Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasileios Kamperidis | -- | 2443 | 2023-12-02 00:03:01 | | | |
| 2 | Jason Zhu | Meta information modification | 2443 | 2023-12-04 03:01:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Anastasiou, V.; Bazmpani, M.; Daios, S.; Moysidis, D.V.; Zegkos, T.; Didagelos, M.; Karamitsos, T.; Toutouzas, K.; Ziakas, A.; Kamperidis, V. Echocardiographic Assessment of Right Ventricular Function. Encyclopedia. Available online: https://encyclopedia.pub/entry/52275 (accessed on 08 February 2026).
Anastasiou V, Bazmpani M, Daios S, Moysidis DV, Zegkos T, Didagelos M, et al. Echocardiographic Assessment of Right Ventricular Function. Encyclopedia. Available at: https://encyclopedia.pub/entry/52275. Accessed February 08, 2026.
Anastasiou, Vasileios, Maria-Anna Bazmpani, Stylianos Daios, Dimitrios V. Moysidis, Thomas Zegkos, Matthaios Didagelos, Theodoros Karamitsos, Konstantinos Toutouzas, Antonios Ziakas, Vasileios Kamperidis. "Echocardiographic Assessment of Right Ventricular Function" Encyclopedia, https://encyclopedia.pub/entry/52275 (accessed February 08, 2026).
Anastasiou, V., Bazmpani, M., Daios, S., Moysidis, D.V., Zegkos, T., Didagelos, M., Karamitsos, T., Toutouzas, K., Ziakas, A., & Kamperidis, V. (2023, December 02). Echocardiographic Assessment of Right Ventricular Function. In Encyclopedia. https://encyclopedia.pub/entry/52275
Anastasiou, Vasileios, et al. "Echocardiographic Assessment of Right Ventricular Function." Encyclopedia. Web. 02 December, 2023.
Copy Citation
Tricuspid regurgitation (TR) is a highly prevalent valvular heart disease that has been long overlooked, but lately its independent association with adverse cardiovascular outcomes was recognized. The time point to intervene and repair the tricuspid valve is defined by the right ventricular (RV) dilation and dysfunction that comes up at a later stage. While guidelines favor tricuspid valve repair before severe RV dysfunction ensues, the definition of RV dysfunction in a universal manner remains vague. As a result, the candidates for transcatheter or surgical TR procedures are often referred late, when advanced RV dysfunction is established, and any derived procedural survival benefit is attenuated.
tricuspid regurgitation
right ventricular function
multimodality imaging
1. Introduction
Clinically significant tricuspid regurgitation (TR) is a common valvular heart disease affecting 0.55% of the general population, with functional TR accounting for the most severe TR cases [1][2]. Despite its rising prevalence, TR has been long overlooked, being largely considered the sequela of advanced left sided heart disease, rather than a valvular entity of independent prognostic relevance. Contemporary data have shed light to its concealed, independent association with excess mortality, outlining the need for drastic therapies [3][4][5]. Although surgical intervention was until recently considered the only interventional approach, transcatheter tricuspid valve repair has emerged as an alternative treatment strategy for inoperable symptomatic patients [6].
Significant TR has a deleterious impact on right ventricular (RV) size and function; TR will progressively induce tricuspid annular dilation and tricuspid leaflet tethering due to RV remodeling, while retaining normal RV function at the compensating phase. When the point of RV adaptation to volume overload is surpassed, patients suffer RV functional compromise and enter a vicious cycle of recurrent heart failure admissions, further aggravating their prognosis (Figure 1) [7]. In this regard, a well-timed selection of candidates for interventional TR treatments in advance of the RV failure is of paramount importance, while at the same time remaining a difficult task. The ESC and AHA/ACC guidelines recommend that the intervention for severe TR treatment takes place before RV dysfunction is established; however, they remain vague in terms of practically defining RV dysfunction [8][9].
