Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Pereira | -- | 5102 | 2023-12-05 10:53:41 | | | |
| 2 | Catherine Yang | -5 word(s) | 5097 | 2023-12-06 01:53:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pereira, J.; Souza, R.; Moreira, A.; Moita, A. Thermophysical Properties of Nanofluids and PCMs. Encyclopedia. Available online: https://encyclopedia.pub/entry/52362 (accessed on 08 February 2026).
Pereira J, Souza R, Moreira A, Moita A. Thermophysical Properties of Nanofluids and PCMs. Encyclopedia. Available at: https://encyclopedia.pub/entry/52362. Accessed February 08, 2026.
Pereira, José, Reinaldo Souza, António Moreira, Ana Moita. "Thermophysical Properties of Nanofluids and PCMs" Encyclopedia, https://encyclopedia.pub/entry/52362 (accessed February 08, 2026).
Pereira, J., Souza, R., Moreira, A., & Moita, A. (2023, December 05). Thermophysical Properties of Nanofluids and PCMs. In Encyclopedia. https://encyclopedia.pub/entry/52362
Pereira, José, et al. "Thermophysical Properties of Nanofluids and PCMs." Encyclopedia. Web. 05 December, 2023.
Copy Citation
Nanofluids can enhance the thermal conductivity of the base fluids by up to 100%. In addition, it was also reported that the simultaneous use of PCMs and nanofluids enhances the overall, thermal, and electrical efficiencies of solar thermal energy storage systems and photovoltaic-nano-enhanced PCM systems. The capability of storing substantial quantities of latent energy of between 100 MJ/m3 and 250 MJ/m3 and energy densities of from 150 MJ/m3 to 430 MJ/m3 for organic and inorganic PCMs, respectively, makes them very promising alternatives for thermal energy storage purposes.
nanofluids
PCMs
heat transfer
thermal energy storage
1. Thermal Conductivity
The thermal conductivity of nanofluids depends on various factors like the type, morphology, and concentration of the added nanoparticles; the type of base fluid; the addition of surfactants; and the operating temperature. Nonetheless, the recent findings about their contribution to the effective thermal conductivity of nanofluids are inconsistent and often persist with some discrepancies and somewhat unexpected results. For example, the researchers Philip et al. [1] investigated thermal conductivity changes with a volumetric concentration of oleic acid-coated magnetite nanoparticles dispersed in kerosene-based fluid. No thermal conductivity increase was reported up to a concentration value of 1.7% vol. Beyond this value, the thermal conductivity of the developed nanofluid changed linearly with the concentration of nanoparticles. The authors stated that the thermal conductivity remained unchanged at low concentrations because of the uniformly dispersed nanoparticles, whereas at higher concentrations the existing nanoparticle clusters in the kerosene could have been the responsible for the verified thermal conductivity increase.
Chon et al. [2] evaluated the impact of nanoparticles added in the sizes of 11 nm, 47 nm, and 150 nm on the thermal conductivity of alumina aqueous nanofluids. The authors observed a greater thermal conductivity enhancement when they added the smaller nanoparticles of 11 nm. This was interpreted based on the enhanced Brownian motion of the smaller nanoparticles.
Beck et al. [3] found that the thermal conductivity of the nanofluids increased with increasing nanoparticle size. The research team argued that the verified thermal conductivity decrease for the smaller particles was caused by the enhanced phonon scattering at the interface between the nanoparticles and the base fluid. Indeed, when dealing with nanoparticle-based fluid suspensions, the actual size of the nanoparticles is their hydrodynamic size because of the contribution from the ordered liquid layer around the nanoparticles and the surfactant molecules around the surface of the dispersed nanoparticles.
Jeong et al. [4] studied the thermal conductivity of aqueous nanofluids containing zinc oxide nanoparticles with different morphologies and in different concentrations. The obtained results revealed a 12% increase in the thermal conductivity of the nanofluids with spherical nanoparticles, whereas the increase was of 18% when the authors added rectangular zinc oxide nanoparticles to water.
Murshed et al. [5] observed an increase in thermal conductivity of 17.5% with respect to that of ethylene glycol alone in the nanofluid composed of 5% vol. titanium oxide spherical nanoparticles dispersed in ethylene glycol-based fluid. The increase was of 20% when the same concentration of titanium oxide cylindrical nanoparticles was added. Also, the authors Zhu et al. [6] verified a higher thermal conductivity enhancement of 38% for iron oxide nanofluids than for titanium oxide, copper oxide, and alumina nanofluids (30% increase), though the thermal conductivity of the iron oxide of 7 W/mK was lower than that of the titanium oxide (11.7 W/mK), copper oxide (20 W/mK), and alumina (36 W/mK). The observed enhancement for Fe3O4-based nanofluid was attributed to the alignment of the nanoparticles as clusters, where the thermal conductivity increased with increasing concentration because of the increased length of the aligned particles.
Hong et al. [7] reported a higher thermal conductivity increase for iron nanofluids than for copper nanofluids, even though the bulk iron had a lower thermal conductivity of 80 W/mK than the bulk copper, of 384 W/mK. In fact, the iron nanofluids demonstrated an 18% increase in the thermal conductivity at a 0.55% vol. of nanoparticles, whereas the copper nanofluid at the same concentration value exhibited a 14% increase.
The last two studies demonstrated that, contrary to what was expected, the incorporation of highly thermal conductive nanoparticles is not always the best choice for enhancing the thermal conductivity of nanofluids. Instead, the wetting of the surface of the nanoparticles and the heat transfer capability at the solid–liquid interface are usually determining factors for the thermal conductivity enhancement verified in the nanofluids.
On the other hand, the researchers Dadwal et al. [8] examined the influence of the size of the nanoparticles on the thermal conductivity of magnetite nanoparticles suspended in kerosene and toluene. The researchers reported distinct tendencies of the thermal conductivity evolution with the size of the nanoparticles in the two base fluids, given that a thermal conductivity increase with increasing nanoparticle size was observed for the kerosene, whereas, with toluene as base fluid, the smaller nanoparticles promoted a thermal conductivity increase. The authors argued that the different evolutions of the size-dependent thermal conductivity could have been caused by the different nanoparticle-based-fluid interactions. The interaction between the nanoparticles and the molecules of the base fluid determines the thickness of the interfacial layer. This interaction is different for different base fluids, and it fundamentally decides the density of the nanolayer at the solid–liquid interface, which in turn affects the heat transfer performance at this interface.
