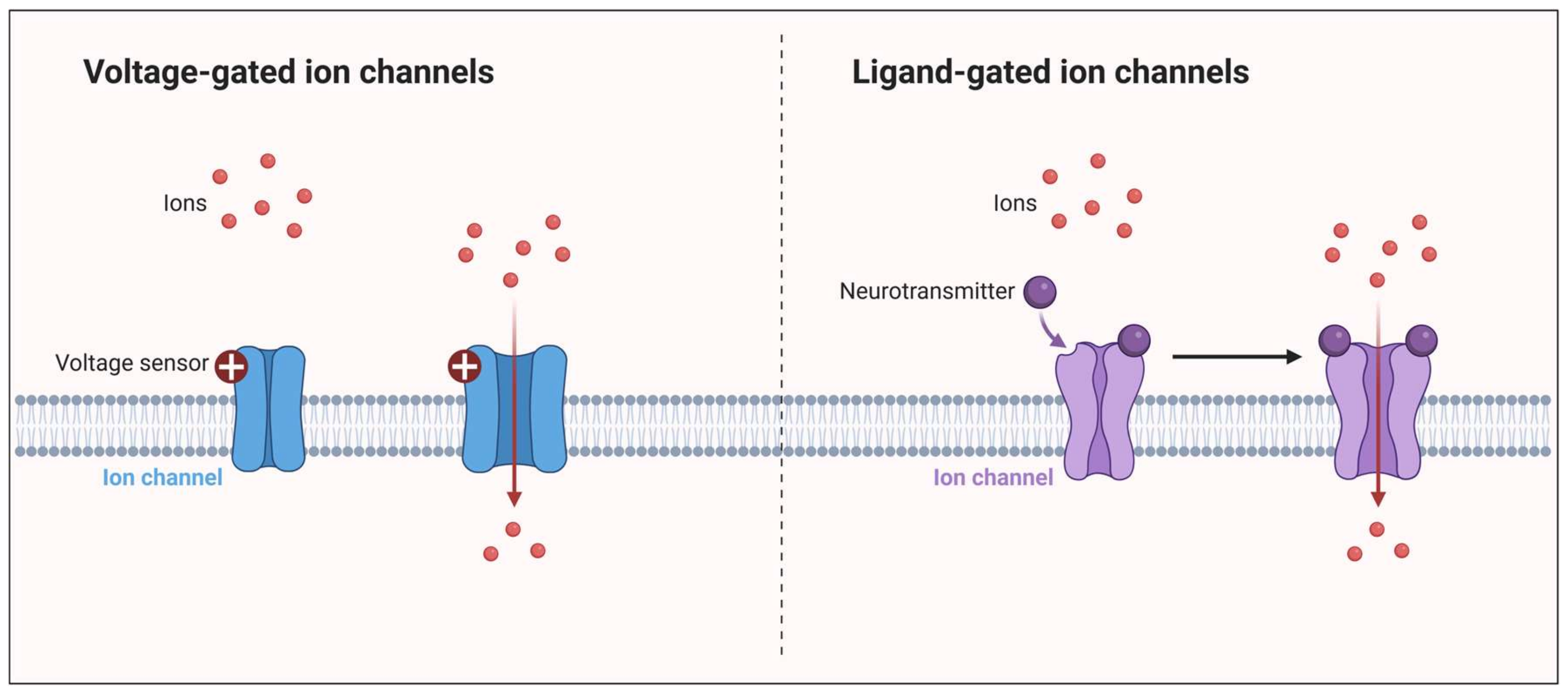
2. Migraine Pathogenesis and the Impact of the Ion Channels
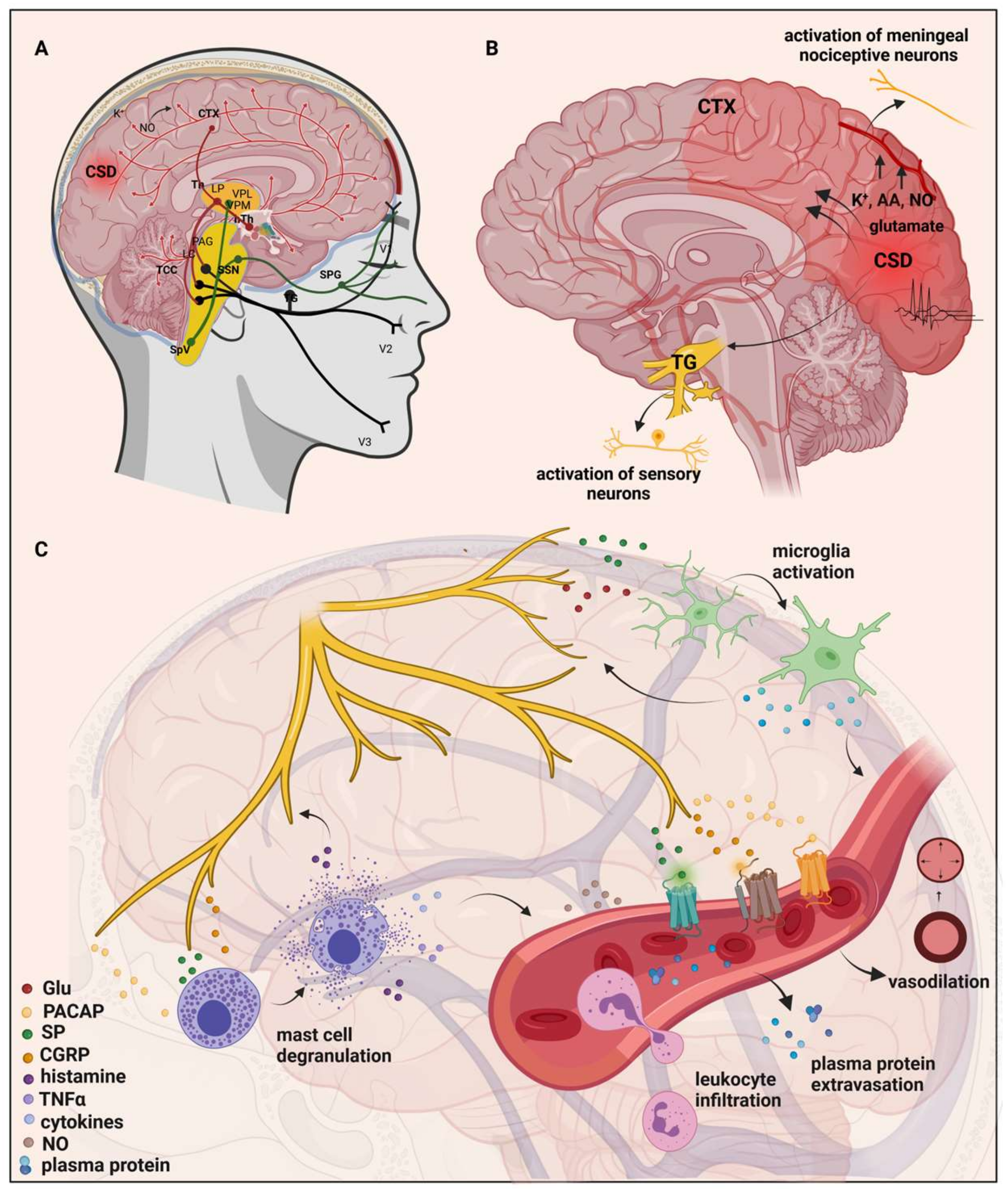
3. Ion Channels in Migraine: Unraveling Pathogenesis and Therapeutic Implications
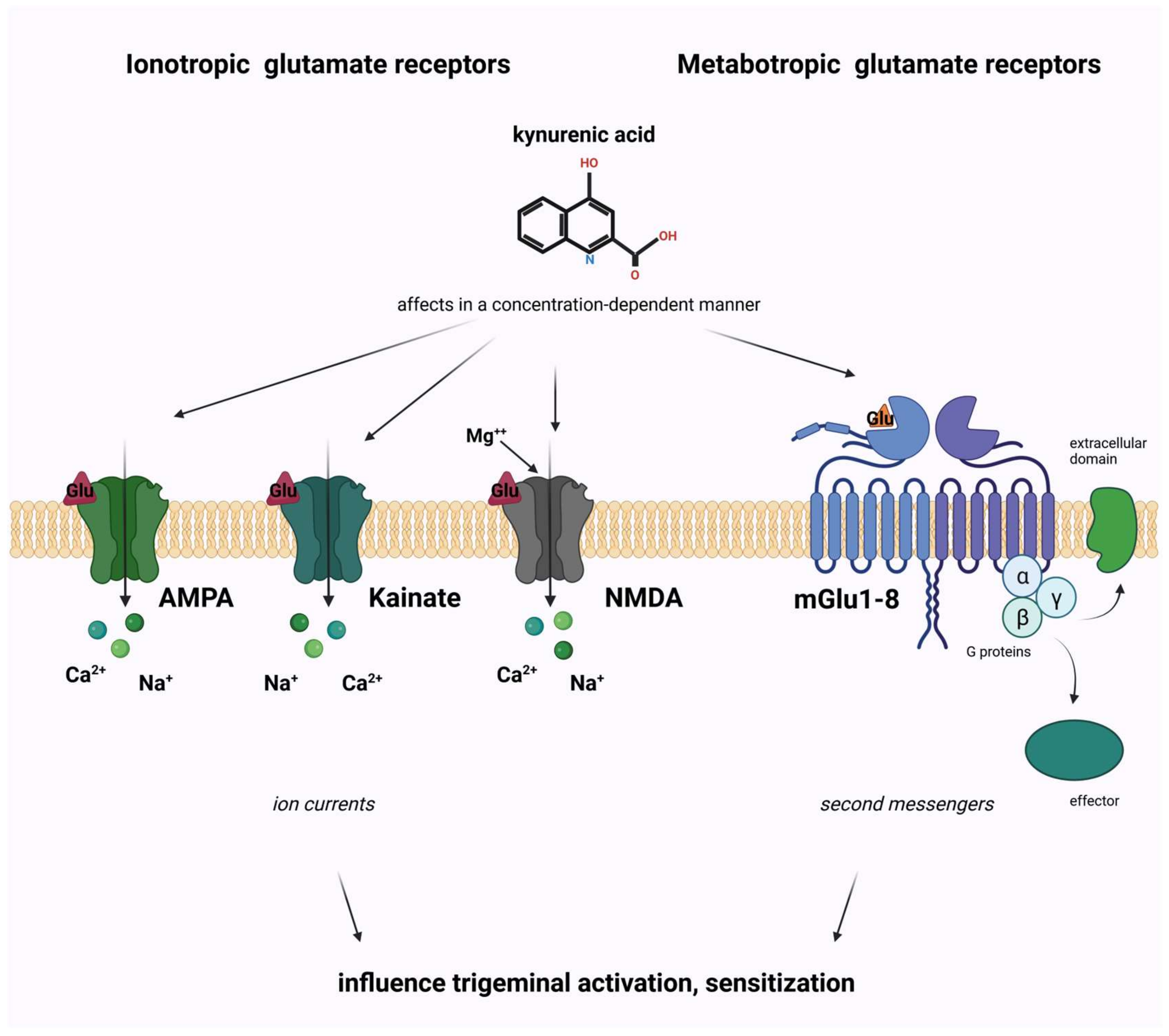
4. The Interplay of Glutamate and the Kynurenine Pathway in Migraine
4.1. Glutamate and Its Receptors
4.2. The Kynurenine Pathway
4.3. The Role of Kynurenine Pathway in Migraine Pathomechanism Connected to Glutamate Receptors
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2013, 33, 629–808.
- Humphries, E.S.A.; Dart, C. Neuronal and Cardiovascular Potassium Channels as Therapeutic Drug Targets: Promise and Pitfalls. SLAS Discov. Adv. Sci. Drug Discov. 2015, 20, 1055–1073.
