
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Temitope Mary Olaleye | -- | 3253 | 2023-12-05 11:25:40 | | | |
| 2 | Peter Tang | Meta information modification | 3253 | 2023-12-06 01:50:40 | | |
Video Upload Options
Orbital angular momentum (OAM) encoding is a promising technique to boost data transmission capacity in optical communications. Azobenzene films have gained attention as a versatile tool for creating and altering OAM-carrying beams. Unique features of azobenzene films make it possible to control molecular alignment through light-induced isomerization about the azo bond. This feature enables the fabrication of diffractive optical devices such as spiral phase plates and holograms by accurately imprinting a phase profile on the incident light. By forming azobenzene sheets into diffractive optical elements, such as spiral phase plates, one can selectively create OAM-carrying beams. Due to the helical wavefront and phase variation shown by these beams, multiple distinct channels can be encoded within a single optical beam. This can significantly increase the data transmission capacity of optical communication systems with this OAM multiplexing technique. Additionally, holographic optical components made from azobenzene films can be used to build and reconstruct intricate wavefronts. It is possible to create OAM-based holograms by imprinting holographic designs on azobenzene films, which makes it simpler to control and shape optical beams for specific communication requirements. In addition, azobenzene-based materials can then be suitable for integration into optical communication devices because of their reconfigurability, compactness, and infrastructure compatibility, which are the main future perspectives for achieving OAM-based technologies for the next generation, among other factors.
1. Introduction
2. Background, Generation, and Application of OAM Light
3. Azobenzene Materials
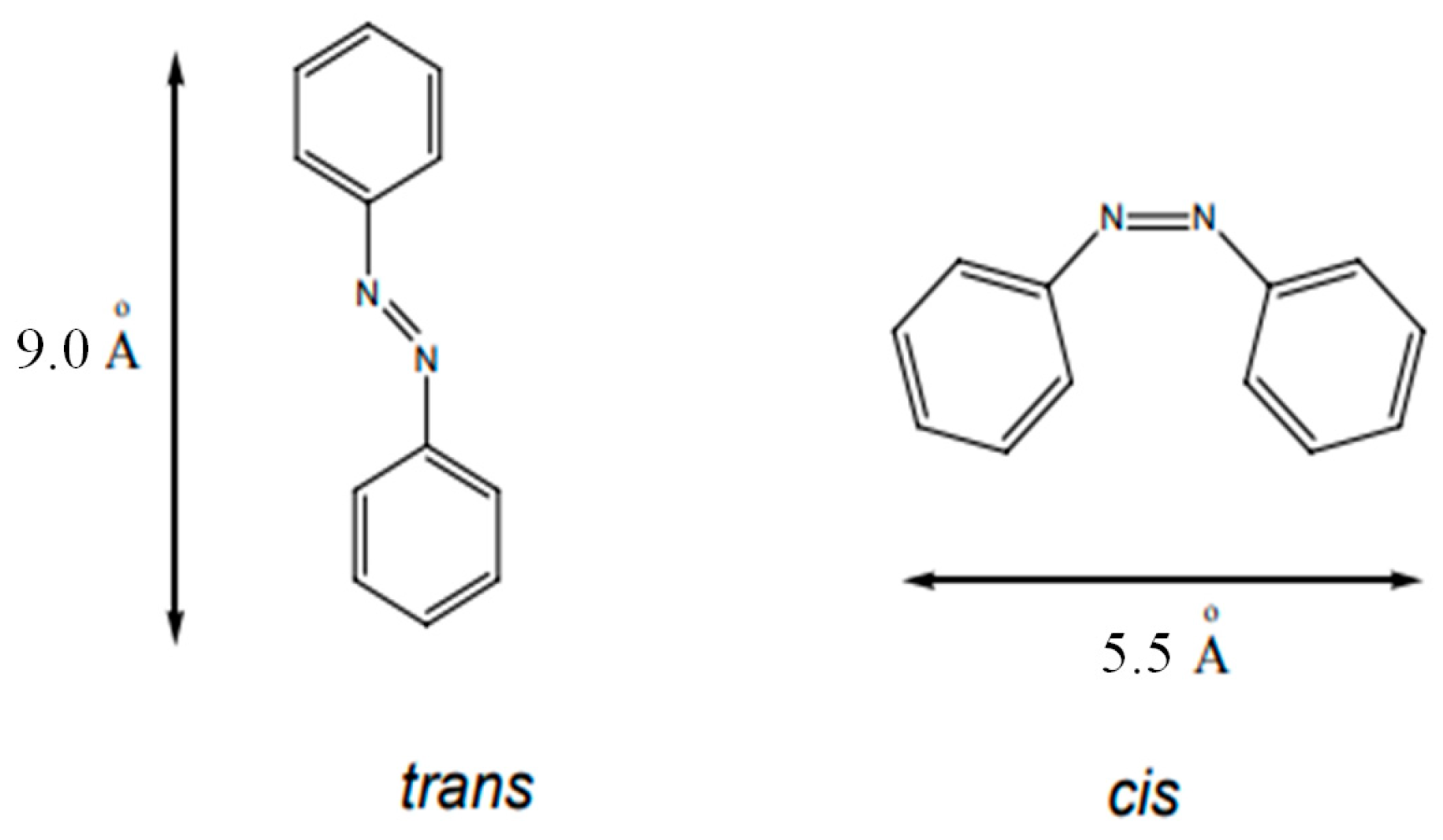
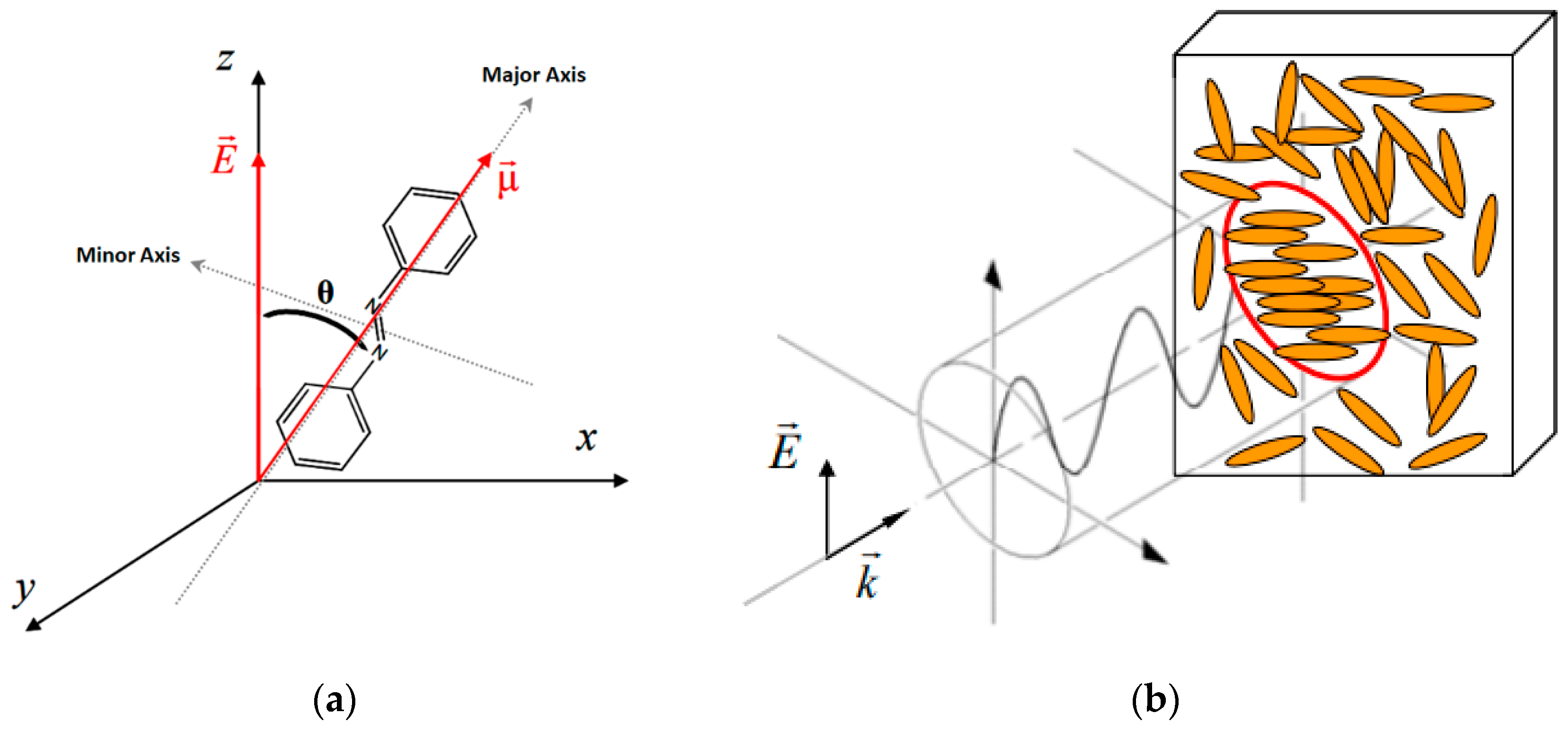
3.1. Azobenzene Thin Films
4. Azobenzene for OAM Generation and Manipulation
- i.
