Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Sanches Silva | -- | 2708 | 2023-12-04 09:58:54 | | | |
| 2 | Ana Sanches Silva | Meta information modification | 2708 | 2023-12-04 11:35:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, �.; Mateus, A.R.S.; Barros, S.C.; Silva, A.S.; Sanches-Silva, A. Ergot Alkaloids on Cereals and Seeds. Encyclopedia. Available online: https://encyclopedia.pub/entry/52316 (accessed on 07 February 2026).
Silva �, Mateus ARS, Barros SC, Silva AS, Sanches-Silva A. Ergot Alkaloids on Cereals and Seeds. Encyclopedia. Available at: https://encyclopedia.pub/entry/52316. Accessed February 07, 2026.
Silva, Ângela, Ana Rita Soares Mateus, Sílvia Cruz Barros, Ana Sanches Silva, Ana Sanches-Silva. "Ergot Alkaloids on Cereals and Seeds" Encyclopedia, https://encyclopedia.pub/entry/52316 (accessed February 07, 2026).
Silva, �., Mateus, A.R.S., Barros, S.C., Silva, A.S., & Sanches-Silva, A. (2023, December 04). Ergot Alkaloids on Cereals and Seeds. In Encyclopedia. https://encyclopedia.pub/entry/52316
Silva, Ângela, et al. "Ergot Alkaloids on Cereals and Seeds." Encyclopedia. Web. 04 December, 2023.
Copy Citation
Ergot alkaloids are secondary metabolites resulting from fungi of the genus Claviceps that have proven to be highly toxic. These mycotoxins commonly infect cereal crops such as wheat, rye, barley, and oats. Due to the increase worldwide consumption of cereal and cereal-based products, the presence of ergot alkaloids in food presents a concern for human safety. For this reason, it is essential to develop several analytical methods that allow the detection of these toxic compounds.
ergot alkaloids
analytical methods
decontamination
cereals
mycotoxins
1. Introduction
Mycotoxins are natural, toxic contaminants resulting from the metabolism of fungi of the genus Aspergillus, Penicillium, Alternaria, and Fusarium. Nowadays, hundreds of mycotoxins are known. Aflatoxins (AFs), ochratoxin A (OTA), patulin (PAT), fumonisins (FUMs), trichothecenes (TCs), zearalenone (ZEA), citrinin (CIT), and ergot alkaloids (EAs) are those with the more relevance [1][2].
Ergot alkaloids are secondary metabolites produced by Claviceps species (principally C. purpurea) and can contaminate seeds and cereal products such as barley, oats, rye, triticale, and wheat, among others [1][3]. Their production depends on many factors, such as temperature, humidity, insect damage in crops, nutrients, and fungal concentration [2][4]. Depending on the concentration of mycotoxins ingested and the frequency of ingestion, these toxins can cause acute and chronic toxic effects on human health. These effects can be aggravated and dangerous if more than one mycotoxin is ingested because of the synergistic or potentiating toxic effects [5][6].
Mycotoxins can contaminate food and feed in many phases of the food chain, and this contamination can occur pre-harvest (by crop contamination with fungi in the field) or post-harvest (during storage, transportation, and industrial food processing) [7]. These compounds are very stable and resistant to degradation [2], so good agricultural and manufacturing processes and industrial or home food processing are not enough to eliminate them [7].
The presence of these toxic compounds in food and feeds needs to be considered because they can cause health concerns, are stable and resistant to decomposition, and at determined concentrations can be associated with acute and chronic health problems [2].
2. Ergot Alkaloids
Production of these compounds depends on the geographic region, as C. purpurea is mainly responsible for its production in Europe [1][8]. Moreover, the production of EAs depends on multiple factors, such as the type of fungi and plants, the concentration of fungus, temperature, humidity, and nutrients, among others; those factors related to climatic conditions are most influential because EAs production is favored in wet soils and rainfall conditions [7][9][10]. Presence of these toxic compounds is noticed essentially in seed and cereals products such as rye, wheat, barley, triticale, oat, and millet, of which rye, triticale, and barley are the most affected [1][11].