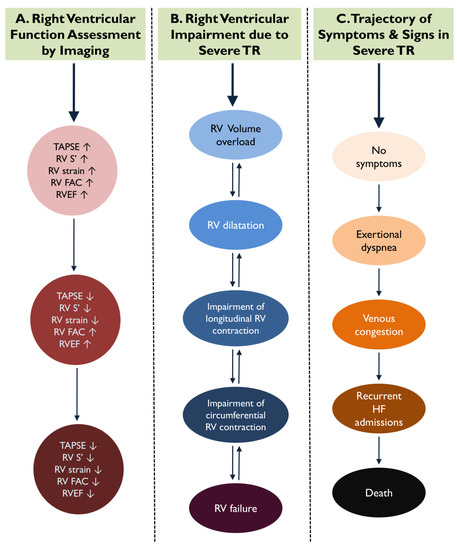
Figure 1. During the course of significant tricuspid regurgitation (TR), indices of longitudinal right ventricular (RV) function are impaired earlier compared to parameters of circumferential contraction. This figure demonstrates the parallel trajectories of RV dysfunction via imaging parameters (A), RV adverse remodeling (B), and the onset of RV failure symptoms and signs (C) in severe TR.
2. Conventional RV Function Indices
Challenges in the evaluation of RV performance stem mostly from its complex anatomy. The RV is a crescent-shaped structure including an outlet, apex, and inlet portion. Hence, incorporating the contractile force of all three components with the measurement of one single index is practically impossible [10]. Conventionally, echocardiography is the mainstay imaging modality for evaluating RV function in the presence of significant TR, owing to its ease of use, safety, availability, and lower costs. Numerous traditional indices of RV systolic function in severe TR have been embraced in clinical practice due to their practicality, despite having fundamental flaws [11].
Tricuspid annular plane systolic excursion (TAPSE) evaluates the distance of systolic excursion of the RV annular segment along its longitudinal plane, while S’ measures the peak systolic annular velocity via Doppler tissue imaging at the tricuspid annulus level. Both are widely established indices, recommended for RV function assessment with a suggested cutoff value of 17 mm and 9.5 cm/s, respectively [11]. In contrast to the aforementioned indices which describe solely the longitudinal contractile force at the tricuspid annular level, fractional area change (FAC) additionally accounts for the circumferential RV contraction. FAC is defined as (end-diastolic area–end-systolic area)/end-diastolic area ×100 and has shown a fair correlation with RV ejection fraction (RVEF) via CMR [12]. Its abnormality cutoff is set at 35% for the general population as dictated by the respective guidelines [11].
Despite their widespread use, evidence of the prognostic value of those indices is contradictory in severe TR. In the study by Karam et al., which included 249 high-risk patients with severe TR undergoing transcatheter repair, neither TAPSE nor FAC assessed pre-intervention could elicit long-term prognostic information [13]. Conversely, for conservatively managed patients, conventional RV function indices offered prognostic information. In a large registry of 1298 patients with significant secondary TR who were medically managed, subjects with TAPSE < 17 mm suffered the worst survival regardless of their RV dimensions [14]. The discrepancy of findings between the two studies might be attributable to the large sample size of the latter, where even the simplified TAPSE might still be indicative of the natural history of RV failure.
Kwon et al. associated reduced preoperative S’ with unfavorable outcomes in patients with severe functional TR, with previous mitral valve surgery having isolated tricuspid valve surgery, but their study was small and underpowered [15]. For surgically managed patients, more contemporary data were obtained by Dreyfus et al. who examined the post-operative incidence of in-hospital mortality in patients with primary or secondary symptomatic severe TR undergoing isolated tricuspid valve surgery.
TAPSE and S’ are simplified estimates of RV function and subjects to significant limitations in the setting of severe TR. Both indices are angle and load dependent and they assume that the displacement of a single point of the tricuspid annulus represents the overall function of the complex, three-dimensional structure of the RV [11]. Therefore, they exhibit only modest a correlation with the volumetric gold standard of RV EF via CMR [16]. Moreover, the movement of tricuspid annulus is usually accentuated in severe TR, resulting in overestimation of RV performance [11]. Even after tricuspid valve surgery TAPSE can be inaccurate and lead to underestimation of RV systolic function [17]. With respect to FAC, it is measured from a single acquisition of the RV from an apical four-chamber view; hence, it is limited by appreciable geometrical assumptions and demonstrates only fair inter-observer variability [18].
3. Three-Dimensional Echocardiography
Left ventricular EF by two-dimensional Simpson’s biplane method is the main echocardiographic parameter implemented to evaluate ventricular function for left-sided valvular heart disease in clinical practice. Simpson’s biplane method cannot be applied for the RV, which can only be assessed from a single plane in an apical RV-focused view via two-dimensional echocardiography. However, echocardiography allows the estimation of RV EF with the use of two-dimensional imaging that allows real RV full-volume capture and it is not dependent on the image plane orientation of the two-dimensional imaging.