Accordingly, the authors Kamalvand et al. [9] analyzed the enhancement in thermal conductivity of nanofluids with the adsorption of the molecules of the solvent onto the nanoparticles at distinct temperature values. The investigation team found a linear increase in thermal conductivity with the adsorption of the base fluid molecules onto the surface of the dispersed nanoparticles. Also, the authors confirmed that the adsorption level of the molecules of the base liquid increased with increasing bulk temperature.
Altan et al. [10] investigated the impact of the base fluid on the thermal conductivity of iron oxide nanoparticles coated with capric acid and oleic acid surfactants and suspended in hexane, heptane, and mineral oil. The research team verified a greater thermal conductivity increase when using hexane as the base fluid, followed by heptane and mineral oil. The researchers stated that the greater increase was not directly linked to the low thermal conductivity of the base fluid, and that, instead, the interactions between the base fluid and the added surfactant at the solid–liquid interface could be the main reason behind the thermal conductivity enhancement.
Shao et al. [11] evaluated an aqueous hybrid nanofluid containing nanoparticles of titanium oxide under the form of nanotubes of 9 nm to 10 nm and nanoplatelets of 50 nm to 80 nm. It was confirmed that the nanofluid at 0.1% wt. of titanium oxide with 25% of titanium nanoparticles showed the greatest enhancement in thermal conductivity, of 22.3%, in comparison to that of the water-based fluid. The impact of the super-cooling degree of the hybrid nanofluids decreased by up to 4.97 ± 0.2 °C and 5.27 ± 0.2 °C, respectively, compared to mono nanofluids. The freezing time for the hybrid nanofluid was also diminished by up to 54.9% in comparison to the titanium nanoplatelet nanofluid and by 56.4% compared with the nanofluid with titanium nanoparticles. Concerning the thermal conductivity of the phase-change materials with the incorporation of nanoparticles, it can be stated that most of the available papers on the subject focus on thermal conductivity enhancement as a direct effect of the addition of nanomaterials to phase-change materials.
The only exception was the study conducted by Colla et al. [12], where the addition of alumina nanoparticles to paraffin caused a 7–8% lower thermal conductivity compared with that of pure paraffin. There is no clear explanation for this phenomenon in the mentioned study. Generally, due to the improved thermal conductivity of nanocomposites and nanofluids, a reduction in heating, melting, and solidification times can be expected. Usually, the expected improvement in thermal conductivity is in the range of 20–100%, but there are examples of even higher improvements being reported—for instance, in the case of the lauric acid phase-change nanocomposite with graphene nanoplatelet [13], carbon-based phase-change material [14], or magnesium chloride hexahydrate phase-change material composites [15]. Generally, it can be concluded that an increase in the concentration of nanomaterials dispersed in the phase-change materials increases the thermal conductivity. This may be misleading, since there is a limit to the amount of nanomaterial in the phase-change material—i.e., nanomaterials in larger fractions tend to agglomerate. Agglomeration may lead to a decrease or increase in thermal conductivity. It is hard to predict when agglomeration will occur since it depends on many parameters, such as the mass or volume fraction, size, and morphology of the nanomaterials, among others. Furthermore, the type, phase, and temperature of the phase-change material may also have a significant influence on this phenomenon.
Sharma et al. [16] prepared a nano-enhanced phase-change material composed of palmitic acid and nanoparticles of titanium oxide. The authors stated that the increase in the mass fraction of the nanoparticles causes the nano-enhanced phase-change material thermal conductivity to increase. A maximum increase in thermal conductivity of about 80% was recorded for 5% wt. loading of nanoparticles. For the same concentration of nanoparticles, there was a 15.5% decrease in latent heat of fusion with respect to the pure phase-change material.
Mayilvelnathan and Arasu [17] reported on the thermal conductivity measured by the laser flash method of pure erythritol phase-change material, i.e., erythritol with 0.1%, 0.5%, and 1% of graphene nanoparticles before as well as after thermal-cycling periods. The thermal conductivity of pure erythritol was 0.733 W/mK, whereas, when considering the erythritol with 0.1% wt., 0.5% wt., and 1% wt. of graphene nanoparticles, the thermal conductivity increased to 1.074, 1.095, and 1.122 W/mK. The thermal conductivity was 0.692, 0.899, 0.921, and 1.020 W/mK for pure erythritol without and with 0.1% wt., 0.5% wt., and 1% wt. of graphene nanoparticles, respectively. Additionally, the authors Putra et al. [18] studied the thermophysical characteristics of nano-enhanced phase-change materials based on Rubitherm paraffin wax, RT22 HC, and graphene nanoplatelets. The thermal conductivity of the phase-change material was increased by around 90% with the addition of 0.3% wt. of graphene.
Nourani et al. [19] modified paraffin with different mass fractions of alumina nanoparticles. The authors in the article claim that the character of the relationship between the effective thermal conductivity and increasing the alumina mass fraction was nonlinear for both the solid and the liquid phase. The enhancement ratio of the effective thermal conductivity was 31% in the solid phase and 13% in the liquid phase for the nano-enhanced phase-change material at 10% wt. of alumina. A reduction of 27% of the heating and melting times was also detected for the same concentration of nanoparticles in the phase-change material.
Wang et al. [20] produced a stable OP10E and water emulsion enhanced by the inclusion of graphite nanoparticles. The authors argued that the supercooling effect of the emulsion could be prevented by the addition of graphite nanoparticles with a concentration superior to 2% wt. Furthermore, it was stated that the addition of graphite nanoparticles to the OP10E/water emulsion did not affect the latent heat. In the same study, it was found that 2% wt. of graphite nanoparticles resulted in a thermal conductivity increase of 88.9%.