-
The azobenzene-enabled amplitude control plays a pivotal role in encoding information onto light by precisely modulating its intensity or brightness at different spatial points. This can be used to create patterns, enhance contrast, improve resolution and encode information. It is especially useful in microscopy and other optical applications. However, historically, lenses, prisms, apertures, and mirrors were the main static optical devices used in light manipulation, since accurate control over optical fields frequently required more complicated modifications [82]. One may accurately control the amplitude of a light beam as a first step toward better control, an idea that was crucial in the creation of holography. Amplitude masks were used in holography to simulate a “writing” laser beam which carries the information that is being encoded onto a holographic plate. Although clearly beneficial, this method is only able to use specified beam patterns [83].
- ii.
-
Azobenzene materials also facilitate phase modulation by altering the timing or phases of different parts of a light wave. This process is integral to enabling beam shaping, producing structured wavefronts, and navigating light in desired directions in applications such as interference patterns, holography [84], and wavefront shaping within optical communication systems [85].
- iii.
-
Polarization control alters the orientation of the electric field vector of light. It is used in applications such as LCDs, 3D cinema for 3D effects, and optical communications for transmission of information. Azobenzene plays a valuable role in enabling the manipulation of light’s polarization state for improving data-carrying capacity using polarization-based multiplexing and demultiplexing techniques in optical communication systems.
References
- Knight, P. Fundamentals of Photonics. J. Mod. Opt. 1992, 39, 1400.
- Chralyvy, A. Plenary paper: The coming capacity crunch. In Proceedings of the 35th European Conference on Optical Communication, Vienna, Austria, 20–24 September 2009; p. 1.
- Allen, L.; Beijersbergen, M.W.; Spreeuw, R.J.C.; Woerdman, J.P. Orbital angular momentum of light and the transformation of Laguerre-Gaussian laser modes. Phys. Rev. A 1992, 45, 8185–8189.
- Abramochkin, E.; Volostnikov, V. Beam transformations and nontransformed beams. Opt. Commun. 1991, 83, 123–135.
- Shen, Y.; Wang, X.; Xie, Z.; Min, C.; Fu, X.; Liu, Q.; Gong, M.; Yuan, X. Optical vortices 30 years on: OAM manipulation from topological charge to multiple singularities. Light Sci. Appl. 2019, 8, 90.
- Yao, A.M.; Padgett, M.J. Orbital angular momentum: Origins, behavior and applications. Adv. Opt. Photonics 2011, 3, 161.
- Ellis, A.D.; Zhao, J.; Cotter, D. Approaching the Non-Linear Shannon Limit. J. Light. Technol. 2010, 28, 423–433.
- Essiambre, R.-J.; Kramer, G.; Winzer, P.J.; Foschini, G.J.; Goebel, B. Capacity Limits of Optical Fiber Networks. J. Light. Technol. 2010, 28, 662–701.
- Willner, A.E.; Huang, H.; Yan, Y.; Ren, Y.; Ahmed, N.; Xie, G.; Bao, C.; Li, L.; Cao, Y.; Zhao, Z.; et al. Optical communications using orbital angular momentum beams. Adv. Opt. Photonics 2015, 7, 66.
- Zhai, Y.; Cao, L.; Liu, Y.; Tan, X. A Review of Polarization-Sensitive Materials for Polarization Holography. Mater. 2020, 13, 5562.
- Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 2021, 6, 51–69.
- Fregoni, J.; Granucci, G.; Coccia, E.; Persico, M.; Corni, S. Manipulating azobenzene photoisomerization through strong light–molecule coupling. Nat. Commun. 2018, 9, 4688.
