
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radoslav Stojchevski | -- | 2066 | 2023-12-01 18:45:23 | | | |
| 2 | Catherine Yang | Meta information modification | 2066 | 2023-12-04 02:08:28 | | |
Video Upload Options
A large body of evidence has demonstrated a significant link between obesity and cancer risk. Adipose tissue, conventionally viewed as a passive reservoir for energy storage, is now recognized as a highly secretory endocrine organ that produces various pro- and anti-inflammatory cytokines, estrogens, and other bioactive molecules. Obesity, characterized by adipose tissue hypertrophy (increase in adipocyte size) and hyperplasia (increase in adipocyte number), causes the dysregulation of adipose tissue hormonal production, leading to chronic low-grade inflammation that can contribute to the initiation and progression of breast cancer, particularly among postmenopausal women. Furthermore, obesity-related metabolic changes can influence the composition of the gut microbiome, leading to dysbiosis, which may further affect breast cancer risk and outcomes. Over the past two decades, following advancements in DNA sequencing technologies, the microbiome has been recognized as a major factor in maintaining health. The interaction between the microbiome and the host organism is a dynamic bidirectional relationship, where disruptions in the microbiome reflect the host’s health and vice versa: modifications to the health status of the host lead to corresponding microbiome changes.
1. Role of the Microbiome in Obesity-Induced Inflammation
The consistent energy overload mainly affects visceral white adipose tissue. Adipose tissue hypertrophy impairs normal adipocyte differentiation and secretion and stimulates tissue infiltration of immune cells, resulting in elevated proinflammatory cytokine secretion and chronic low-grade inflammation [1], leading to the development of metabolic conditions such as metabolic syndrome, dyslipidemia, insulin resistance, and type 2 diabetes [2][3][4]. The level of adiposity also strongly correlates with an increased incidence and worse outcomes in many different types of cancer [5]. Obesity is thought to be related to a 30% increase in breast cancer risk [6][7], and various studies have found intriguing associations between microbiota and obesity [8]. Although obesity plays a protective role in the development of breast cancer in premenopausal (particularly European) women, it shows a strong positive correlation with breast cancer risk in postmenopausal settings [9]. Among the multiple factors involved in this association, cytokines released by the white adipocytes per se, or activated macrophages, may directly promote the invasive potential and aggressiveness of breast cancer cells [10][11].
The gut microbiome composition is tightly modulated by metabolic signals and plays a significant role in the development of obesity. The level of adiposity is positively associated with changes in the microbiome composition (referred to as dysbiosis), characterized by generally reduced diversity and a shift in the abundance of dominant species [12][13][14][15]. For example, a cohort study involving primary school students in China revealed that obese children had lower species diversity and a relative abundance of typically dominant bacterial strands but a higher abundance of other genera [16]. Obese leptin-deficient (ob/ob) mice have a higher Firmicutes/Bacteroidetes (F/B) bacterial ratio than their wild-type counterparts [17]. Similar changes in the F/B ratio were observed in obese and lean humans [12][17][18]. Differences in abundance between lean and obese individuals have also been detected in other bacterial groups, such as those from the Oscillospira genus or the Christensenellaceae family [19][20]. Lv et al. [15] demonstrated a linear relationship between the body mass index (BMI) and several bacterial families (Porphyromonadaceae, Acidaminococcaceae, Rikenellaceae, and Desulfovibrionaceae). White et al. [21] suggested that gut microbiota is a modifiable factor linked to early rapid weight gain during infancy, and early weight gain has been identified as a risk factor for obesity during adulthood. The connection between the gut microbiome and adiposity extends to preterm infants, where the microbial composition was found to correlate with weight gain and subsequent growth, showing the influence of the microbiota from the earliest stages of life [22]. Similar to the gut microbiome, the breast tissue microbiome shows disparities between lean and obese individuals, with obese individuals exhibiting reduced bacterial diversity [23][24].