2. Specific Heat
The specific heat of nanofluids depends primarily on the type, morphology, and concentration of the incorporated nanoparticles in the base fluid. Considering water as a base fluid, the published studies confirmed a considerable reduction in the specific heat of the water-based nanofluids, with the fundamental influencing factor for that to happen being the concentration of the nanoparticles added to the water. In this scope, the authors Zhou and Ni [21] prepared an aqueous alumina nanofluid at different concentrations ranging from 1.4% vol. to 21.7% vol. The differential scanning calorimetry technique was used to measure the specific heat of the nanofluids in a temperature range of between 20 °C and 45 °C. The research team observed decreases in the specific heat capacity of between 6% and 45% at concentrations of 1.4% vol. and 21.7% vol., respectively. Several other studies have investigated aqueous nanofluids with silica particles with dimensions of 32 nm [22] and 20 nm [23] and copper oxide particles with dimensions of 30 nm [24] and 23–37 nm [25] at various particle concentrations. These studies reported large decreases in the specific heat value with increasing nanoparticle concentrations. These lower specific heat values could have been a result of agglomerated nanoparticles in these samples, but a comprehensive characterization was reported. Like water-based nanofluids, ethylene glycol and ethylene glycol–water nanofluids showed decreases in specific heat from the values of pristine fluid. Although ethylene glycol had a lower specific heat value than water, it was still much higher than that of the particle materials. Thus, the proper trend of decreasing specific heat at higher particle concentrations was upheld in these studies. Additionally, the researchers Teng et al. [26] evaluated the specific heat of multi-walled carbon nanotubes dispersed in a mixture of ethylene glycol and water nanofluid at concentrations of 0.1% wt., 0.2% wt., and 0.4 wt.%, employing chitosan as a surfactant. The authors found that the inclusion of the multi-walled carbon nanotubes reduced the specific heat capacity of the base fluid mixture by between 2% and 8%, with the highest reduction shown by the most concentrated nanofluid. Furthermore, the researchers De Robertis et al. [27] analyzed the specific heat of a copper–ethylene glycol nanofluid at a 0.5% wt. of nanoparticles and at two different pH values. The pH of the primary nanofluid was 2.2, and a solution of sodium hydroxide was used to increase the pH to 10. The specific heat measurement revealed a decrease in specific heat in the nanofluids compared to the ethylene glycol-based fluid. The experiments showed a decrease of around 4% for the copper–ethylene glycol with the pH adjusted to 2.2 and a 12% decrease when the pH was adjusted to 10. It is likely that an increase in the pH moved the solution around the isoelectric point, perhaps resulting in an agglomeration of the nanoparticles in this case. From these available studies, it can be concluded that water and ethylene glycol nanofluids exhibit a decrease in specific heat with an increase in particle concentration and an increase in specific heat with an increase in temperature. In a more comprehensive pursuit of enhanced nanofluid-specific heat, a study by Starace et al. [28] explored various nanoparticle and base fluid combinations at different concentrations. The study used 36 different nanofluids with negligible changes in the heat capacity with respect to the base fluid. The authors used nanoparticles of silica, fumed silica, alumina needles, aluminum nitride, exfoliated graphite, iron core/iron oxide shell, bismuth, and mesoporous silica. The nanoparticles were dispersed in mineral oil, poly-α olefin, ethylene glycol, 60/40 by mass ethylene glycol/water, and calcium nitrate tetrahydrate. As some nanoparticles were suspended easily in some fluids and others required the use of surfactants, the researchers defined specific mixing procedures for each combination. The results showed only small changes in the specific heat considering the overall scatter of measured specific heats over the temperature range investigated. It was suggested that a change in overall specific heat could be possible only if a rearrangement of the base fluid molecules to a higher heat capacity configuration was caused by the nanoparticles. Molecular dynamic simulations of copper nanoparticles in ethylene glycol [29] have shown an ordering layer of 0.75 nm thick around the nanoparticle; however, the specific heat of this predicted ordered layer has not been studied. Despite the high number of available experimental methods for evaluating the thermal transport of PCMs during the phase-change process, numerical simulations are also powerful tools for the optimized design of PCMs. Numeric simulations can accurately describe the phase-change heat transfer process by exploring diverse models like the energy balance method, finite element simulation, or commercially available computational fluid dynamics (CFD) software such as Ansys Fluent or COMSOL Multiphysics with Heat Transfer Module. The structural and operational parameters after optimization can provide guidance for the thermal enhancement of PCMs and thermal energy storage systems containing PCMs. As the most common analytical methods for solving engineering problems, the energy balance method and the enthalpy method are widely used to simulate the thermal transport behavior of PCMs during the solidification/melting stages. To simulate the thermal transport of PCMs in thermal energy storage systems more efficiently, the finite element method and the CFD modeling method are introduced, which can easily accomplish the simulation. The energy balance method is usually used to study the flow of energy based on energy conservation and to analyze the thermal enhancement effect in PCMs. The energy equation for a system without energy generation can be given by Equation (1):
where, in the cases of solar energy modules operating with PCMs, Est is the energy transfer and energy retention between the different modules of the system, Ein is the received solar energy, and Eout is the energy loss of the system. When Est of the PCMs needs to be considered, the energy equation between the PCMs and other parts should be used. Based on the flow and change in energy, the thermal enhancement effect can be reliably addressed and regulated, which further improves the thermal energy storage ability of PCMs. The enthalpy method is based on the enthalpy modifications of the different modules of a solar thermal energy system. The fundamental benefit of the enthalpy method is closely related to the capability of avoiding direct front tracking when dealing with materials that melt or solidify over a range of temperatures, which enable numeric simulation using complex geometries. The enthalpy equation for the solidification and melting processes can be expressed by Equation (2):
 where H is the total enthalpy, ρ is the density of the operating fluid, ν is the velocity of the operating fluid, and S is the source term defined by the condition of the sample. The total enthalpy can be determined by Equation (3):
where h is the sensible heat enthalpy and ΔH is the latent heat enthalpy. The sensible heat enthalpy is expressed by Equation (4):
where H is the total enthalpy, ρ is the density of the operating fluid, ν is the velocity of the operating fluid, and S is the source term defined by the condition of the sample. The total enthalpy can be determined by Equation (3):
where h is the sensible heat enthalpy and ΔH is the latent heat enthalpy. The sensible heat enthalpy is expressed by Equation (4):