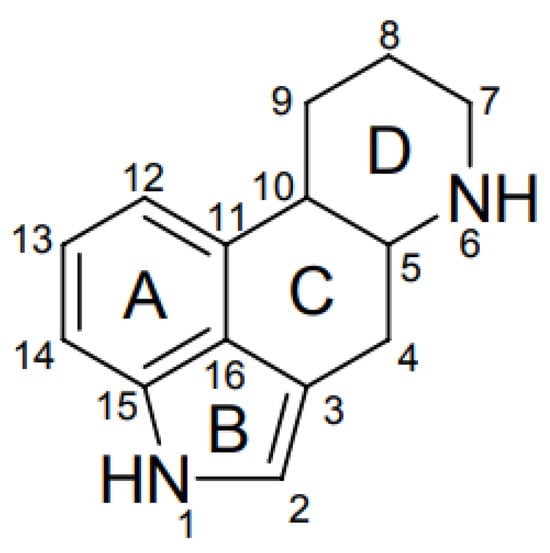
To date, more than 80 EAs are known and can be divided into three main groups: clavinet-type (hydroxyl- and dehydro-derivatives of 6,8-dimethylergoline), simple lysergic acid amines, and peptide-type (which have an additional cyclic tripeptide linked through an amide bond to the lysergic acid) [5][10]. All EAs have an ergoline ring as the main structure and a nitrogen atom at position 6 (that can be methylated in some structures), differing in the substitution on the C8 position of the ergoline ring, and the possession of a double bond between C8 and C8 or C9 and C10, as shown in Figure 1 [9][10].

Figure 1. Ergot alkaloid common chemical structure [12].
EFSA published a scientific opinion on ergot alkaloids in food and feed where the clavine type is described as the most common and toxic EAs, with ergometrine, ergosine, ergotamine, ergocornine, ergokryptine, and ergocristine and their -inine forms being the most important ones [12]. The suffix -inine is a result of the epimerization process of the C8 position of the ergoline ring to C8 (S)-configuration, and the suffix -ine corresponds to the (R)-configuration [9].
The epimerization process of ergot alkaloids is still not yet totally understood, but several factors that influence this process are known. Factors like temperature, humidity, light, pH, and solvent characteristics can affect this process [8][13][14]. Many studies reveal that temperature of −20 °C or lower, non-protic solvents, and the use of amber glass or aluminum foil can minimize the epimerization process [8][13][15].
Epimerization can occur rapidly, especially in aqueous solutions, and the conversion on -inine forms can also convert back into -ine forms or vice versa [10]. Some studies evaluated the activity of the -inine forms and concluded that this form is biologically active [13][16], although -ine forms are considered more active in regard to toxicity [17].
3. Factors Associated with Contamination by Ergot Alkaloids
After infection of the host plant, filamentous fungi invade the ovule of the plant and colonize the whole ovary, and after some weeks, when the wintering body of the fungus turns visible, the wintering body containing alkaloids is replaced on the developing grain or seed [8][9]. This wintering body is known as the ergot body or sclerotium, which has a dark color and crescent, tubular shape [10][14]. The content of ergot alkaloids in the sclerotia depends on many factors, such as the maturity of the ergot bodies, the fungal strain, the host plant, the geographical region, and the climatic conditions [9][10][14].
Sclerotia can be harvested together with grain, seeds, and grasses, resulting in contamination of food and feed cereal-based products. Ergot alkaloid contamination can also occur in different phases of the food chain since sclerotia can be broken during transportation, which facilitates their entrance into the food chain [5][14].
Nowadays, a considerable amount (up to 80%) [15] of EAs can be eliminated by effective cleaning and milling techniques such as grading, sieving, and sorting [8]. However, their presence cannot be totally eliminated even with fungicides, which makes methods for their determination very relevant [18].