Dietary patterns may cause gut dysbiosis, which can lead to chronic inflammation [25]. A growing body of evidence has revealed that obesity-induced inflammation is associated with changes in microbiome composition. For example, using a high-fat diet (HFD)-induced obesity C57Bl/6 mouse model, Albornoz et al. [26] showed that obesity increases the susceptibility, pulmonary inflammation, and interferon-gamma (INF-γ) levels, following an infection with Mycobacterium tuberculosis. Gut bacteria metabolize dietary fiber into short-chain fatty acids (SCFAs), primarily butyrate, acetate, and propionate [27]. Butyrate has beneficial effects against obesity, including the promotion of lipolysis and an increase in energy expenditure. It also possesses anti-inflammatory properties by inhibiting proinflammatory cytokine production and reducing the translocation of lipopolysaccharides (LPSs) from the gut lumen to the bloodstream [28][29]. Butyrate also inhibits the expression of nitric oxide synthase (NOS) in intestinal cells by activating peroxisome proliferator-activated receptor gamma (PPARγ) signaling, thus limiting the growth of certain bacteria (such as those of the Enterobacteriaceae family) [30].
2. Microbiota and Breast Cancer
The microbiome profile has been linked to many types of cancers (stomach, colon, liver, lung, and skin, among others). The most robust connections are observed in cancers of the gastrointestinal tract, which are primarily associated with Helicobacter pylori and Fusobacterium bacteria [24][31][32][33][34][35][36].
Breast cancer patients are characterized by decreased microbial diversity, as reported in several studies [37][38][39][40]. Early observational studies detected impaired intestinal microbiota in breast cancer patients, represented by a higher proportion of fecal Enterobacteriaceae, aerobic Streptococci, Lactobacilli, and anaerobic species such as Clostridia, Lactobacilli, and Bacteroides [41]. A comparative analysis by Xuan et al. [37] showed the enrichment of Methylobacterium radiotolerans in breast tumor tissues and Sphingomonas yanoikuyae in paired normal breast tissues (Figure 1). Using 16S rRNA gene amplicon sequencing, Chan et al. [42] investigated microbiota from nipple aspirates from healthy women and those with breast cancer and reported a higher incidence of Sphingomonadaceae family in the healthy subject group and a higher proportion of the genus Alistipes in breast cancer patients (Figure 1). The microbiota of breast tissue adjacent to the tumor showed higher presence of the phylum Bacteroidetes and the genera Bacillus and Staphylococcus than those in healthy tissues [43]. Similarly, Meng et al. [44] analyzed breast tissue samples using needle biopsies from patients with breast cancer and benign tumors and observed an increase in the genus Propionicimonas and the families Micrococcaceae, Caulobacteraceae, Rhodobacteraceae, Nocardioidaceae, and Methylobacteriaceae (Figure 1). The microbial characterization of samples from 25 breast cancer patients showed lower abundance of Firmicutes and Bacteroidetes and higher abundance of Proteobacteria, Actinobacteria, and Verrucomicrobia bacteria, accompanied by a reduction in Faecalibacterium prausnitzii [45]. Another comparative study between patients with breast cancer and healthy individuals [46] demonstrated greater proportion of Enterobacteriaceae and Pseudomonadaceae families (such as the genera Pseudomonas, Proteus, Azomonas, and Porphyromonas) in breast tumors and predominance of the genera Staphylococcus and Propionibacterium in healthy controls (Figure 1). A comparison of the breast tissues adjacent to the tumor showed a higher abundance of Bacteroidetes (Bacillus and Staphylococcus). Additionally, the F/B ratio was found to be significantly higher in patients with breast cancer than in controls [45].
Furthermore, microbial profiles vary during the progression of breast cancer. A comparison of the microbiome profiles of malignant tissues of different histological grades revealed that the development of breast cancer was associated with a decreased proportion of bacteria from the Bacteroidaceae family and an increased proportion of bacteria from the Agrococcus genus [44]. Stage I breast cancers exhibit an abundance of Proteobacteria, Ruminococcaceae, and Hyphomicrobium; stage II breast cancers show higher presence of Euryarchaeota, Firmicutes, Spirochaetes, and Sporosarcina, whereas stages III and IV breast cancers are more abundant on Thermi, Gemmatimonadetes, Tenericutes, and Bosea [47].