 where Cp is the specific heat capacity of the operating fluid and Href and Tref are reference values. The initial condition Ti = T(x,y,z,τ0) and boundary conditions like k·∂T/∂n = q0 and −k·∂T/∂n = hs(T − To) should be used in the numeric simulation, where q0 is the heat flux, hs is the air surface heat transfer coefficient, and To is the ambient temperature. The governing equation of the enthalpy method is similar to the single-phase equation, providing an easier solution to the phase-change problems. Also, in the enthalpy method, there are no conditions to be satisfied at the solid–liquid interface, and this method yields a solution involving both solid and liquid phases [30]. For example, the researchers Han et al. studied the thermal energy storage and release processes in multi-cavity-microstructured PCM microcapsules and found that the charge and discharge velocity of PCMs were accelerated by increasing the number of cavities and cavity interlayers in the microcapsules [31]. The feature is that the enthalpy method possesses great suitability to be applied in the field of engineering thermal energy storage systems, providing a reliable evaluation for engineering applications of PCMs. The finite element simulation method subdivides a large phase-change energy storage system into smaller and simpler elements and simulates them separately, which is called the discretization strategy. There are many types of discretization strategies, such as the h-version and the p-version. Both of these versions are numerical methods for solving partial differential equations. Their main difference is that the polynomial degrees of the elements are fixed and the mesh is refined in the h-version, whereas the finite element mesh is fixed and the polynomial degrees of elements are enhanced in the p-version [32]. After the simulation, the subdivided elements are then assembled into a larger system, which makes it easier to model the thermal transport problem during the phase-change process. The finite element simulation method offers many options for regulating the complexity of both modeling and analysis in a heat transfer system. At the same time, it can also balance the necessary accuracy and computational processing time that can assess many of the concerns associated with engineering applications. The thermal performance of PCMs, as one of the most studied research topics in the technological field, can be reliably described via finite element simulations [33]. For instance, the researchers Wang et al. [34] studied the impact of the porosity and thermal conductivity of a metal foam on the thermal conductivity and melting of PCMs. The optimum fin structure was attained, which considerably increased the rate of solidification of the PCMs, opposite to the velocity increase via the action of the dispersed copper nanoparticles studied by the authors Lohrasbi et al. [35]. The finite element simulation method provides accurate prediction of the thermal enhancement effect for micro-/nano-PCMs. To achieve a highly efficient finite element simulation, CFD modeling was developed to analyze engineering situations with inherent complex problems, such as the solidification and melting processes in PCMs for solar thermal purposes. This method also involves fluid mechanics such as turbulence models and two-phase flow, which focus on the flow of gas and liquid and can resolve the intricate restriction of the fluid flows in solar thermal research and technology, like the flow of heat transfer fluids at various flow rates. Therefore, to analyze the thermal enhancement during the solidification process of PCMs or the heat exchange process of colloidal suspensions, CFD is undoubtedly an ideal numerical simulation method. Moreover, the researchers Mahdi et al. [36] combined nanoparticles with metal foam or metal fins in a thermal energy storage system containing triplex-tube PCMs and confirmed the thermal enhancement provoked by this solution for the improvement of the thermal energy storage system. Further work on PCM thermal energy storage analysis will undoubtedly require the development of CFD modeling with enhanced computing processing capacity. Also, to seize the internal and external thermal energy exchange of PCMs or PCMs operating in thermal energy storage systems, the combined exploration of experimental findings and numeric simulations is a vital tendency that should be maintained. For thermal conductivity and the drastic augmentation of the latent heat of PCMs or the use of the thermal energy in a thermal energy storage system, this combination provides accurate data and reliable evidence for the optimized design of thermal energy storage systems using PCMs. Figure 1 summarizes the fundamental formulations for the numerical simulations involving PCMs and nano-enhanced PCMs.
where Cp is the specific heat capacity of the operating fluid and Href and Tref are reference values. The initial condition Ti = T(x,y,z,τ0) and boundary conditions like k·∂T/∂n = q0 and −k·∂T/∂n = hs(T − To) should be used in the numeric simulation, where q0 is the heat flux, hs is the air surface heat transfer coefficient, and To is the ambient temperature. The governing equation of the enthalpy method is similar to the single-phase equation, providing an easier solution to the phase-change problems. Also, in the enthalpy method, there are no conditions to be satisfied at the solid–liquid interface, and this method yields a solution involving both solid and liquid phases [30]. For example, the researchers Han et al. studied the thermal energy storage and release processes in multi-cavity-microstructured PCM microcapsules and found that the charge and discharge velocity of PCMs were accelerated by increasing the number of cavities and cavity interlayers in the microcapsules [31]. The feature is that the enthalpy method possesses great suitability to be applied in the field of engineering thermal energy storage systems, providing a reliable evaluation for engineering applications of PCMs. The finite element simulation method subdivides a large phase-change energy storage system into smaller and simpler elements and simulates them separately, which is called the discretization strategy. There are many types of discretization strategies, such as the h-version and the p-version. Both of these versions are numerical methods for solving partial differential equations. Their main difference is that the polynomial degrees of the elements are fixed and the mesh is refined in the h-version, whereas the finite element mesh is fixed and the polynomial degrees of elements are enhanced in the p-version [32]. After the simulation, the subdivided elements are then assembled into a larger system, which makes it easier to model the thermal transport problem during the phase-change process. The finite element simulation method offers many options for regulating the complexity of both modeling and analysis in a heat transfer system. At the same time, it can also balance the necessary accuracy and computational processing time that can assess many of the concerns associated with engineering applications. The thermal performance of PCMs, as one of the most studied research topics in the technological field, can be reliably described via finite element simulations [33]. For instance, the researchers Wang et al. [34] studied the impact of the porosity and thermal conductivity of a metal foam on the thermal conductivity and melting of PCMs. The optimum fin structure was attained, which considerably increased the rate of solidification of the PCMs, opposite to the velocity increase via the action of the dispersed copper nanoparticles studied by the authors Lohrasbi et al. [35]. The finite element simulation method provides accurate prediction of the thermal enhancement effect for micro-/nano-PCMs. To achieve a highly efficient finite element simulation, CFD modeling was developed to analyze engineering situations with inherent complex problems, such as the solidification and melting processes in PCMs for solar thermal purposes. This method also involves fluid mechanics such as turbulence models and two-phase flow, which focus on the flow of gas and liquid and can resolve the intricate restriction of the fluid flows in solar thermal research and technology, like the flow of heat transfer fluids at various flow rates. Therefore, to analyze the thermal enhancement during the solidification process of PCMs or the heat exchange process of colloidal suspensions, CFD is undoubtedly an ideal numerical simulation method. Moreover, the researchers Mahdi et al. [36] combined nanoparticles with metal foam or metal fins in a thermal energy storage system containing triplex-tube PCMs and confirmed the thermal enhancement provoked by this solution for the improvement of the thermal energy storage system. Further work on PCM thermal energy storage analysis will undoubtedly require the development of CFD modeling with enhanced computing processing capacity. Also, to seize the internal and external thermal energy exchange of PCMs or PCMs operating in thermal energy storage systems, the combined exploration of experimental findings and numeric simulations is a vital tendency that should be maintained. For thermal conductivity and the drastic augmentation of the latent heat of PCMs or the use of the thermal energy in a thermal energy storage system, this combination provides accurate data and reliable evidence for the optimized design of thermal energy storage systems using PCMs. Figure 1 summarizes the fundamental formulations for the numerical simulations involving PCMs and nano-enhanced PCMs.