Three-dimensional echocardiography has shown less volume underestimation than two-dimensional echocardiography and acceptable performance for volume quantification, comparable to CMR [19]. A head-to-head comparison between conventional RV function indices and three-dimensional RV EF demonstrated superior association of the latter in outcomes [20]. Among all other measurements of RV function, three-dimensional RVEF is the only index that allows assessment of all regions of the RV as well as the tricuspid valve leaflets [21][22]. In a cohort of 75 patients with severe TR undergoing transcatheter repair, preoperative three-dimensional RV EF predicted 1-year mortality, whereas TAPSE and FAC did not [23]. Moreover, higher pre-operative three-dimensional RV EF was associated with more pronounced improvement of functional capacity after the procedure [23].
Despite the benefits of three-dimensional echocardiography, the use of RV EF can markedly overestimate RV function for patients with severe TR, as it does not account for the relationship between contractility and afterload, but only describes the percentage of volume change per cardiac cycle [24]. Three-dimensional RV EF is an accurate expression of volumetric RV changes but fails to account for the direction of blood flow; forward to the pulmonary artery or backwards to the right atrium. In the case of severe TR, the RV largely empties in the low-pressure right atrium through the regurgitant jet, and this may mask a reduced RV inotropic state. Thus, the RV EF may not demonstrate an impaired RV myocardial performance since the volumetric change reflects both the forward flow and the amount of regurgitant volume and not active myocardial shortening per se. These limitations have stimulated the development and application of novel indices such as strain imaging for the RV.
4. Longitudinal Strain
In patients with severe TR presenting with RV volume overload, the contraction of subendocardial longitudinal fibers might be the first to be insulted indicating an early phase of ventricular damage, while contractility of the mid-circumferential RV layers can still be preserved or even augmented to compensate for the loss of longitudinal force (Figure 1) [25]. When circumferential contraction is lost, this represents an advanced stage of RV failure, and any attempt to intervene and change the natural history of TR might come with little clinical benefit [25]. Hence, appropriate assessment of RV longitudinal contraction is of paramount importance and might be the most pertinent to set optimal cutoff values to indicate the best time for intervention.
For over two decades, two-dimensional speckle-tracking echocardiography has emerged as a novel imaging technique assessing intrinsic myocardial function. Despite being initially utilized for the left ventricle, its applicability has recently been extended to the RV. RV global longitudinal strain (RVGLS) and RV free-wall longitudinal strain (RVFWLS) are the two main indices of speckle-tracking echocardiography for the assessment of RV performance, with a strong correlation to CMR-derived RV EF [26]. These indices offer substantial benefits over traditional indices of RV longitudinal function such as TAPSE or S’, as they are less dependent on geometry, cardiac translational motion, and loading conditions [18]. Since they follow the movement of myocardial speckles across the whole range of myocardial wall, they more accurately reflect more the overall RV performance [18]. However, a considerable limitation is the absence of unified, robust normality threshold both for RV GLS and RV FWLS [27].
RV longitudinal strain has provided evidence of refined risk stratification for TR patients. Prihadi et al. studied a large cohort of 896 patients with moderate or severe functional TR and disclosed that RV FWLS identified a higher number of patients with abnormal RV function compared to the traditional TAPSE and FAC [28]. This observation denotes the superior sensitivity of this index to capture subtle myocardial damage compared to the conventional echocardiography parameters of RV function [28]. In line with those findings, in a group of severe TR patients mainly functional in etiology, Ancona et al. showed that RV FWLS reclassified almost 50% of patients with apparently normal RV function, via TAPSE, FAC, or S’, to impaired RV function [29].
Apart from the ability to detect RV dysfunction in advance of the conventional parameters, RV longitudinal strain provides prognostic information for patients with severe TR treated conservatively. Prihadi et al. demonstrated that RV FWLS is associated, over and above TAPSE and FAC, with long-term outcomes [28]. In the study by Ancona et al., an optimal cutoff value of RV FWLS of −14% was identified to predict all-cause mortality [29]. Similar findings were published by Hinojan et al., who demonstrated superiority of RV GLS and RV FWLS to predict outcomes compared to conventional RV parameters in a cohort of secondary, at least severe TR patients without indication for intervention, implying that speckle-tracking echocardiography might be best suited to inform regarding intervention [30]. Downstream RV impairment will affect right atrial function, and subjects with a phenotype of impaired RV and right atrial strain are at higher risk for events [31].