- Al-Karagholi, M.A.-M. Involvement of Potassium Channel Signalling in Migraine Pathophysiology. Pharmaceuticals 2023, 16, 438.
- Wang, J.; Ou, S.-W.; Wang, Y.-J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554.
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2011, 4, a011353.
- Longden, T.A.; Hill-Eubanks, D.C.; Nelson, M.T. Ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 2015, 36, 492–512.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622.
- Edvinsson, L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 2011, 31, 737–747.
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76.
- Cross, S.A. Pathophysiology of Pain. Mayo Clin. Proc. 1994, 69, 375–383.
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154, S44–S53.
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache J. Head Face Pain 2018, 58, 4–16.
- Pietrobon, D.; Moskowitz, M.A. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat. Rev. Neurosci. 2014, 15, 379–393.
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of Meningeal Nociceptors by Cortical Spreading Depression: Implications for Migraine with Aura. J. Neursci. 2010, 30, 8807–8814.
- Gursoy-Ozdemir, Y.; Qiu, J.; Matsuoka, N.; Bolay, H.; Bermpohl, D.; Jin, H.; Wang, X.; Rosenberg, G.A.; Lo, E.H.; Moskowitz, M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Investig. 2004, 113, 1447–1455.
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of Migraine. Annu. Rev. Physiol. 2013, 75, 365–391.
- Cohen, C.F.; Roh, J.; Lee, S.H.; Park, C.-K.; Berta, T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int. J. Mol. Sci. 2023, 24, 7897.