Est = Ein − Eout

H = h + ΔH

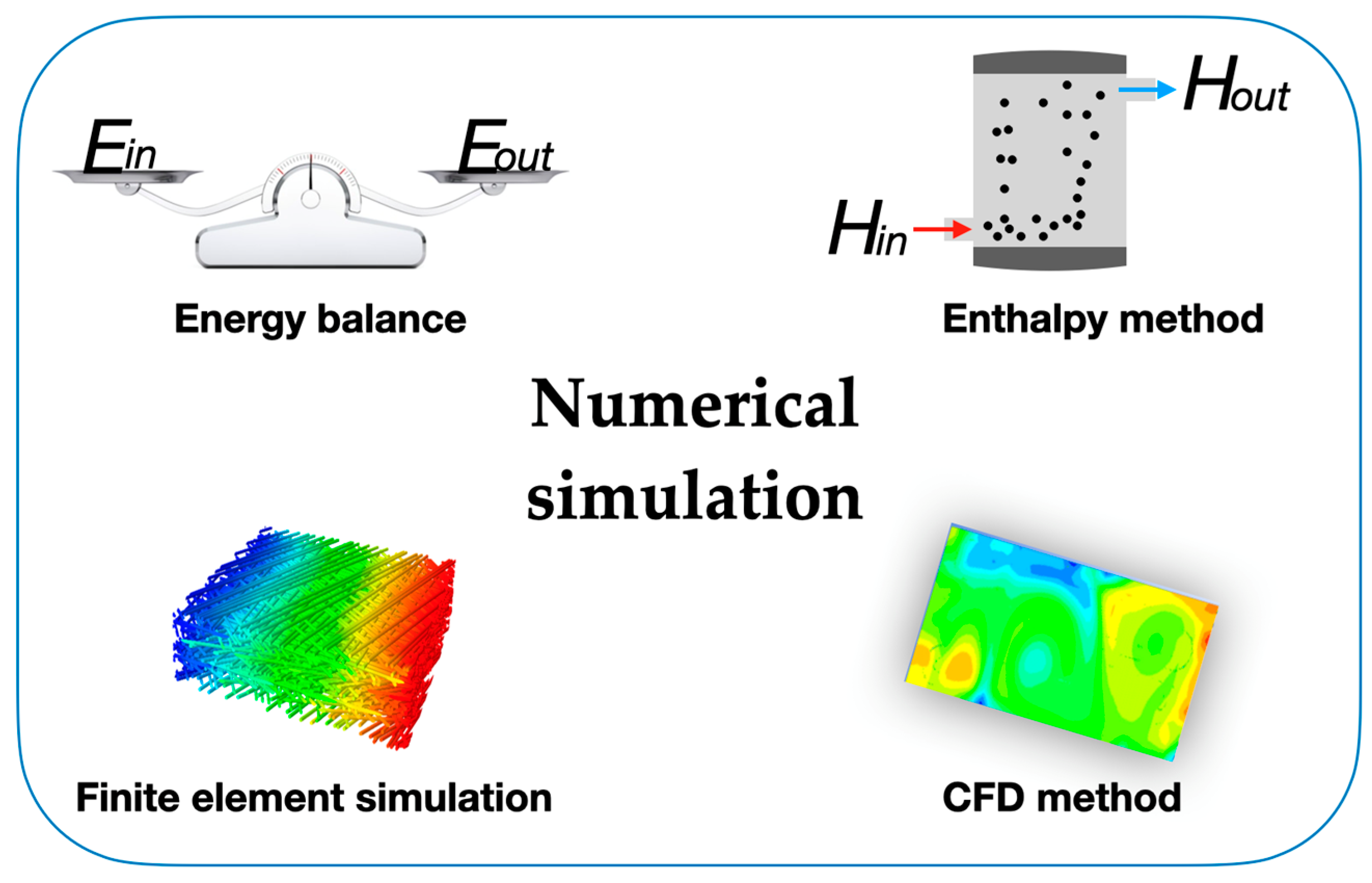
Figure 1. Main formulations for numerical simulations involving PCMs.