- El Halabieh, R.H.; Mermut, O.; Barrett, C.J. Using light to control physical properties of polymers and surfaces with azobenzene chromophores. Pure Appl. Chem. 2004, 76, 1445–1465.
- Forbes, A. Structured Light from Lasers. Laser Photonics Rev. 2019, 13, 1900140.
- Nakayama, K.; Jiang, L.; Iyoda, T.; Hashimoto, K.; Fijishima, A. Photo-induced structural transformation on the surface of azobenzene crystals. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 1997, 36, 3898–3902.
- Harada, K.; Itoh, M.; Yatagai, T.; Kamemaru, S.I. Application of surface relief hologram using azobenzene containing polymer film. Opt. Rev. 2005, 12, 130–134.
- De Martino, S.; Mauro, F.; Netti, P.A. Photonic applications of azobenzene molecules embedded in amorphous polymer. Riv. Nuovo Cim. 2020, 43, 599–629.
- Sekkat, Z.; Wood, J.; Aust, E.F.; Knoll, W.; Volksen, W.; Miller, R.D. Light-induced orientation in a high glass transition temperature polyimide with polar azo dyes in the side chain. J. Opt. Soc. Am. B 1996, 13, 1713–1724.
- Liu, J.; Han, Z.; Wu, P.; Shang, Y.; Chen, J.; Jia, P. Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern. Molecules 2023, 28, 5881.
- Yang, X.; Jin, H.; Tao, X.; Yao, Y.; Xie, Y.; Lin, S. Photo-Responsive Azobenzene-Containing Inverse Opal Films for Information Security. Adv. Funct. Mater. 2023, 33, 2304424.
- Curtis, D.G.-P. Review Letters, and Undefined 2003, “Structure of Optical Vortices,” APS. Available online: https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.90.133901 (accessed on 31 October 2023).
- Ramachandran, S.; Kristensen, P. Optical vortices in fiber. Nanophotonics 2013, 2, 455–474.
- Berkhout, G.C.; Beijersbergen, M.W. Method for probing the orbital angular momentum of optical vortices in electromagnetic waves from astronomical objects. Phys. Rev. Lett. 2008, 101, 100801.
- Cunha, A.; Figueira, G.; André, P. Enabling the study of photons orbital angular momentum for optical communications: Orbital angular momentum carrying beam modulation and demodulation using twsited nematic liquid crystal spatial light modulators. Opt. Quantum Electron. 2016, 48, 425.
- Leach, J.; Padgett, M.J.; Barnett, S.M.; Franke-Arnold, S.; Courtial, J. Measuring the Orbital Angular Momentum of a Single Photon. Phys. Rev. Lett. 2002, 88, 4.
- Veysi, M.; Guclu, C.; Capolino, F.; Rahmat-Samii, Y. Revisiting orbital angular momentum beams: Fundamentals, reflectarray generation, and novel antenna applications. IEEE Antennas Propag. Mag. 2018, 60, 68–81.
- Gibson, G.; Courtial, J.; Padgett, M.J.; Vasnetsov, M.; Pas’ko, V.; Barnett, S.M.; Franke-Arnold, S. Free-space information transfer using light beams carrying orbital angular momentum. Opt. Express 2004, 12, 5448.
- Molina-Terriza, G.; Torres, J.P.; Torner, L. Twisted photons. Nat. Phys. 2007, 3, 305–310.
- Erhard, M.; Fickler, R.; Krenn, M.; Zeilinger, A. Twisted photons: New quantum perspectives in high dimensions. Light Sci. Appl. 2018, 7, 17146.
- Yang, Y.; Li, Y.; Wang, C. Generation and expansion of Laguerre–Gaussian beams. J. Opt. 2022, 51, 910–926.
- Qiu, S.; Wang, C.; Liu, T.; Ren, Y. Generation of spiral optical vortex with varying OAM for micro-manipulation. Opt. Commun. 2022, 524, 128767.
- Cojoc, D.; Garbin, V.; Ferrari, E.; Proietti, R.; Cabrini, S.; Di Fabrizio, E. Laser trapping and micro-manipulation using optical vortices. Dig. Pap. Microprocess. Nanotechnol. 2004, 2004, 260–261.