4. Toxicity and Mechanisms of Action
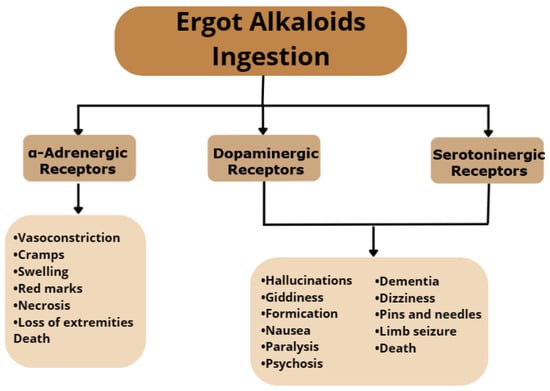
The effects of ergot alkaloids consumption depend on the amount ingested and the frequency of ingestion and can vary from acute to chronic diseases and in several cases can cause death. These effects can be manifested in several forms, as these compounds are known to interact with adrenergic, serotonergic, and dopaminergic receptors (Figure 2) [15]. One of the effects caused by excessive ingestion of EAs is vasoconstriction, mediated by α-adrenergic receptors interaction, which is characterized by cramps, swelling, red marks, necrosis, loss of extremities, and death. Interaction with serotoninergic and dopaminergic receptors affects the central nervous system, causing symptoms such as hallucinations, giddiness, formication, nausea, paralysis, psychosis, dementia, dizziness, pins and needles, limb seizure, and death [11][16].

Figure 2. Effects of excessive ingestion of ergot alkaloids.
Intoxication by EAs is known as ergotism; this condition has been known since the Middle Ages, when intoxications occurred for ingestion of contaminated grains, flour, and bread [8]. These intoxications were known as St. Anthony’s Fire or Holy Fire because of the intensive pain caused by the vasoconstriction effect as well as the neurotoxic effects [9]. There are two symptomatic forms of ergotism (gangrenous and convulsive); in the gangrenous form, tingling effects are felt in peripheral tissues and can lead to loss of limbs, while the convulsive form is characterized by tingling followed by hallucinations, delirium, and epileptic-type seizures [19].
5. Legislation with Focus on EU
Due to the health problems caused by mycotoxins, governmental authorities such as the WHO, FAO, and EFSA are paying attention to these toxic compounds. Some controlling strategies have been reported by the authorities, and regulatory levels of mycotoxins in foodstuffs have been established around the world, including for ergot alkaloids.
In Europe, the European Commission has established a maximum level for the most frequent mycotoxins in foodstuffs. In Commission Regulation (EU) no. 2023/915 of 25 April 2023, the maximum levels for mycotoxins, including for ergot sclerotia and ergot alkaloids, are established in certain foodstuffs [20].
Although a maximum level of 500 μg/kg was established in European Union for EAs, on 1 July 2024, there will be a reduction of the maximum levels of EAs for some categories of foods to provide a high level of human health protection. To safeguard human and animal health, the CONTAM panel of the EFSA has established a group acute reference dose of 1 μg/kg body weight and a group tolerable daily intake (TDI) for total ergot alkaloids of 0.6 μg/kg of body weight/day [8].
The limits established by the European Commission are more restrictive when compared to other countries around the world. In 2004, the FAO published “Worldwide regulations for mycotoxins in food and feed in 2003”, where legal mycotoxin limits can be accessed in several countries around the world [21]. Australia has established 500 mg/kg for the maximum limit for ergot alkaloids, which is extremely higher than the actual limits in Europe [21].
6. Determination of Ergot Alkaloids
6.1. Sampling
Sampling is a crucial step in ergot alkaloids determination, as their heterogeneous distribution influences the precision of the determination. Concerning cereal samples, matrices can contain tiny fragments of sclerotia or bulks of EAs, making sampling a step of higher importance [22].
6.2. Sample Pre-Treatment
Extraction is a step of great importance, as it is responsible for the separation of the analyte from the matrix and sometimes can be followed by a clean-up procedure to eliminate possible interference with the analysis. This pre-treatment of samples is required not only to remove interferences but to pre-concentrate the analytes [9].