Moreover, the authors Starace et al. [28] also referred to many experimental works investigating the ordered layer of the water around proteins and hydrophobic molecules, where the water molecules are almost immobile in that layer, providing an increase in the specific heat of the system proportional to the volume of the ordered layer interface. Hence, the authors concluded that, to have an increase in the specific heat of nanofluids, the layered structure should have a specific heat many times higher than that of the base fluid. This ordered interfacial structure will be discussed in more detail in the next section. Furthermore, the authors Shin and Banerjee [37] and Tiznobaik and Shin [38] investigated the binary eutectic salt Li2CO3-K2CO3 (62:38) with the addition of 1% wt. of silica nanoparticles in different sizes. Their experimental works reported enhancements of around 25% in the specific heat regardless of the size of the nanoparticles. In addition, the obtained TEM images of the nano-enhanced salts after a melting and freezing process showed the formation of needle-like nanostructures in the salt, which could be the main reason behind the improved specific heat. Nonetheless, these special nanostructures within the molten salt were not observed in other published studies. Moreover, the researchers Dudda and Shin [39] investigated the binary eutectic salt NaNO3-KNO3 (60:40), commonly designated as solar salt given its suitability for commercial solar thermal power plants as a thermal energy storage medium, with the incorporation of silica nanoparticles. In their first study, the authors added 1% wt. of silica nanoparticles in two different sizes and confirmed a 19% enhancement in the specific heat for the 5 nm nanoparticles and a 25% increase when adding 30 nm nanoparticles. In their subsequent experimental work [40], the researchers added the same fraction of silica nanoparticles and found enhancements in the specific heat of 8%, 12%, 19%, and 27% with nanoparticles in sizes of 5 nm, 10 nm, 30 nm, and 60 nm, respectively. Furthermore, the authors Lu and Huang [41] evaluated the specific heat of molten NaNO3-KNO3 (60:40) salt containing alumina nanoparticles 9 nm and 13 nm in size. The concentrations used were superior to 1% wt., and a decrease in the specific heat capacity of the nanofluid was found. Also, it was verified by the authors that the specific heat decreased with increasing concentration and size of the nanoparticles. Additionally, the researchers Chieruzzi et al. [42] studied NaNO3-KNO3 (60:40) salt with the addition of alumina nanoparticles at concentrations of between 0.5% wt. and 1.5% wt. and reported an increase in the specific heat of 5.9% at 1% wt. and decreases in the specific heat at 0.5% wt. and 1.5% wt. Also, the researchers Ho and Pan [43] confirmed a nearly 20% enhancement in the specific heat at a very reduced concentration of 0.063% wt. of alumina nanoparticles in the ternary eutectic salt KNO3-NaNO2-NaNO3. Interestingly, increases in temperature were shown to decrease the specific heat of the salt, which is not in agreement with any other study, all of which showed constant or increasing specific heat with temperature. The specific heat enhancement of the molten salts with the addition of nanoparticles was attributed by the authors Shin and Banerjee [44][45] to three different possible mechanisms. The first one is related to the higher specific heat capacities of nanoparticles themselves, which is possible when the size of the particles is decreased. At present, nanoparticle-specific heat values have only been investigated up to 350 K [46], indicating that their properties at the high operating temperatures of molten salts is still largely unknown—a topic that should be investigated further. Also, the authors suggested that a high surface area per unit mass of nanoparticles increases the interfacial thermal resistance between nanoparticles and the surrounding liquid molecules. This high interfacial thermal resistance acts as additional thermal storage due to the interfacial interaction of the vibration energies between nanoparticle atoms and the interfacial molecules. This phenomenon could cause an increase in the specific heat of the salts [45]. Finally, the semi-solid behavior of layered liquid molecules at the surface of solid particles must be considered. The semi-solid layer can be visualized as a liquid–solid phase change at the surface and has been shown to have higher thermal properties than the bulk liquid and be equivalent to a latent phase change heat. Also, the researchers Jung and Banerjee [47] carried out molecular dynamics simulations to investigate this mechanism and introduced an analytical model to determine the specific heat of the salts. The empirical model for this mechanism simply assumes values for the semi-solid layer thickness, which could vary for different salts and particle materials and sizes. Moreover, the authors Shin and Banerjee [45] estimated that the semi-solid layer of liquid molecules on a crystalline surface can reach 2–5 nm in thickness—with this range depending on the surface energy of the crystalline interface. Thus, the mass fraction of the semi-solid layer should increase proportionally with the reduction in the size of the nanoparticles (for the same concentration of nanoparticles). Consequently, smaller nanoparticles should cause a greater enhancement in the specific heat of nanofluids. However, constant enhancement with different sizes of nanoparticles [45] and more enhancements with larger particles [42] have been reported, which seems to contradict this part of the theory. It is possible, though, that these unexpected trends could be explained by clustering at high temperatures, resulting in different particle size distributions during testing. The ionic liquid-based solvation force stability, as previously described, is also based on the existence of layered structures of ions in the vicinity of the solid–liquid boundary. Therefore, the common concept of the ionic liquid interfacial structure and semi-solid layers indicate a plausible mechanism to produce a stable colloidal system with enhanced specific heat. However, this is unproven for molten salts and still requires further investigation. On the other hand, the nanocomposites had lower specific heat compared to the base PCM, such as, for example, in the case of the improvement in photo-thermal performance [20], for PCM-filled cylinders [18], and in thermoelectric applications [48]. On the contrary, the authors Chieruzzi et al. [49] found that nanocomposites had a higher specific heat than that of the corresponding base PCM. The researchers prepared a nanofluid based on a nitrate salt mixture of 60% wt. NaNO3 and 40% wt. KNO3, Tm 220 °C. To create the nano-enhanced PCM, silica (7 nm), alumina (13 nm), and a mixture of silica and alumina (2–200 nm) nanoparticles at 1% wt. were added to the nitrate salt mixtures. The best results were achieved for the nanofluid with 1% wt. of silica/alumina nanoparticles. The specific heat in the solid phase was improved by around 52 and in the liquid phase by around 19%. The stored heat was increased by 13.5% compared with that of the PCM itself. On the other hand, the researchers Liu and Yang [50] enhanced the thermal properties of inorganic hydrate salt with titanium oxide–P25 nanoparticles with a dimension of 21 nm. The specific heat was improved by 83.5% in the solid phase and by 15.1% in the liquid phase by adding 0.3% wt. of nanoparticles. Furthermore, the latent heat was increased by 6.4%.