- Zhang, X.-C.; Strassman, A.M.; Burstein, R.; Levy, D. Sensitization and Activation of Intracranial Meningeal Nociceptors by Mast Cell Mediators. Experiment 2007, 322, 806–812.
- Levy, D. Endogenous Mechanisms Underlying the Activation and Sensitization of Meningeal Nociceptors: The Role of Immuno-Vascular Interactions and Cortical Spreading Depression. Curr. Pain Headache Rep. 2012, 16, 270–277.
- Liu, Y.; Broman, J.; Zhang, M.; Edvinsson, L. Brainstem and Thalamic Projections from a Craniovascular Sensory Nervous Centre in the Rostral Cervical Spinal Dorsal Horn of Rats. Cephalalgia 2009, 29, 935–948.
- Weiller, C.; May, A.; Limmroth, V.; Jüptner, M.; Kaube, H.; Schayck, R.; Coenen, H.; Dlener, H. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995, 1, 658–660.
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584.
- Settle, M. The Hypothalamus. Neonatal Netw. 2000, 19, 9–14.
- Goadsby, P.J.; Holland, P.R. Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671.
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564.
- Kullmann, D.M. The neuronal channelopathies. Brain 2002, 125, 1177–1195.
- Yan, J.; Dussor, G. Ion Channels and Migraine. Headache J. Head Face Pain 2014, 54, 619–639.
- Carrera, P.; Stenirri, S.; Ferrari, M.; Battistini, S. Familial hemiplegic migraine: A ion channel disorder. Brain Res. Bull. 2001, 56, 239–241.
- Uchitel, O.D.; Inchauspe, C.G.; Di Guilmi, M.N. Calcium channels and synaptic transmission in familial hemiplegic migraine type 1 animal models. Biophys. Rev. 2013, 6, 15–26.
- Pietrobon, D. Ion channels in migraine disorders. Curr. Opin. Physiol. 2018, 2, 98–108.
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72.
- Barker, B.S.; Young, G.T.; Soubrane, C.H.; Stephens, G.J.; Stevens, E.B.; Patel, M.K. Chapter 2—Ion Channels. In Conn’s Translational Neuroscience; Michael Conn, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 11–43.
- De Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118.
- Alexander, S.P.; Peters, J.A.; Kelly, E.; Marrion, N.V.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The Concise Guide to Pharmacology 2017/18: Ligand-gated ion channels. Br. J. Pharmacol. 2017, 174, S130–S159.
- Szalardy, L.; Klivenyi, P.; Zadori, D.; Fulop, F.; Toldi, J.; Vecsei, L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: Medicinal chemistry aspects. Curr. Med. Chem. 2012, 19, 1899–1920.
- Egerton, A.; Grace, A.A.; Stone, J.; Bossong, M.G.; Sand, M.; McGuire, P. Glutamate in schizophrenia: Neurodevelopmental perspectives and drug development. Schizophr. Res. 2020, 223, 59–70.
- Henter, I.D.; Park, L.T.; Zarate, C.A. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 2021, 35, 527–543.
- Vecsei, L.; Majlath, Z.; Balog, A.; Tajti, J. Drug targets of migraine and neuropathy: Treatment of hyperexcitability. CNS Neurol. Disord. Drug Targets 2015, 14, 664–676.
- Martínez, F.; Castillo, J.; Rodríguez, J.R.; Leira, R.; Noya, M. Neuroexcitatory Amino Acid Levels in Plasma and Cerebrospinal Fluid During Migraine Attacks. Cephalalgia 1993, 13, 89–93.
- Olney, J.W.; Sharpe, L.G. Brain Lesions in an Infant Rhesus Monkey Treated with Monosodium Glutamate. Science 1969, 166, 386–388.
- Choi, D.W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci. Lett. 1985, 58, 293–297.
- Koga, K.; Li, S.; Zhuo, M. Metabotropic Glutamate Receptor Dependent Cortical Plasticity in Chronic Pain. Curr. Neuropharmacol. 2016, 14, 427–434.
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87.
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A Glycine Site Associated with N-Methyl-d-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem. 1989, 52, 1319–1328.
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112.
- Rózsa, Á.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. 2008, 115, 1087–1091.
- Kemp, J.A.; Foster, A.C.; Leeson, P.D.; Priestley, T.; Tridgett, R.; Iversen, L.L.; Woodruff, G.N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc. Natl. Acad. Sci. USA 1988, 85, 6547–6550.