- Tao, S.H.; Yuan, X.C.; Lin, J.; Sun, Y.Y. Influence of geometric shape of optically trapped particles on the optical rotation induced by vortex beams. J. Appl. Phys. 2006, 100, 043105.
- Kai, C.; Feng, Z.; Dedo, M.I.; Huang, P.; Guo, K.; Shen, F.; Gao, J.; Guo, Z. The Performances of Different OAM Encoding Systems. Opt. Commun. 2019, 430, 151–157.
- Picón, A.; Benseny, A.; Mompart, J.; Vázquez De Aldana, J.R.; Plaja, L.; Calvo, G.F.; Roso, L. Transferring orbital and spin angular momenta of light to atoms. New J. Phys. 2010, 12, 83053.
- Papathanasopoulos, A.; Rahmat-Samii, Y. A Review on Orbital Angular Momentum (OAM) Beams: Fundamental Concepts, Potential Applications, and Perspectives. In Proceedings of the 2021 XXXIVth General Assembly and Scientific Symposium of the International Union of Radio Science (URSI GASS), Rome, Italy, 28 August–4 September 2021; IEEE: New York, NY, USA, 2021; pp. 1–4.
- Mahimwalla, Z.; Yager, K.G.; Mamiya, J.I.; Shishido, A.; Priimagi, A.; Barrett, C.J. Azobenzene photomechanics: Prospects and potential applications. Polym. Bull. 2012, 69, 967–1006.
- Yamashita, S.; Ono, H.; Toyama, O. The cis-trans Photoisomerization of Azobenzene. Bull. Chem. Soc. Jpn. 1962, 35, 1849–1853.
- Yu, X.; Wang, Z.; Buchholz, M.; Füllgrabe, N.; Grosjean, S.; Bebensee, F.; Bräse, S.; Wöll, C.; Heinke, L. cis-to-trans isomerization of azobenzene investigated by using thin films of metal–organic frameworks. Phys. Chem. Chem. Phys. 2015, 17, 22721–22725.
- Siampiringue, N.; Guyot, G.; Monti, S.; Bortolus, P. The cis → trans photoisomerization of azobenzene: An experimental re-examination. J. Photochem. 1987, 37, 185–188.
- Hartley, G.S. The Cis-Form of Azobenzene. Nature 1937, 140, 281.
- Todorov, T.; Nikolova, L.; Tomova, N. Polarization holography 1: A new high-efficiency organic material with reversible photoinduced birefringence. Appl. Opt. 1984, 23, 4309.
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261.
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175.
- Chyla, A.; Bieńkowski, M.; Sworakowski, J.; Koźlecki, T.; Wilk, K.A. Photochromic properties of anionic azobenzene amphiphiles in solution and Langmuir Blodgett films. Trends Colloid Interface Sci. XI 2007, 105, 153–159.
- Rochon, P.; Batalla, E.; Natansohn, A. Optically induced surface gratings on azoaromatic polymer films. Appl. Phys. Lett. 1995, 66, 136–138.
- Kim, D.; Tripathy, S.; Li, L.; Kumar, J. Laser-Induced Holographic Surface Relief Gratings on Nonlinear Optical Polymer Films. Available online: https://pubs.aip.org/aip/apl/article-abstract/66/10/1166/64201 (accessed on 26 October 2023).
- Oliveira, O.N.; Li, L.; Kumar, J.; Tripathy, S.K. Surface-Relief Gratings on Azobenzene-Containing Films. In Photoreactive Organic Thin Films; Photoreact, Elsevier: Amsterdam, The Netherlands, 2002; pp. 429–486.
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Berichte Bunsenges. Phys. Chem. 1991, 95, 1430–1434.
- Decher, G.; Schmitt, J. Fine-Tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. Trends Colloid Interface Sci. VI 2007, 89, 160–164.
- Oliveira, O.N., Jr.; Raposo, M.; Dhanabalan, A. Langmuir-Blodgett and Self-assembled polymeric films. In Handbook of Surfaces and Interfaces of Materials: Solid Thin Films and Layers; Academic Press: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2001; Volume 4, pp. 1–63.
- Seki, T.; Sakuragi, M.; Kawanishi, Y.; Suzuki, Y.; Tamaki, T.; Fukuda, R.; Ichimura, K.; Suzuki, Y. “Command Surfaces” of Langmuir-Blodgett Films. Photoregulations of Liquid Crystal Alignment by Molecularly Tailored Surface Azobenzene Layers. Langmuir 1993, 9, 211–218.