Evidence suggests that shifts in microbial assemblages in the breast are related to breast cancer development, aggressiveness, and progression [24]. Using next-generation sequencing techniques and quantitative PCR analysis, Xuan et al. [37] demonstrated that breast tumor tissue has a reduced expression of antibacterial response genes, compared with adjacent healthy breast tissue. The observed dysbiosis in breast cancer suggests that bacteria may play a role in maintaining the normal cellular processes in the breast. Thus, it is speculated that the microbial components present in the breast may influence the local microenvironment. It is hypothesized that chronic exposure to low-residue antimicrobial drugs ingested from the diet could disrupt the gut microbiota equilibrium, which can contribute to corresponding physiological changes [48]. Dysbiosis caused by antibiotic use may increase the risk of breast cancer; however, more extensive studies are needed to confirm this hypothesis [49][50].
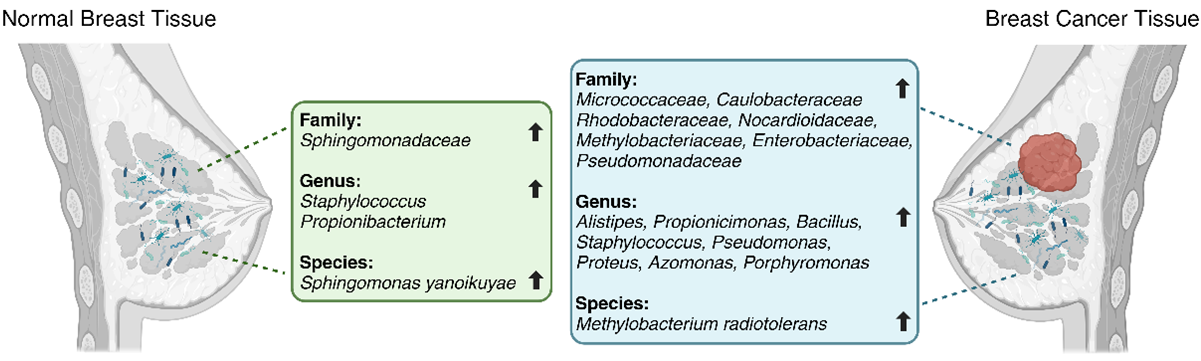
Figure 1. Differences in microbial taxa between normal breast tissue (on the left) and breast cancer tissue (on the right) per various studies (Created with BioRender).
3. The Microbiome as a Biomarker and Treatment Target
Based on current knowledge, the microbiome has emerged as a promising biomarker for evaluating breast cancer risk and prognosis or predicting the surgical outcomes and survival of patients with breast cancer [39][51][52][53]. For example, the F/B ratio can be used as an indicator of breast cancer risk [43][54]. Evaluation of the microbiome profile could have broad implications for the diagnosis and staging of breast cancer [44]. Meng et al. [44] showed that glycerophospholipid levels and ribosome biogenesis are higher in grade III breast cancers than in grades I and II. Additionally, the microbiome involved in human estrogen metabolism (also known as the estrobolome) can be used as another target for breast cancer treatment [54]. Microbial communities can alter the response to breast cancer therapy [55]. Gut microbe dysbiosis undermines the outcome of both immune and non-immune chemotherapeutic cancer treatment modalities [56][57][58]. The microbiota may potentially be targeted to enhance the efficacy and reduce the toxicity of conventional anticancer therapies. Taken together, the complex scenario linking microbiome composition to oncogenesis and the response to anticancer treatments defines the frame of a new “oncobiotic” perspective.