3. Latent Heat
In most studies, the addition of nanomaterials caused a slight decrease in the latent heat, except in [49] in relation to the passive cooling application and in [50] for the case of hydrate salts, where increases of 10.8% and 6.4% were reported, respectively, whereas in [51], the addition of graphite nanoparticles to an OP10E/water emulsion did not affect the latent heat. Also, the authors Warzoha et al. [52] found that the latent heat of fusion of organic paraffin decreased with the percentage of herringbone graphite nanofibers. The latent heat of fusion of the pure paraffin PCM was 271.6 J/g, whereas the latent heat of fusion of the nano-enhanced PCM with 11.4 vol% of graphite nanofibers was 242.7 J/g. The thermal conductivity and diffusivity increased with the increase in volume fraction of the HGNF. Furthermore, the authors Colla et al. [12] added alumina and carbon black nanoparticles to the paraffin waxes RUBITHERM®RT20 and RUBITHERM®RT25. The authors claimed that the addition of 1% wt. of alumina nanoparticles caused a degradation in thermal conductivity in both PCMs by 7–8%, whereas 1% wt. of carbon black nanoparticles increased the thermal conductivity by more than 25%. When alumina nanoparticles were added to RT20, the improvement in the latent heat was 10.8%, whereas in the case of carbon black nanoparticles the improvement was only 3.4%. The addition of carbon black nanoparticles to RT25 caused a reduction in latent heat of 11.6%. Moreover, the authors Muthoka et al. [53] prepared barium chloride dehydrate solutions in which nanoparticles of magnesium oxide and multi-walled carbon nanotubes were added. The authors stated that a 7% reduction in latent heat was detected for 1% wt. of multi-walled carbon nanotube0-enhanced fluid and a 5.2% reduction for 1% wt. magnesium oxide-enhanced fluid. Furthermore, the researchers Ebadi et al. [48] enhanced coconut oil with nanoparticles of copper oxide to create biobased nano-enhanced PCMs. A nanocomposite with 1% wt. of nanoparticles had 7.5% higher thermal conductivity than the base PCM, whereas the specific heat and latent heat of fusion decreased by 0.75% and 8.2%, respectively.
References
- Philip, J.; Shima, P.D.; Raj, B. Evidence for Enhanced Thermal Conduction through Percolating Structures in Nanofluids. Nanotechnology 2008, 19, 305706.
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U.; Chon, C.H.; Kihm, K.D. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107.
- Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S. The Effect of Particle Size on the Thermal Conductivity of Alumina Nanofluids. J. Nanoparticle Res. 2009, 11, 1129–1136.
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle Shape Effect on the Viscosity and Thermal Conductivity of ZnO Nanofluids. Int. J. Refrig. 2013, 36, 2233–2241.
- Murshed, S.M.S. Simultaneous Measurement of Thermal Conductivity, Thermal Diffusivity, and Specific Heat of Nanofluids. Heat Transf. Eng. 2012, 33, 722–731.
- Zhu, H.; Zhang, C.; Liu, S.; Tang, Y.; Yin, Y. Effects of Nanoparticle Clustering and Alignment on Thermal Conductivities of Fe3O4 Aqueous Nanofluids. Appl. Phys. Lett. 2006, 89, 23123.
- Hong, T.-K.; Yang, H.-S.; Choi, C.J. Study of the Enhanced Thermal Conductivity of Fe Nanofluids. J. Appl. Phys. 2005, 97, 064311.
- Dadwal, A.; Joy, P.A. Particle Size Effect in Different Base Fluids on the Thermal Conductivity of Fatty Acid Coated Magnetite Nanofluids. J. Mol. Liq. 2020, 303, 112650.
- Kamalvand, M.; Karami, M. A Linear Regularity between Thermal Conductivity Enhancement and Fluid Adsorption in Nanofluids. Int. J. Therm. Sci. 2013, 65, 189–195.
- Altan, C.L.; Gurten, B.; Sommerdijk, N.A.J.M.; Bucak, S. Deterioration in Effective Thermal Conductivity of Aqueous Magnetic Nanofluids. J. Appl. Phys. 2014, 116, 224904.
- Shao, X.-F.; Mo, S.-P.; Chen, Y.; Yin, T.; Yang, Z.; Jia, L.-S.; Cheng, Z.-D. Solidification behavior of hybrid TiO2 nanofluids containing nanotubes and nanoplatelets for cold thermal energy storage. Appl. Therm. Eng. 2017, 117, 427–436.
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for Enhanced Energy Storage and Passive Cooling Applications. Appl. Therm. Eng. 2017, 110, 584–589.
- Harish, S.; Orejon, D.; Takata, Y.; Kohno, M. Thermal Conductivity Enhancement of Lauric Acid Phase Change Nanocomposite with Graphene Nanoplatelets. Appl. Therm. Eng. 2015, 80, 205–211.
- Sarı, A.; Biçer, A.; Hekimoğlu, G. Effects of Carbon Nanotubes Additive on Thermal Conductivity and Thermal Energy Storage Properties of a Novel Composite PCM. J. Compos. Mater. 2018, 53, 2967–2980.
- Yadav, A.; Barman, B.; Kardam, A.; Narayanan, S.S.; Verma, A.; Jain, V.K. Thermal Properties of Nano-Graphite-Embedded Magnesium Chloride Hexahydrate Phase Change Composites. Energy Environ. 2017, 28, 651–660.
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Thermal Properties and Heat Storage Analysis of Palmitic Acid-TiO2 Composite as Nano-Enhanced Organic PCM (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262.
- Vivekananthan, M.; Amirtham, V.A. Characterization and Thermophysical Properties of Graphene Nanoparticles Dispersed Erythritol PCM for Medium Temperature Thermal Energy Storage Applications. Thermochim. Acta 2019, 676, 94–103.
- Putra, N.; Amin, M.; Kosasih, E.A.; Luanto, R.A.; Abdullah, N.A. Characterization of the Thermal Stability of RT 22 HC/Graphene Using a Thermal Cycle Method Based on Thermoelectric Methods. Appl. Therm. Eng. 2017, 124, 62–70.
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal Behavior of Paraffin-Nano-Al2O3 Stabilized by Sodium Stearoyl Lactylate as a Stable PCM with High Thermal Conductivity. Renew. Energy 2016, 88, 474–482.
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite Nanoparticles-Dispersed Paraffin/Water Emulsion with Enhanced Thermal-Physical Property and Photo-Thermal Performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107.