- Uznanski, P.; Pecherz, J. Surface plasmon resonance of azobenzene-incorporated polyelectrolyte thin films as an H+ indicator. J. Appl. Polym. Sci. 2002, 86, 1459–1464.
- Han, M.; Ichimura, K. In-plane and tilt reorientation of p-methoxyazobenzene side chains tethered to liquid crystalline polymethacrylates by irradiation with 365 nm light. Macromolecules 2001, 34, 90–98.
- Madruga, C.; Filho, P.A.; Andrade, M.M.; Gonçalves, M.; Raposo, M.; Ribeiro, P.A. Birefringence dynamics of poly cast films. Thin Solid Film. 2011, 519, 8191–8196.
- Balasubramanian, S.; Wang, X.; Wang, H.C.; Yang, K.; Kumar, J.; Tripathy, S.K.; Li, L. Azo Chromophore-Functionalized Polyelectrolytes. 2. Acentric Self-Assembly through a Layer-by-Layer Deposition Process. Chem. Mater. 1998, 10, 1554–1560.
- Tripathy, S.; Kumar, J.; Nalwa, H.S. Handbook of Polyelectrolytes and Their Applications. Available online: https://cir.nii.ac.jp/crid/1130282271512206592 (accessed on 27 October 2023).
- Ferreira, Q.; Ribeiro, P.A.; Oliveira, O.N.; Raposo, M. Long-term stability at high temperatures for birefringence in PAZO/PAH layer-by-layer films. ACS Appl. Mater. Interfaces 2012, 4, 1470–1477.
- Bobrovsky, A.; Shibaev, V.; Cigl, M.; Hamplová, V.; Pociecha, D.; Bubnov, A. Azobenzene-containing LC polymethacrylates highly photosensitive in broad spectral range. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2962–2970.
- Leibold, J.; Sabat, R.G. Fabrication of micrometer-scale surface relief gratings in azobenzene molecular glass films using a modified Lloyd’s mirror interferometer. Opt. Mater. 2019, 96, 109315.
- Kamenjicki, M.; Lednev, I.K.; Asher, S.A. Photoresponsive azobenzene photonic crystals. J. Phys. Chem. B 2004, 108, 12637–12639.
- Bisoyi, H.; Li, Q. Light-directing chiral liquid crystal nanostructures: From 1D to 3D. Acc. Chem. Res. 2014, 47, 3184–3195.
- Mosquera, J.; Zhao, Y.; Jang, H.J.; Xie, N.; Xu, C.; Kotov, N.A.; Liz-Marzán, L.M. Plasmonic Nanoparticles with Supramolecular Recognition. Adv. Funct. Mater. 2020, 30, 1902082.
- Mahmoud, M.A. Electromagnetic field of plasmonic nanoparticles extends the photoisomerization lifetime of azobenzene. J. Phys. Chem. C 2017, 121, 18144–18152.
- Bruder, F.K.; Hagen, R.; Rölle, T.; Weiser, M.S.; Fäcke, T. From the surface to volume: Concepts for the next generation of optical-holographic data-storage materials. Angew. Chem. Int. Ed. 2011, 50, 4552–4573.
- Hagen, R.; Bieringer, T. Photoaddressable Polymers for Optical Data Storage. Adv. Mater. 2001, 13, 1805–1810.
- Mazloumi, M.; Dawson, E.; Sabat, R.G. Hierarchical concentric surface patterns and metasurfaces on azobenzene molecular glass films using axicon interference lithography. Opt. Mater. 2023, 136, 113428.
- Kang, H.S.; Yang, S. Photopatterning via photofluidization of azobenzene polymers. Light Adv. Manuf. 2022, 3, 3.
- Yager, K.G.; Barrett, C.J. Azobenzene Polymers for Photonic Applications. In Smart Light Responsive Materials: Azobenzene Containing Polymers and Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–46.
- Yu, H. Recent advances in photoresponsive liquid-crystalline polymers containing azobenzene chromophores. J. Mater. Chem. C 2014, 2, 3047–3054.
- Yamamoto, T.; Hasegawa, M.; Kanazawa, A.; Shiono, T.; Ikeda, T. Phase-type gratings formed by photochemical phase transition of polymer azobenzene liquid crystals: Enhancement of diffraction efficiency by spatial modulation of molecular alignment. ACS Publ. 1999, 103, 9873–9878.