Probiotics have been shown to improve gut microbiota composition and function, suggesting their potential implications in cancer prevention and treatment [59]. Lactobacillus bacteria can modulate dysregulated SCFA levels in obesity by influencing other gut microbiota, energy absorption, and chronic low-grade inflammation [60]. Lactic acid bacteria (LAB) have been reported to exert anti-obesity effects. Thus, targeting the microbiome could be considered a potential treatment option for obesity [61]. Animal and cell-based studies have shown that probiotics may have anticancer effects because they can modulate the immune system and reduce obesity-induced low-grade chronic inflammation, potentially inhibiting cancer cell growth [59][62]. A study investigating the effect of oral administration of probiotics for 12 weeks, involving 18 patients with breast cancer, demonstrated an improved microbiome profile and serum tests (ANC (absolute neutrophil count), fasting blood glucose (FBG), and low-density lipoprotein cholesterol (LDL-C) levels) [63]. The most prominent changes observed in this study were for Ruminococcus and Streptococcus spp. The effects of probiotics, prebiotics, and synbiotics on breast cancer have been reviewed in randomized controlled trials [59][64]. A systematic review and meta-analysis of randomized clinical trials of probiotic and prebiotic use in breast cancer patients and survivors by Thu et al. [65] demonstrated the beneficial effects of a combination of pro- and prebiotics on obesity and dyslipidemia, as well as the reduction of tumor-necrosis factor alpha (TNFα) levels, thus highlighting their potential against breast cancer. However, using probiotics to improve the gut microbiome as a treatment strategy for obesity is likely more complicated than anticipated and may require a long-term complex program [60].
Fecal microbiota transplantation (FMT) is another promising strategy for reducing obesity. Dietary interventions or FMT have emerged as promising strategies to help patients maintain a healthy weight [66]. FMT has been shown to reverse the effects of antibiotics and re-establish microbiota balance, resulting in the restoration of the normal functioning microbiome [67].
Furthermore, diet is known to influence the microbiota. The Mediterranean diet (characterized by a high content of plant-based foods and healthy fats) has been associated with a distinctive shift in the mammary gland microbiota, suggesting possible anti-breast cancer effects [68]. Long-term breast cancer risk is associated with diet-related plasma metabolic signatures involving exogenous steroid metabolites and microbiota-related compounds [69]. SCFAs are produced by two major groups of bacteria: Firmicutes bacteria produce butyrate, while Bacteroidetes bacteria – acetate and propionate. It has been shown that SCFAs, more specifically butyrate, inhibit tumor growth [70]. A typical Western diet decreases the generation of SCFAs, causing a leaky gut and leading to an increase in inflammatory marker levels in the bloodstream, which results in the progression of breast cancer. Conversely, healthy diets with a higher fiber content may decrease inflammation by increasing SCFA production [71].
Further studies should focus on elucidating the mechanisms underlying the impact of the microbiome on breast cancer and exploring its potential as a biomarker and treatment agent.
Abbreviations
ANC Absolute neutrophil count
F/B Firmicutes/Bacteroidetes bacterial ratio
FBG Fasting blood glucose
FMT Fecal microbiota transplantation
HFD High-fat diet
INF-γ Interferon-gamma
LAB Lactic acid bacteria
LDL-C Low-density lipoprotein cholesterol
LPS Lipopolysaccharide
NOS Nitric oxide synthase
ob/ob mice Leptin-deficient mice
PPARγ Peroxisome proliferator-activated receptor gamma
SCFA Short-chain fatty acid
TNFα Tumor necrosis factor-alpha
References
- Sonali Sengupta; Dimiter Avtanski. Obesity and Inflammation; Springer Science and Business Media LLC: Dordrecht, GX, Netherlands, 2023; pp. 15-53.
- Gökhan S. Hotamisligil; Inflammation and metabolic disorders. Nat. 2006, 444, 860-867.
- Tatsuo Kawai; Michael V. Autieri; Rosario Scalia; Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2020, 320, C375-C391.
- Dimiter Avtanski; Valentin A. Pavlov; Kevin J. Tracey; Leonid Poretsky; Characterization of inflammation and insulin resistance in high‐fat diet‐induced male C57BL/6J mouse model of obesity. Anim. Model. Exp. Med. 2019, 2, 252-258.
- Graham A Colditz; Lindsay L. Peterson; Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154-162.
- Harri Vainio; Rudolf Kaaks; Franca Bianchini; Weight control and physical activity in cancer prevention: international evaluation of the evidence.. European Journal of Cancer Prevention 2002, 11, S94-100.