RV strain could serve as a tool to select appropriate surgical candidates for TR repair at an early stage of the disease, before irreversible RV damage ensues, to optimize outcomes. Kim et al. indicated in a cohort of 115 patients with severe functional TR undergoing isolated tricuspid valve surgery that a pre-operative RV FWLS > −24% could independently predict dismal post-operative outcomes [32]. Furthermore RV strain provided incremental information on top of baseline clinical characteristics, whereas RV end-systolic area and RV FAC did not [32]. Similarly, post-operatively impaired RV GLS was independently associated with poor outcomes, highlighting the prognostic role of post-operative RV failure [33].
RV longitudinal strain provides information on the myocardial contraction beyond a mere description of volumetric RV changes. As such, it appears to be superior to other RV indices and could potentially refine risk stratification and optimal thresholds for intervention in TR. Figure 2 illustrates the progression from conventional RV indices to contemporary RV strain imaging via echocardiography in three diverse phenotypes of TR. A summary of studies addressing the prognostic value of RV strain in TR is shown in Table 2.
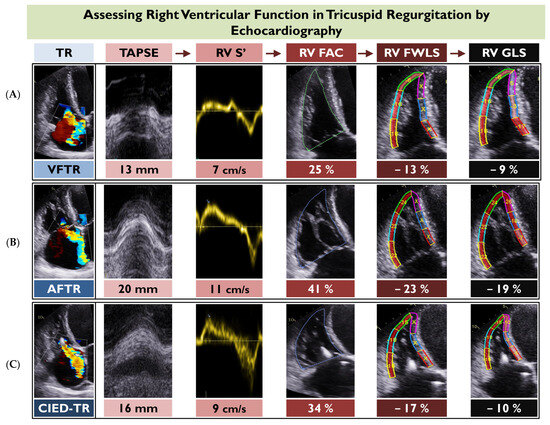
Figure 2. This figure depicts the application of the main two-dimensional echocardiographic indices of right ventricular (RV) function for three different case examples of tricuspid regurgitation, including ventricular functional TR (VFTR) (A), atrial functional TR (AFTR) (B), and cardiovascular implantable electronic device-related TR (CIED-TR) (C). Of note, all indices of RV function appear more preserved for AFTR compared to the other two TR types. FAC, fractional area change; RV FWLS, right ventricular free wall longitudinal strain; RV GLS; right ventricular global longitudinal strain; TAPSE; tricuspid annular systolic plane excursion.
5. Right Ventricular-Pulmonary Arterial Coupling
Although RV function assessment is paramount in severe TR, it fails to account for the subtending RV afterload, which may vary substantially between patients. Besides RV dysfunction, the prognostic role of pulmonary hypertension, which is a common hemodynamic complication of long-standing heart failure and TR, has been recognized [34]. RV-pulmonary arterial (PA) coupling has emerged as a novel, comprehensive index that allows the evaluation of RV function in relation to the underlying RV afterload [35]. This index can be readily assessed non-invasively through the ratio of two standard echocardiographic measurements: TAPSE over pulmonary artery systolic pressure (PASP). RV FWLS has also been used instead of TAPSE, but currently, there is less evidence available [36].
TAPSE/PASP ratio has demonstrated good correlation with the invasive pressure-volume loop-derived end-systolic/arterial elastance, which is the gold-standard measure of RV-PA coupling [37]. This index was initially applied as an outcome predictor for heart failure patients [35][38] but has recently been expanded to valvular heart disease [36][39][40], including patients with severe TR [41]. Fortuni et al. disclosed in a cohort of 1149 patients with at least moderate functional TR treated mostly conservatively, that RV-PA uncoupling, as defined via TAPSE/PASP < 0.31 mm/mmHg, was associated with significantly reduced survival [41]. Brener et al. expanded on those findings by demonstrating that impaired TAPSE/PASP was an independent predictor of poor post-operative outcomes for patients with severe TR undergoing transcatheter tricuspid valve repair [36]. More recently, a single-center study by Ancona et al. indicated that RV FWLS/PASP might be a superior prognosticator than TAPSE/PASP in severe TR, with a threshold of 0.26%/mmHg predicting outcomes [42].