Some pre-treatment techniques such as Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) [9][23][24] procedures and solid–liquid extractions [10][25][26] have been applied over the years to ergot alkaloids. Independently of the extraction technique, the choice of the extraction solvent and optimisation of the procedure conditions are critical to obtain satisfactory results [14].
6.3. Analytical Methods
Many methods have been reported for ergot alkaloids determination, such as liquid chromatography (LC), enzyme-linked immunosorbent assay (ELISA), capillary electrophoresis (CE), gas chromatography (GC), and thin-layer chromatography (TLC) [9][10]. Gas chromatography is usually coupled with electron capture detection (ECD), and liquid chromatography can be coupled with different detectors, such as ultraviolet light (UV), fluorescence detector (FLD), evaporative light scattering detector (ELSD), and mass spectrometry (MS) [7][10][26].
Chromatographic methods are based on the separation of components depending on their affinity to a mobile or stationary phase. These different affinities make different movements in the column, leading to a possible separation of the compounds [27]. This method makes possible the determination of the major EAs individually and summary of them in order to obtain the total ergot alkaloid content; however, this requires a lot of standards, making this process costly. A more cost-effective approach is to transform the EAs into a common structure before the analyses, which can be achieved by a hydrolysis process where EAs and their epimers are cleaved to an uniform lysergic acid hydrolyze [28].
Since EAs are non-volatile and can decompose in the injector once they are susceptible to heat, gas chromatographic (GC) techniques have become less applied to these compounds. On the other hand, liquid chromatographic methods are commonly used for polar, non-volatile, or thermally labelled mycotoxins such as EAs [13][15].
Liquid chromatographic methods such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and ultra-high-performance liquid chromatography (UHPLC) have been applied for EAs determination. With its technological advances, UHPLC has shown to be rapid and efficient for compounds separation, which can be justified for the use of columns packed with submicron particles, making this technique more applied to mycotoxin determination [12][26]. In respect to detectors, UV is used for EAs quantification; however, UV light conducts the epimerization process, interfering with quantification. Thus, FLD detectors began to be applied not only to offer more specificity and sensitivity but because some EAs are naturally fluorescent. However, mass spectrometry (MS) detectors have become widely used for EAs quantification [14].
Although chromatographic methods are important for official and reference laboratories to control EAs concentration, it seems to be necessary to develop a fast and cost-effective test system for application in the production locations to make a primary screening for EAs possible. In this sense, the enzyme-linked immunosorbent assay (ELISA) has been applied as solid basis for rapid and sensitive screening of ergot alkaloids. This method is based on the interaction between the mycotoxin and antibodies marked with a conjugate toxin enzyme, as binding of the mycotoxin to the conjugate produces color depending on the amount of binding.
7. Rapid Alert System for Food and Feed (RASFF) Notifications
In the European Union, a safety tool named the Rapid Alert System for Food and Feed (RASFF) was established in order to facilitate the rapid notification and response in case of risk to human health related to food and feed [29]. This is an important tool that shares rapid information about direct or indirect risk to humans between the member states, the commission, and the authority [30].
When a member state identifies a risk and reports it to the RASFF, the first notification is received by the European Commission, which verifies the notification and immediately transmits it to the other members, allowing them to take the necessary actions [31].
To date, only nine RASFF notifications for ergot alkaloids contaminations have been generated, all of them in very recent years (between September 2021 and March 2023). Looking at the results, it can conclude that from all the cereal and cereal-based products, there is a higher incidence of notifications for rye-flour products. A notification from a product from Ireland was the only one whose notification was not related to cereal or cereal-based products but to dietetic foods, food supplements, and fortified foods. Additionally, all the samples were originally from EU countries, with France having with most notifications. The highest values were found in Belgian and German rye flours, and in addition to this, six of the nine notifications were classified as serious risk; however, two of the notifications are still undecided.