- Huang, J.; Beckemper, S.; Wu, S.; Shen, J.; Zhang, Q.; Wang, K.; Gillner, A. Light driving force for surface patterning on azobenzene-containing polymers. Phys. Chem. Chem. Phys. 2011, 13, 16150–16158.
- Bégin, J.-L.; Jain, A.; Parks, A.; Hufnagel, F.; Corkum, P.; Karimi, E.; Brabec, T.; Bhardwaj, R. Nonlinear helical dichroism in chiral and achiral molecules. Nat. Photonics 2023, 17, 82–88.
- Sánchez, C.; Alcalá, R.; Hvilsted, S.; Ramanujam, P.S. Biphotonic holographic gratings in azobenzene polyesters: Surface relief phenomena and polarization effects. Appl. Phys. Lett. 2000, 77, 1440–1442.
- Sobolewska, A.; Zawada, J.; Bartkiewicz, S. Biphotonic Photochromic Reaction Results in an Increase in the Efficiency of the Holographic Recording Process in an Azo Polymer. Langmuir 2014, 30, 17–21.
- Wu, P.; Zou, B.; Wu, X.; Xu, J.; Gong, X.; Zhang, G.; Tang, G.; Chen, W. Biphotonic self-diffraction in azo-doped polymer film. Appl. Phys. Lett. 1997, 70, 1224–1226.
- Wu, P.; Wang, L.; Xu, J.; Zou, B.; Gong, X.; Zhang, G.; Tang, G.; Chen, W. Transient biphotonic holographic grating in photoisomerizative azo materials. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 57, 3874–3880.
- Wu, P.; Wu, X.; Wang, L.; Xu, J.; Zou, B.; Gong, X.; Huang, W. Image storage based on biphotonic holography in azo/polymer system. Appl. Phys. Lett. 1998, 72, 418–420.
- Kim, S.; Ishii, S.; Yagi, R.; Kuwahara, Y.; Ogata, T.; Kurihara, S. Photo-induced orientation behaviors of azobenzene liquid crystal copolymers for photonic crystals. RSC Adv. 2017, 7, 51978–51985.
- Pang, X.; Lv, J.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable Azobenzene-Containing Liquid Crystal Polymers and Soft Actuators. Adv. Mater. 2019, 31, 1904224.
- Andrews, D.L. Structured Light and Its Applications: An Introduction to Phase-Structured Beams and Nanoscale Optical Forces; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123740274.
- Angelsky, O.V.; Bekshaev, A.Y.; Dragan, G.S.; Maksimyak, P.P.; Zenkova, C.Y.; Zheng, J. Structured Light Control and Diagnostics Using Optical Crystals. Front. Phys. 2021, 9, 715045.
- Clark, T.W.; Offer, R.F.; Franke-Arnold, S.; Arnold, A.S.; Radwell, N. Comparison of beam generation techniques using a phase only spatial light modulator. Opt. Express 2016, 24, 6249.
- Zheng, S.; Zhao, Z.; Zhang, W. Versatile generation and manipulation of phase-structured light beams using on-chip subwavelength holographic surface gratings. Nanophotonics 2023, 12, 55–70.
- Angelsky, O.V.; Bekshaev, A.Y.; Hanson, S.G.; Zenkova, C.Y.; Mokhun, I.I.; Jun, Z. Structured Light: Ideas and Concepts. Front. Phys. 2020, 8, 114.
- Porfirev, A.; Khonina, S.; Khorin, P.; Ivliev, N. Polarization-Sensitive Direct Laser Patterning of Azopolymer Thin Films with Vortex Beams. Opt. Lett. 2022, 47, 5080–5083.
- Berkowitz, R. Unpolarized Light Could Separate Chiral Molecules. Physics 2022, 15, 66.
- Dumont, M.; El Osman, A. On spontaneous and photoinduced orientational mobility of dye molecules in polymers. Chem. Phys. 1999, 245, 437–462.
- Sekkat, Z.; Yasumatsu, D.; Kawata, S. Pure Photoorientation of Azo Dye in Polyurethanes and Quantification of Orientation of Spectrally Overlapping Isomers. J. Phys. Chem. B 2002, 106, 12407–12417.