While TAPSE/PASP could play a role to select appropriate candidates for percunatenous tricuspid valve interventions with severe TR, its utility is questionable for cases with greater than severe (massive or torrential) TR. In such cases, TAPSE/PASP may overestimate the true coupling of the RV-PA circuit, as echocardiographic PASP is underestimated and correlates poorly with the respective invasive PASPs. In a cohort of 126 patients with greater than severe TR undergoing transcatheter repair, only the TAPSE/PASP using the invasive PASP could provide prognostic information, whereas the echocardiographic TAPSE/PASP could not [43].
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442.
- Prihadi, E.A.; Van Der Bijl, P.; Gursoy, E.; Abou, R.; Vollema, E.M.; Hahn, R.T.; Stone, G.W.; Leon, M.B.; Marsan, N.A.; Delgado, V.; et al. Development of significant tricuspid regurgitation over time and prognostic implications: New insights into natural history. Eur. Heart J. 2018, 39, 3574–3581.
- Topilsky, Y.; Inojosa, J.M.; Benfari, G.; Vaturi, O.; Maltais, S.; Michelena, H.; Mankad, S.; Enriquez-Sarano, M. Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur. Heart J. 2018, 39, 3584–3592.
- Messika-Zeitoun, D.; Verta, P.; Gregson, J.; Pocock, S.J.; Boero, I.; Feldman, T.E.; Abraham, W.T.; Lindenfeld, J.; Bax, J.; Leon, M.; et al. Impact of tricuspid regurgitation on survival in patients with heart failure: A large electronic health record patient-level database analysis. Eur. J. Heart Fail. 2020, 22, 1803–1813.
- Benfari, G.; Antoine, C.; Miller, W.L.; Thapa, P.; Topilsky, Y.; Rossi, A.; Michelena, H.I.; Pislaru, S.; Enriquez-Sarano, M. Excess Mortality Associated with Functional Tricuspid Regurgitation Complicating Heart Failure with Reduced Ejection Fraction. Circulation 2019, 140, 196–206.
- Deuschl, F.; Schäfer, U. Tricuspid Valve Regurgitation: A Challenge for Interventional Treatment. JACC Cardiovasc. Interv. 2018, 11, 1129–1130.
- Bartko, P.E.; Arfsten, H.; Frey, M.K.; Heitzinger, G.; Pavo, N.; Cho, A.; Neuhold, S.; Tan, T.C.; Strunk, G.; Hengstenberg, C.; et al. Natural History of Functional Tricuspid Regurgitation: Implications of Quantitative Doppler Assessment. JACC Cardiovasc. Imaging 2019, 12, 389–397.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632.
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197.
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482.
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713.
- Anavekar, N.S.; Gerson, D.; Skali, H.; Kwong, R.Y.; Yucel, E.K.; Solomon, S.D. Two-Dimensional Assessment of Right Ventricular Function: An Echocardiographic? MRI Correlative Study. Echocardiography 2007, 24, 452–456.
- Karam, N.; Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; et al. Value of Echocardiographic Right Ventricular and Pulmonary Pressure Assessment in Predicting Transcatheter Tricuspid Repair Outcome. JACC: Cardiovasc. Interv. 2020, 13, 1251–1261.
- Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; Goedemans, L.; Mertens, B.J.; Gursoy, E.; van Genderen, O.S.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Remodeling and Function in Patients with Significant Secondary Tricuspid Regurgitation. Circulation 2019, 140, 836–845.
- Kwon, D.-A.; Park, J.-S.; Chang, H.-J.; Kim, Y.-J.; Sohn, D.-W.; Kim, K.-B.; Ahn, H.; Oh, B.-H.; Park, Y.-B.; Choi, Y.-S. Prediction of Outcome in Patients Undergoing Surgery for Severe Tricuspid Regurgitation Following Mitral Valve Surgery and Role of Tricuspid Annular Systolic Velocity. Am. J. Cardiol. 2006, 98, 659–661.