8. Decontamination of Mycotoxins
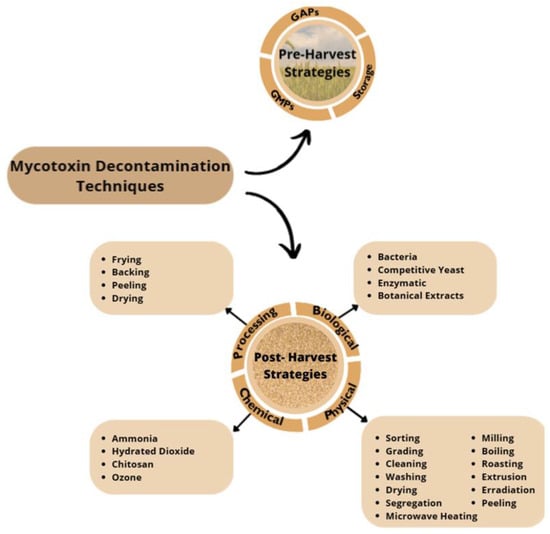
Since mycotoxins contamination leads to economic losses and health concerns, the search for effective decontamination and detoxification has been of great interest [32]. Decontamination and detoxification methods for mycotoxins should be effective, simple, and inexpensive; use existing technology; and not alter the nutritional value [33]. The search for an efficient and effective process for the decontamination of mycotoxins from food and feed still remains a practical and scientific global challenge [34]. When talking about controlling the levels of EAs in cereals, it need to take into account two main stages. The first stage includes pre-harvest practices, which focus on prevention of mycotoxin production or contamination and are mainly based on good agricultural practices (GAP), good manufacturing practices (GMP), and favorable storage practices [32][35][36]. Pre-harvest strategies are the best way to prevent mycotoxin production in the field, but once mycotoxin contamination occurs, these strategies might not eliminate them, so post-harvest strategies must be applied [32]. Therefore, post-harvest strategies are the second stage and are based on processing, chemical, physical, and biological techniques, and application of these strategies aims to decontaminate contaminated products [32][34]. At both stages, hazard analysis and critical control points (HACCP) plays an important role, which involves strategies for mycotoxin prevention, control, and GMPs for all stages of product management; storage strategies; and sorting, segregation, and cleaning procedures [32].
A compilation of the pre- and post-harvest strategies applied to mycotoxins decontamination is shown at Figure 3.

Figure 3. Pre- and Post- Harvest Mycotoxin Decontamination Techniques; GAPs, good agricultural practices; GMPs, good manufacturing practices.
Specifically concerning ergot alkaloids decontamination, pre-harvest strategies remain the most important stage, as they are based on GMPs, GAPs, and favorable storage practices. Relative to post-harvest strategies, only a few have been applied to ergot alkaloid decontamination, namely sorting and cleaning as a physical strategy; frying, baking, and peeling as processing techniques; and ammonization as a chemical strategy [33][35][37][38][39][40].
9. Conclusions
Cereals and seeds have a high risk of contamination by mycotoxins, namely by ergot alkaloids. Due to climate change and the increase in cereal and cereal-based product consumption, it is one of today’s worldwide food safety concerns. For that reason, monitoring, prevention, and control are imperative to minimizing their occurrence.
Good agricultural and manufacturing practices and controlled storage and transport conditions can prevent ergot alkaloid contamination. These preventive strategies together with control analysis of critical points are fundamental. However, when products are already contaminated, physical, chemical, and biological processes are needed for mycotoxins decontamination. Although decontamination processes can be used, many of them can only reduce the toxicity of the ergot alkaloids by promoting the epimerization process. Therefore, the quantification of both epimers must be taken into account.
Many methods have been developed for the determination and quantification of ergot alkaloids in the search for an efficient, sensitive, and cost-effective method for the quantification of both epimers. QuEChERS has been the preferred method for extraction and purification steps, along with chromatographic methods for quantification, like HPLC and UHPLC. The preference for the tandem mass spectrometry (MS/MS) detector is well known over the years due to its unequivocal advantages.