- Sas, K.; Robotka, H.; Rózsa, E.; Ágoston, M.; Szénási, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vécsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308.
- Fejes-Szabó, A.; Bohár, Z.; Vámos, E.; Nagy-Grócz, G.; Tar, L.; Veres, G.; Zádori, D.; Szentirmai, M.; Tajti, J.; Szatmári, I.; et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J. Neural Transm. 2014, 121, 725–738.
- Vamos, E.; Pardutz, A.; Fejes, A.; Tajti, J.; Toldi, J.; Vecsei, L. Modulatory effects of probenecid on the nitroglycerin-induced changes in the rat caudal trigeminal nucleus. Eur. J. Pharmacol. 2009, 621, 33–37.
- Vámos, E.; Párdutz, Á.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429.
- Nagy-Grócz, G.; Tar, L.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Vécsei, L.; Párdutz, Á. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2015, 36, 849–861.
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 8, 278.
- Lukács, M.; Warfvinge, K.; Kruse, L.S.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L.; Edvinsson, L. KYNA analogue SZR72 modifies CFA-induced dural inflammation- regarding expression of pERK1/2 and IL-1β in the rat trigeminal ganglion. J. Headache Pain 2016, 17, 64.
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17.
- Cseh, E.K.; Veres, G.; Körtési, T.; Polyák, H.; Nánási, N.; Tajti, J.; Párdutz, Á.; Klivényi, P.; Vécsei, L.; Zádori, D. Neurotransmitter and tryptophan metabolite concentration changes in the complete Freund’s adjuvant model of orofacial pain. J. Headache Pain 2020, 21, 35.
- Fejes-Szabo, A.; Bohar, Z.; Nagy-Grocz, G.; Vamos, E.; Tar, L.; Podor, B.; Tajti, J.; Toldi, J.; Vecsei, L.; Pardutz, Á. Effect of probenecid on the pain-related behaviour and morphological markers in orofacial formalin test of the rat. CNS Neurol. Disord. Drug Targets 2015, 14, 350–359.
- Veres, G.; Fejes-Szabó, A.; Zádori, D.; Nagy-Grócz, G.; László, A.M.; Bajtai, A.; Mándity, I.; Szentirmai, M.; Bohár, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: A behavioral, immunohistochemical and pharmacokinetic study. J. Neural Transm. 2016, 124, 99–112.
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Redavide, E.; Pampalone, S.; Toldi, J.; Fülöp, F.; Blandini, F.; Nappi, G.; Sandrini, G.; et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia 2016, 37, 1272–1284.
- Knyihár-Csillik, E.; Chadaide, Z.; Okuno, E.; Krisztin-Péva, B.; Toldi, J.; Varga, C.; Molnár, A.; Csillik, B.; Vécsei, L. Kynurenine aminotransferase in the supratentorial dura mater of the rat: Effect of stimulation of the trigeminal ganglion. Exp. Neurol. 2004, 186, 242–247.
- Knapp, L.; Szita, B.; Kocsis, K.; Vécsei, L.; Toldi, J. Nitroglycerin enhances the propagation of cortical spreading depression: Comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des. Dev. Ther. 2016, 11, 27–34.
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745.
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47.
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27.
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J. Headache Pain 2021, 22, 60.
- Tuka, B.; Körtési, T.; Nánási, N.; Tömösi, F.; Janáky, T.; Veréb, D.; Szok, D.; Tajti, J.; Vécsei, L. Cluster headache and kynurenines. J. Headache Pain 2023, 24, 35.
- Curto, M.; Lionetto, L.; Fazio, F.; Mitsikostas, D.-D.; Martelletti, P. Fathoming the kynurenine pathway in migraine: Why understanding the enzymatic cascades is still critically important. Intern. Emerg. Med. 2015, 10, 413–421.
- Sato, K.; Kiyama, H.; Park, H.T.; Tohyama, M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. NeuroReport 1993, 4, 1263–1265.
- Mecs, L.; Tuboly, G.; Nagy, E.; Benedek, G.; Horvath, G. The Peripheral Antinociceptive Effects of Endomorphin-1 and Kynurenic Acid in the Rat Inflamed Joint Model. Obstet. Anesthesia Dig. 2009, 109, 1297–1304.
- Zhang, Y.-Q.; Ji, G.-C.; Wu, G.-C.; Zhao, Z.-Q. Kynurenic acid enhances electroacupuncture analgesia in normal and carrageenan-injected rats. Brain Res. 2003, 966, 300–307.