- Pavlicek, M.; Wahl, A.; Rutz, T.; de Marchi, S.F.; Hille, R.; Wustmann, K.; Steck, H.; Eigenmann, C.; Schwerzmann, M.; Seiler, C. Right ventricular systolic function assessment: Rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur. J. Echocardiogr. 2011, 12, 871–880.
- de Agustin, J.A.; Martinez-Losas, P.; de Diego, J.J.G.; Mahia, P.; Marcos-Alberca, P.; Nuñez-Gil, I.J.; Rodrigo, J.L.; Luaces, M.; Islas, F.; Garcia-Fernandez, M.A.; et al. Tricuspid annular plane systolic excursion inaccuracy to assess right ventricular function in patients with previous tricuspid annulopasty. Int. J. Cardiol. 2016, 223, 713–716.
- Aalen, J.M.; Smiseth, O.A. Strain identifies pseudo-normalized right ventricular function in tricuspid regurgitation. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 876–877.
- Gopal, A.S.; Chukwu, E.O.; Iwuchukwu, C.J.; Katz, A.S.; Toole, R.S.; Schapiro, W.; Reichek, N. Normal Values of Right Ventricular Size and Function by Real-time 3-Dimensional Echocardiography: Comparison with Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2007, 20, 445–455.
- Sayour, A.A.; Tokodi, M.; Celeng, C.; Takx, R.A.; Fábián, A.; Lakatos, B.K.; Friebel, R.; Surkova, E.; Merkely, B.; Kovács, A. Association of Right Ventricular Functional Parameters with Adverse Cardiopulmonary Outcomes: A Meta-analysis. J. Am. Soc. Echocardiogr. 2023, 36, 624–633.e8.
- Addetia, K.; Maffessanti, F.; Muraru, D.; Singh, A.; Surkova, E.; Mor-Avi, V.; Badano, L.P.; Lang, R.M. Morphologic Analysis of the Normal Right Ventricle Using Three-Dimensional Echocardiography–Derived Curvature Indices. J. Am. Soc. Echocardiogr. 2018, 31, 614–623.
- Addetia, K.; Muraru, D.; Veronesi, F.; Jenei, C.; Cavalli, G.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Badano, L.P. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc. Imaging 2019, 12, 401–412.
- Orban, M.; Wolff, S.; Braun, D.; Stolz, L.; Higuchi, S.; Stark, K.; Mehr, M.; Stocker, T.J.; Dischl, D.; Scherer, C.; et al. Right Ventricular Function in Transcatheter Edge-to-Edge Tricuspid Valve Repair. JACC Cardiovasc. Imaging 2021, 14, 2477–2479.
- Hirasawa, K.; van Rosendael, P.J.; Dietz, M.F.; Marsan, N.A.; Delgado, V.; Bax, J.J. Comparison of the Usefulness of Strain Imaging by Echocardiography Versus Computed Tomography to Detect Right Ventricular Systolic Dysfunction in Patients with Significant Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2020, 134, 116–122.
- Kresoja, K.-P.; Rommel, K.-P.; Lücke, C.; Unterhuber, M.; Besler, C.; von Roeder, M.; Schöber, A.R.; Noack, T.; Gutberlet, M.; Thiele, H.; et al. Right Ventricular Contraction Patterns in Patients Undergoing Transcatheter Tricuspid Valve Repair for Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 1551–1561.
- Focardi, M.; Cameli, M.; Carbone, S.F.; Massoni, A.; De Vito, R.; Lisi, M.; Mondillo, S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 47–52.
- Landzaat, J.W.D.; van Heerebeek, L.; Jonkman, N.H.; van der Bijl, E.M.; Riezebos, R.K. The quest for determination of standard reference values of right ventricular longitudinal systolic strain: A systematic review and meta-analysis. J. Echocardiogr. 2023, 21, 1–15.
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients with Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666.
- Ancona, F.; Melillo, F.; Calvo, F.; El Halabieh, N.A.; Stella, S.; Capogrosso, C.; Ingallina, G.; Tafciu, E.; Pascaretta, A.; Ancona, M.B.; et al. Right ventricular systolic function in severe tricuspid regurgitation: Prognostic relevance of longitudinal strain. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 868–875.
- Hinojar, R.; Zamorano, J.L.; González Gómez, A.; García-Martin, A.; Monteagudo, J.M.; García Lunar, I.; Sanchez Recalde, A.; Fernández-Golfín, C. Prognostic Impact of Right Ventricular Strain in Isolated Severe Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 615–623.