- Sophia A. Stone; Claire J. Han; Taurence Senn; Larissa A. Korde; Kristen Allott; Scott Reding; Dale Whittington; Kerryn W. Reding; Sex Hormones in Women With Elevated Breast Cancer Risk Undergoing Weight Loss. West. J. Nurs. Res. 2019, 41, 1602-1622.
- Hannah Wang; Jessica Altemus; Farshad Niazi; Holly Green; Benjamin C. Calhoun; Charles Sturgis; Stephen R. Grobmyer; Charis Eng; Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 2017, 8, 88122-88138.
- Dehesh, T.; Fadaghi, S.; Seyedi, M.; Abolhadi, E.; Ilaghi, M.; Shams, P.; Ajam, F.; Mosleh-Shirazi, M.A.; Dehesh, P The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: A systematic review and meta-analysis. BMC Women's Health 2023, 23, 392.
- Dan Yan; Dimiter Avtanski; Neeraj K. Saxena; Dipali Sharma; Leptin-induced Epithelial-Mesenchymal Transition in Breast Cancer Cells Requires β-Catenin Activation via Akt/GSK3- and MTA1/Wnt1 Protein-dependent Pathways. J. Biol. Chem. 2012, 287, 8598-8612.
- Dimiter Avtanski; Anabel Garcia; Beatriz Caraballo; Priyanthan Thangeswaran; Sela Marin; Julianna Bianco; Aaron Lavi; Leonid Poretsky; Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms. Cytokine 2019, 120, 155-164.
- Froukje J. Verdam; Susana Fuentes; Charlotte de Jonge; Erwin G. Zoetendal; Runi Erbil; Jan Willem Greve; Wim A. Buurman; Willem M. de Vos; Sander S. Rensen; Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obes. 2013, 21, E607-E615.
- John S. Davis Connecting Female Infertility to Obesity, Inflammation, and Maternal Gut Dysbiosis. Endocrinology 2016, 157, 1725–1727.
- Jonathan Tam; Thomas Hoffmann; Sabine Fischer; Stefan Bornstein; Jürgen Gräßler; Barbara Noack; Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLOS ONE 2018, 13, e0204724.
- Yanrong L.; Xiangxiang Q.; Huaijie J.; Sirui C.; Weiwei S.; Xiaoxia W. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. British Journal of Nutrition 2019, 122, 986-995.
- L F Jiang; Y Y Wang; H Peng; R Li; F Zhang; N Wang; Q W Shao; Qingwu Jiang; [Association between obesity with the diversity and genus of gut microbiota in school-aged children].. null 2022, 43, 260-268.
- Ruth E. Ley; Peter J. Turnbaugh; Samuel Klein; Jeffrey I. Gordon; Human gut microbes associated with obesity. Nat. 2006, 444, 1022-1023.
- Emily R Leeming; Abigail J Johnson; Tim D Spector; Caroline I Le Roy; Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutr. 2019, 11, 2862.
- Tom Konikoff; Uri Gophna; Oscillospira : a Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523-524.
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell 2014, 159, 789-799.
- Richard A. White; Jørgen V. Bjørnholt; Donna D. Baird; Tore Midtvedt; Jennifer R. Harris; Marcello Pagano; Winston Hide; Knut Rudi; Birgitte Moen; Nina Iszatt; et al.Shyamal D. PeddadaMerete Eggesbø Novel Developmental Analyses Identify Longitudinal Patterns of Early Gut Microbiota that Affect Infant Growth. PLOS Comput. Biol. 2013, 9, e1003042.
- Jun Qiu; Changci Zhou; Shiting Xiang; Jie Dong; Qifeng Zhu; Jieyun Yin; Xiulan Lu; Zhenghui Xiao; Association Between Trajectory Patterns of Body Mass Index Change Up to 10 Months and Early Gut Microbiota in Preterm Infants. Front. Microbiol. 2022, 13, 828275.