References
- Malir, F.; Pickova, D.; Toman, J.; Grosse, Y.; Ostry, V. Hazard Characterisation for Significant Mycotoxins in Food. Mycotoxin Res. 2023, 39, 81–93.
- Liao, C.D.; Wong, J.W.; Zhang, K.; Hayward, D.G.; Lee, N.S.; Trucksess, M.W. Multi-Mycotoxin Analysis of Finished Grain and Nut Products Using High-Performance Liquid Chromatography-Triple-Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2013, 61, 4771–4782.
- Food Contaminants: Ninetieth Meeting-Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://www.who.int/news-room/articles-detail/call-for-data-jecfa-90-meeting (accessed on 13 September 2023).
- Shi, H.; Yu, P. Correlation Patterns Prevalence, and Co-Occurrence of Ergot Alkaloids in Cool-Season Adapted Cereal Grains Revealed with Molecular Spectroscopy and LC-MS/MS Equipped HPLC System. Food Chem. 2022, 393, 133322.
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654.
- Rai, A.; Das, M.; Tripathi, A. Occurrence and Toxicity of a Fusarium Mycotoxin, Zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729.
- de Sá, S.V.M.; Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Emerging Mycotoxins in Infant and Children Foods: A Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 1707–1721.
- Gürbüzel, M.; Uysal, H.; Kızılet, H. Assessment of Genotoxic Potential of Two Mycotoxins in the Wing Spot Test of Drosophila Melanogaster. Toxicol. Ind. Health 2015, 31, 261–267.
- Carbonell-Rozas, L.; Mahdjoubi, C.K.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Ergot Alkaloids in Barley and Wheat from Algeria. Toxins 2021, 13, 316.
- Guo, Q.; Shao, B.; Du, Z.; Zhang, J. Simultaneous Determination of 25 Ergot Alkaloids in Cereal Samples by Ultraperformance Liquid Chromatography−Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 7033–7039.
- Diana, J.; Mavungu, D.; Malysheva, S.V.; Sanders, M.; Larionova, D.; Robbens, J.; Dubruel, P.; Van Peteghem, C.; De Saeger, S. Analytical Methods Development and Validation of a New LC-MS/MS Method for the Simultaneous Determination of Six Major Ergot Alkaloids and Their Corresponding Epimers. Application to Some Food and Feed Commodities. Food Chem. 2012, 135, 292–303.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Ergot Alkaloids in Food and Feed. EFSA J. 2012, 10, 2798.
- Müller, C.; Kemmlein, S.; Klaffke, H.; Krauthause, W.; Preiß-Weigert, A.; Wittkowski, R. A Basic Tool for Risk Assessment: A New Method for the Analysis of Ergot Alkaloids in Rye and Selected Rye Products. Mol. Nutr. Food Res. 2009, 53, 500–507.
- Chung, S.W.C. A Critical Review of Analytical Methods for Ergot Alkaloids in Cereals and Feed and in Particular Suitability of Method Performance for Regulatory Monitoring and Epimer-Specific Quantification. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess 2021, 38, 997–1012.
- Lenain, P.; Diana Di Mavungu, J.; Dubruel, P.; Robbens, J.; De Saeger, S. Development of Suspension Polymerized Molecularly Imprinted Beads with Metergoline as Template and Application in a Solid-Phase Extraction Procedure toward Ergot Alkaloids. Anal. Chem. 2012, 84, 10411–10418.
- Crews, C. Analysis of Ergot Alkaloids. Toxins 2015, 7, 2024.
- Köppen, R.; Rasenko, T.; Merkel, S.; Mönch, B.; Koch, M. Novel Solid-Phase Extraction for Epimer-Specific Quantitation of Ergot Alkaloids in Rye Flour and Wheat Germ Oil. J. Agric. Food Chem. 2013, 61, 10699–10707.
- Storm, I.D.; Rasmussen, P.H.; Strobel, B.W.; Hansen, H.C.B. Ergot Alkaloids in Rye Flour Determined by Solid-Phase Cation-Exchange and High-Pressure Liquid Chromatography with Fluorescence Detection. Food Addit. Contam. Part A 2008, 25, 338–346.