- Vely, M.; L’Official, G.; Galli, E.; Kosmala, W.; Guerin, A.; Chen, E.; Sportouch, C.; Dreyfus, J.; Oger, E.; Donal, E. Functional tricuspid regurgitation: A clustering analysis and prognostic validation of three echocardiographic phenotypes in an external cohort. Int. J. Cardiol. 2022, 365, 140–147.
- Kim, M.; Lee, H.; Park, J.; Kim, J.; Lee, S.; Kim, Y.; Chang, S.; Kim, H. Preoperative Right Ventricular Free-Wall Longitudinal Strain as a Prognosticator in Isolated Surgery for Severe Functional Tricuspid Regurgitation. J. Am. Heart Assoc. 2021, 10, e019856.
- Kim, D.-Y.; Seo, J.; Cho, I.; Lee, S.H.; Lee, S.; Hong, G.-R.; Ha, J.-W.; Shim, C.Y. Prognostic Implications of Biventricular Global Longitudinal Strain in Patients with Severe Isolated Tricuspid Regurgitation. Front. Cardiovasc. Med. 2022, 9, 908062.
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017, 69, 1718–1734.
- Guazzi, M.; Naeije, R.; Arena, R.; Corrà, U.; Ghio, S.; Forfia, P.; Rossi, A.; Cahalin, L.P.; Bandera, F.; Temporelli, P. Echocardiography of Right Ventriculoarterial Coupling Combined with Cardiopulmonary Exercise Testing to Predict Outcome in Heart Failure. Chest 2015, 148, 226–234.
- Brener, M.I.; Grayburn, P.; Lindenfeld, J.; Burkhoff, D.; Liu, M.; Zhou, Z.; Alu, M.C.; Medvedofsky, D.A.; Asch, F.M.; Weissman, N.J.; et al. Right Ventricular-Pulmonary Arterial Coupling in Patients with HF Secondary MR: Analysis From the COAPT Trial. JACC Cardiovasc. Interv. 2021, 14, 2231–2242.
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047.
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur. J. Heart Fail. 2017, 19, 873–879.
- Rubbio, A.P.; Testa, L.; Granata, G.; Salvatore, T.; De Marco, F.; Casenghi, M.; Guerrini, M.; Oliva, O.A.; Stefanini, E.; Barletta, M.; et al. Prognostic significance of right ventricle to pulmonary artery coupling in patients with mitral regurgitation treated with the MitraClip system. Catheter. Cardiovasc. Interv. 2022, 99, 1277–1286.
- Cahill, T.J.; Pibarot, P.; Yu, X.; Babaliaros, V.; Blanke, P.; Clavel, M.-A.; Douglas, P.S.; Khalique, O.K.; Leipsic, J.; Makkar, R.; et al. Impact of Right Ventricle-Pulmonary Artery Coupling on Clinical Outcomes in the PARTNER 3 Trial. JACC Cardiovasc. Interv. 2022, 15, 1823–1833.
- Fortuni, F.; Butcher, S.C.; Dietz, M.F.; van der Bijl, P.; Prihadi, E.A.; De Ferrari, G.M.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right Ventricular–Pulmonary Arterial Coupling in Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2021, 148, 138–145.
- Ancona, F.; Margonato, D.; Menzà, G.; Bellettini, M.; Melillo, F.; Stella, S.; Capogrosso, C.; Ingallina, G.; Biondi, F.; Boccellino, A.; et al. Ratio between right ventricular longitudinal strain and pulmonary arterial systolic pressure: A novel prognostic parameter in patients with severe tricuspid regurgitation. Int. J. Cardiol. 2023, 384, 55–61.
- Gerçek, M.; Körber, M.I.; Narang, A.; Friedrichs, K.P.; Puthumana, J.J.; Rudolph, T.K.; Thomas, J.D.; Pfister, R.; Davidson, C.J.; Rudolph, V. Echocardiographic Pulmonary Artery Systolic Pressure Is Not Reliable for RV-PA Coupling in Transcatheter Tricuspid Valve Annuloplasty. JACC: Cardiovasc. Interv. 2022, 15, 2578–2580.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
442
Revisions:
2 times
(View History)
Update Date:
04 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