- Raul Cabrera-Rubio; M Carmen Collado; Kirsi Laitinen; Seppo Salminen; Erika Isolauri; Alex Mira; Alexander D Miras; Robert N Jackson; Sabrina N Jackson; Anthony P Goldstone; et al.Torsten OlbersTimothy HackenbergAlan C SpectorCarel W le Roux The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544-551.
- Tina J. Hieken; Jun Chen; Tanya L. Hoskin; Marina Walther-Antonio; Stephen Johnson; Sheri Ramaker; Jian Xiao; Derek C. Radisky; Keith L. Knutson; Krishna R. Kalari; et al.Janet Z. YaoLarry M. BaddourNicholas ChiaAmy C. Degnim The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751.
- Varun Reddy; Dimiter Avtanski. Environmental and Lifestyle Factors Influencing Inflammation and Type 2 Diabetes; Springer Science and Business Media LLC: Dordrecht, GX, Netherlands, 2023; pp. 165-183.
- Sandra Patricia Palma Albornoz; Thais Fernanda de Campos Fraga-Silva; Ana Flávia Gembre; Rômulo Silva de Oliveira; Fernanda Mesquita de Souza; Tamara Silva Rodrigues; Isis Do Carmo Kettelhut; Camila Sanches Manca; Alceu Afonso Jordao; Leandra Naira Zambelli Ramalho; et al.Paulo Eduardo Martins RibollaDaniela CarlosVânia Luiza Deperon Bonato Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection. Cells 2021, 10, 1732.
- Yimin Jia; Jian Hong; Huifang Li; Yun Hu; Longfei Jia; Demin Cai; Ruqian Zhao; Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation‐associated β3‐adrenergic receptor activation in high‐fat diet‐induced obese mice. Exp. Physiol. 2017, 102, 273-281.
- Hardi Lührs; Tobias Gerke; Jürgen Schauber; Gerda Dusel; Ralf Melcher; Wolfgang Scheppach; Thomas Menzel; Cytokine-activated degradation of inhibitory κB protein α is inhibited by the short-chain fatty acid butyrate. Int. J. Color. Dis. 2001, 16, 195-201.
- Annick V. Hartstra; Kristien E.C. Bouter; Fredrik Bäckhed; Max Nieuwdorp; Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2014, 38, 159-165.
- Mariana X. Byndloss; Erin E. Olsan; Fabian Rivera-Chávez; Connor R. Tiffany; Stephanie A. Cevallos; Kristen L. Lokken; Teresa P. Torres; Austin J. Byndloss; Franziska Faber; Yandong Gao; et al.Yael LitvakChristopher A. LopezGege XuEleonora NapoliCecilia GiuliviRenée M. TsolisAlexander RevzinCarlito B. LebrillaAndreas J. Bäumler Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Sci. 2017, 357, 570-575.
- D Forman; D G Newell; F Fullerton; J W Yarnell; A R Stacey; N Wald; F Sitas; Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation.. BMJ 1991, 302, 1302-1305.
- A.C. Wotherspoon; T.C. Diss; L. Pan; P.G. Isaacson; C. Doglioni; A. Moschini; M. de Boni; Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575-577.
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Research 2012, 22, 292-298.
- Robert F. Schwabe; Christian Jobin; The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800-812.
- Jiyoung Ahn; Rashmi Sinha; Zhiheng Pei; Christine Dominianni; Jing Wu; Jianxin Shi; James J. Goedert; Richard B. Hayes; Liying Yang; Human Gut Microbiome and Risk for Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2013, 105, 1907-1911.
- Fei Wang; Wenbo Meng; Bingyuan Wang; Liang Qiao; Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196-202.
- Caiyun Xuan; Jaime M. Shamonki; Alice Chung; Maggie L. DiNome; Maureen Chung; Peter A. Sieling; Delphine J. Lee; Microbial Dysbiosis Is Associated with Human Breast Cancer. PLOS ONE 2014, 9, e83744.
- Emily Klann; Jessica M. Williamson; Massimiliano S. Tagliamonte; Maria Ukhanova; Jaya Ruth Asirvatham; Harvey Chim; Lusine Yaghjyan; Volker Mai; Microbiota composition in bilateral healthy breast tissue and breast tumors. Cancer Causes Control. 2020, 31, 1027-1038.