- Kokkonen, M.; Jestoi, M. Determination of Ergot Alkaloids from Grains with UPLC-MS/MS. J. Sep. Sci. 2010, 33, 2322–2327.
- EUR-Lex-32023R0915-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 26 June 2023).
- Worldwide Regulations for Mycotoxins in Food and Feed in 2003. Available online: https://www.fao.org/3/y5499e/y5499e02.htm (accessed on 26 June 2023).
- EUR-Lex-32006R0401-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R0401 (accessed on 26 June 2023).
- Rubert, J.; Dzuman, Z.; Vaclavikova, M.; Zachariasova, M.; Soler, C.; Hajslova, J. Analysis of Mycotoxins in Barley Using Ultra High Liquid Chromatography High Resolution Mass Spectrometry: Comparison of Efficiency and Efficacy of Different Extraction Procedures. Talanta 2012, 99, 712–719.
- Singh, J.; Mehta, A. Rapid and Sensitive Detection of Mycotoxins by Advanced and Emerging Analytical Methods: A Review. Food Sci. Nutr. 2020, 8, 2183.
- Bryła, M.; Szymczyk, K.; Jędrzejczak, R.; Roszko, M. Application of Liquid Chromatography/Ion Trap Mass Spectrometry Technique to Determine Ergot Alkaloids in Grain Products. Food Technol. Biotechnol. 2015, 53, 18–28.
- Veršilovskis, A.; Mulder, P.P.J.; Pereboom-de Fauw, D.P.K.H.; de Stoppelaar, J.; de Nijs, M. Simultaneous Quantification of Ergot and Tropane Alkaloids in Bread in the Netherlands by LC-MS/MS. Food Addit. Contam. Part B 2020, 13, 215–223.
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic Effects of Mycotoxins. Toxicon 2020, 185, 104–113.
- Höfs, S.; Jaut, V.; Schneider, R.J. Ergometrine Sensing in Rye Flour by a Magnetic Bead-Based Immunoassay Followed by Flow Injection Analysis with Amperometric Detection. Talanta 2023, 254, 124172.
- RASFF. Available online: https://food.ec.europa.eu/safety/rasff_en#Learn (accessed on 27 June 2023).
- EUR-Lex-32002R0178-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002R0178 (accessed on 7 July 2023).
- Questions and Answers: Rapid Alert System for Food and Feed (RASFF). Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_17_2461 (accessed on 5 July 2023).
- Luo, Y.; Liu, X.; Li, J. Updating Techniques on Controlling Mycotoxins—A Review. Food Control 2018, 89, 123–132.
- Young, J.C.; Chen, Z.J.; Marquardt, R.R. Reduction in Alkaloid Content of Ergot Sclerotia by Chemical and Physical Treatment. J. Agric. Food Chem. 1983, 31, 413–415.
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279.
- Agriopoulou, S.; Tundo, S.; Kuzmanovi´c, L.K. Ergot Alkaloids Mycotoxins in Cereals and Cereal-Derived Food Products: Characteristics, Toxicity, Prevalence, and Control Strategies. Agronomy 2021, 11, 931.
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137.
- Cherewyk, J.E.; Grusie-Ogilvie, T.J.; Parker, S.E.; Blakley, B.R.; Al-Dissi, A.N. Ammonization of the R- and S-Epimers of Ergot Alkaloids to Assess Detoxification Potential. J. Agric. Food Chem. 2022, 70, 8931–8941.
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Turnock, D.; Taylor, D.; Fu, B.X. Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production. Toxins 2019, 11, 195.
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K. Stability of Ergot Alkaloids during the Process of Baking Rye Bread. LWT 2019, 110, 269–274.
- Merkel, S.; Dib, B.; Maul, R.; Köppen, R.; Koch, M.; Nehls, I. Degradation and Epimerization of Ergot Alkaloids after Baking and in Vitro Digestion. Anal. Bioanal. Chem. 2012, 404, 2489–2497.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
04 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