- Zhou, S.Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123.
- O’Hanley, H.; Buongiorno, J.; McKrell, T.; Hu, L.W. Measurement and model correlation of specific heat capacity of water-based nanofluids with silica, alumina and copper oxide nanoparticles. ASME Int. Mech. Eng. Congr. Expo. 2011, 54969, 1209–1214.
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transfer. 2009, 131, 071601.
- O’Hanley, H.; Buongiorno, J.; McKrell, T.; Hu, L.W. Measurement and model validation of nanofluid specific heat capacity with differential scanning calorimetry. Adv. Mech. Eng. 2012, 4, 181079.
- Barbés, B.; Páramo, R.; Blanco, E.; Casanova, C. Thermal conductivity and specific heat capacity measurements of CuO nanofluids. J. Therm. Anal. Calorim. 2014, 115, 1883–1891.
- Teng, T.P.; Lin, L.; Yu, C.C. Preparation and Characterization of Carbon Nanofluids by Using a Revised Water-Assisted Synthesis Method. J. Nanomater. 2013, 2013, 133.
- De Robertis, E.; Cosme, E.H.H.; Neves, R.S.; Kuznetsov, A.Y.; Campos, A.P.C.; Landi, S.M.; Achete, C.A. Application of the modulated temperature differential scanning calorimetry technique for the determination of the specific heat of copper nanofluids. Appl Therm Eng 2012, 41, 10–17.
- Starace, A.K.; Gomez, J.C.; Wang, J.; Pradhan, S.; Glatzmaier, G.C. Nanofluid heat capacities. J. Appl. Phys. 2011, 110, 124323.
- Lin, Y.S.; Hsiao, P.Y.; Chieng, C.C. Roles of nanolayer and particle size on thermophysical characteristics of ethylene glycol-based copper nanofluids. Appl. Phys. Lett. 2011, 98, 153105.
- Wang, C.; Lin, T.; Li, N.; Zheng, H. Heat transfer enhancement of phase change composite material: Copper foam/paraffin. Renew. Energy 2016, 96, 960–965.
- Han, P.; Zheng, X.; Hou, W.; Qiu, L.; Tang, D. Study on heat-storage and release characteristics of multi-cavity-structured phase change microcapsules. Phase Transit. 2015, 88, 704–715.
- Babuska, I.; Szabo, B.A.; Katz, I.N. The p-version of the finite element method. SIAM J. Numer. Anal. 1981, 18, 515–545.
- Reddy, J. An Introduction to the Finite Element Method; McGraweHill: New York, NY, USA, 2013.
- Wang, G.; Wei, G.; Xu, C.; Ju, X.; Yang, Y.; Du, X. Numerical simulation of effective thermal conductivity and pore-scale melting process of PCMs in foam metals. Appl. Therm. Eng. 2019, 147, 464–472.
- Lohrasbi, S.; Sheikholeslami, M.; Ganji, D.D. Multi-objective RSM optimization of fin assisted latent heat thermal energy storage system based on solidification process of phase change Material in presence of copper nanoparticles. Appl. Therm. Eng. 2017, 118, 430–447.
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E.C. Simultaneous energy storage and recovery in the triplex-tube heat exchanger with PCM, copper fins and Al2O3 nanoparticles. Energy Convers. Manag. 2019, 180, 949–961.
- Shin, D.; Banerjee, D. Enhanced Specific Heat of Silica Nanofluid. ASME. J. Heat Transfer. 2011, 133, 024501.
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548.
- Dudda, B.; Shin, D. Investigation of molten salt nanomaterial as thermal energy storage in concentrated solar power. In Proceedings of the ASME 2012 International Mechanical Engineering Congress & Exposition, IMECE2012, Houston, TX, USA, 9–15 November 2012.
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42.
- Lu, M.C.; Huang, C.H. Specific heat capacity of molten salt-based alumina nanofluid. Nanoscale Res. Lett. 2013, 8, 292.
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448.
- Ho, M.X.; Pan, C. Optimal concentration of alumina nanoparticles in molten Hitec salt to maximize its specific heat capacity. Int. J. Heat Mass Transf. 2014, 70, 174–184.
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070.
- Shin, D.; Banerjee, D. Experimental investigation of molten salt nanofluid for solar thermal energy application. ASME/JSME Therm. Eng. Jt. Conf. 2011, 38921, T30024.
- Likhachev, V.N.; Vinogradov, G.A.; Alymov, M.I. Anomalous heat capacity of nanoparticles. Phys. Lett. A 2006, 357, 236–239.
- Jung, S.; Banerjee, D. A simple analytical model for specific heat of nanofluid with tube shaped and disc shaped nanoparticles. ASME/JSME Therm. Eng. Jt. Conf. 2011, 38921, T30023.
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Geometry and nanoparticle loading effects on the bio-based nano-PCM filled cylindrical thermal energy storage system. Appl. Therm. Eng. 2018, 141, 724–740.
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M.; Torre, L. Heat capacity of nanofluids for solar energy storage produced by dispersing oxide nanoparticles in nitrate salt mixture directly at high temperature. Sol. Energy Mater. Sol. Cells 2017, 167, 60–69.
- Liu, Y.; Yang, Y. Investigation of specific heat and latent heat enhancement in hydrate salt based TiO2 nanofluid PCM. Appl. Therm. Eng. 2017, 124, 533–538.
- Wang, F.; Zhang, C.; Liu, J.; Fang, X.; Zhang, Z. Highly stable graphite nanoparticle-dispersed phase change emulsions with little supercooling and high thermal conductivity for cold energy storage. Appl. Energy 2017, 188, 97–106.
- Warzoha, R.J.; Weigand, R.M.; Fleischer, A.S. Temperature-dependent thermal properties of a paraffin PCM embedded with herringbone style graphite nanofibers. Appl. Energy 2015, 137, 716–725.
- Muthoka, M.J.; Xuelai, Z.; Yuyang, Y.; Yue, C.; Xiaofeng, X. Latent heat of fusion prediction for nanofluid based PCM. Appl. Therm. Eng. 2018, 130, 1590–1597.
More
Information
Subjects:
Engineering, Mechanical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
794
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