- Sabine Dieleman; Romy Aarnoutse; Janine Ziemons; Loes Kooreman; Annemarie Boleij; Marjolein Smidt; Exploring the Potential of Breast Microbiota as Biomarker for Breast Cancer and Therapeutic Response. Am. J. Pathol. 2021, 191, 968-982.
- Na Wang; Tao Sun; Junnan Xu; Tumor-related Microbiome in the Breast Microenvironment and Breast Cancer. J. Cancer 2021, 12, 4841-4848.
- E. Bertazzoni Minelli; A. M. Beghini; S. Vesentini; L. Marchiori; G. Nardo; R. Cerutti; E. Mortani; Intestinal Microflora as an Alternative Metabolic Source of Estrogens in Women with Uterine Leiomyoma and Breast Cancer. Ann. New York Acad. Sci. 1990, 595, 473-479.
- Chan, A., Bashir, M., Rivas, M. et al. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Scientific Reports 2016, 6, 28061.
- Camilla Urbaniak; Gregory B. Gloor; Muriel Brackstone; Leslie Scott; Mark Tangney; Gregor Reid; The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039-5048.
- Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, Zhang L. Study of Microbiomes in Aseptically Collected Samples of Human Breast Tissue Using Needle Biopsy and the Potential Role of in situ Tissue Microbiomes for Promoting Malignancy. Frontiers in Oncology 2018, 8, 318.
- Chuan He; Yue Liu; Shandong Ye; Shiwu Yin; Junfei Gu; Changes of intestinal microflora of breast cancer in premenopausal women. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 503-513.
- Alice Tzeng; Naseer Sangwan; Margaret Jia; Chin-Chih Liu; Karen S. Keslar; Erinn Downs-Kelly; Robert L. Fairchild; Zahraa Al-Hilli; Stephen R. Grobmyer; Charis Eng; et al. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 1-17.
- Alana Smith; Joseph F. Pierre; Liza Makowski; Elizabeth Tolley; Beverly Lyn-Cook; Lu Lu; Gregory Vidal; Athena Starlard-Davenport; Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019, 9, 1-10.
- Lee W. Riley; Eva Raphael; Eduardo Faerstein; Obesity in the United States – Dysbiosis from Exposure to Low-Dose Antibiotics?. Front. Public Heal. 2013, 1, 69.
- Christine M. Velicer; Susan R. Heckbert; Johanna W. Lampe; John D. Potter; Carol A. Robertson; Stephen H. Taplin; Antibiotic Use in Relation to the Risk of Breast Cancer. JAMA 2004, 291, 827-35.
- Alastair M. McKee; Benjamin M. Kirkup; Matthew Madgwick; Wesley J. Fowler; Christopher A. Price; Sally A. Dreger; Rebecca Ansorge; Kate A. Makin; Shabhonam Caim; Gwenaelle Le Gall; et al.Jack PaveleyCharlotte LeclaireMatthew DalbyCristina Alcon-GinerAnna AndrusaiteTzu-Yu FengMartina Di ModicaTiziana TriulziElda TagliabueSimon W.F. MillingKatherine N. WeilbaecherMelanie R. RutkowskiTamás KorcsmárosLindsay J. HallStephen D. Robinson Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience 2021, 24, 103012.
- Safae Terrisse; Lisa Derosa; Valerio Iebba; François Ghiringhelli; Ines Vaz-Luis; Guido Kroemer; Marine Fidelle; Stergios Christodoulidis; Nicola Segata; Andrew Maltez Thomas; et al.Anne-Laure MartinAude SirvenSibille EverhardFanny AprahamianNitharsshini NirmalathasanRomy AarnoutseMarjolein SmidtJanine ZiemonsCarlos CaldasSibylle LoiblCarsten DenkertSylvere DurandClaudia IglesiasFilippo PietrantonioBertrand RoutyFabrice AndréEdoardo PasolliSuzette DelalogeLaurence Zitvogel Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021, 28, 2778-2796.
- Jonathan Sholl; Gregory D. Sepich-Poore; Rob Knight; Thomas Pradeu; Redrawing therapeutic boundaries: microbiota and cancer. Trends Cancer 2021, 8, 87-97.
- Khalid El Bairi; Rachid Jabi; Dario Trapani; Hanae Boutallaka; Bouchra Ouled Amar Bencheikh; Mohammed Bouziane; Mariam Amrani; Said Afqir; Adil Maleb; Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut. Expert Rev. Clin. Pharmacol. 2020, 13, 403-421.
- Jeongshin An; Hyungju Kwon; Woosung Lim; Byung-In Moon; Staphylococcus aureus-Derived Extracellular Vesicles Enhance the Efficacy of Endocrine Therapy in Breast Cancer Cells. J. Clin. Med. 2022, 11, 2030.
- Sheetal Parida; Dipali Sharma; The power of small changes: Comprehensive analyses of microbial dysbiosis in breast cancer. Biochim. et Biophys. Acta (BBA) - Rev. Cancer 2019, 1871, 392-405.
- Jessica R. Lakritz; Theofilos Poutahidis; Sheyla Mirabal; Bernard J. Varian; Tatiana Levkovich; Yassin M. Ibrahim; Jerrold M. Ward; Ellen C. Teng; Brett Fisher; Nicola Parry; et al.Stephanie LesageNatalie AlbergSravya GourishettiJames G. FoxZhongming GeSusan E. Erdman Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 2015, 6, 9387-9396.
- Noriho Iida; Amiran Dzutsev; C. Andrew Stewart; Loretta Smith; Nicolas Bouladoux; Rebecca A. Weingarten; Daniel A. Molina; Rosalba Salcedo; Timothy Back; Sarah Cramer; et al.Ren-Ming DaiHiu KiuMarco CardoneShruti NaikAnil K. PatriEna WangFrancesco M. MarincolaKaren M. FrankYasmine BelkaidGiorgio TrinchieriRomina S. Goldszmid Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Sci. 2013, 342, 967-970.
- Sophie Viaud; Fabiana Saccheri; Grégoire Mignot; Takahiro Yamazaki; Romain Daillère; Dalil Hannani; David P. Enot; Christina Pfirschke; Camilla Engblom; Mikael J. Pittet; et al.Andreas SchlitzerFlorent GinhouxLionel ApetohElisabeth ChachatyPaul-Louis WoertherGérard EberlMarion BérardChantal EcobichonDominique ClermontChantal BizetValérie Gaboriau-RouthiauNadine Cerf-BensussanPaule OpolonNadia YessaadEric VivierBernhard RyffelCharles O. ElsonJoël DoréGuido KroemerPatricia LepageIvo Gomperts BonecaFrançois GhiringhelliLaurence Zitvogel The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Sci. 2013, 342, 971-976.
- Luis Mendoza; Potential effect of probiotics in the treatment of breast cancer. Oncol. Rev. 2019, 13, 134-138.
- Sang Man Kim; Obesity and Dysbiosis. Korean J. Obes. 2015, 24, 121-125.
- Hongjie Li; Diet, Gut Microbiota and Obesity. J. Nutr. Heal. Food Sci. 2015, 3, 1-6.
- Samia Abd El‐Atti; Kelley Wasicek; Scott Mark; Refaat Hegazi; Use of Probiotics in the Management of Chemotherapy‐Induced Diarrhea: A Case Study. J. Parenter. Enter. Nutr. 2009, 33, 569-570.
- J. An; B. Moon; 85P Microbiome analysis in patients with breast cancer via an oral prebiotics therapy. ESMO Open 2023, 8, 101308.
- Dan Duan; Maojun Chen; Wenyao Cui; Wenjie Liu; Xinrong Chen; Application of probiotics, prebiotics and synbiotics in patients with breast cancer: a systematic review and meta-analysis protocol for randomised controlled trials. BMJ Open 2022, 12, e064417.
- May S. Thu; Thunnicha Ondee; Tanawin Nopsopon; Izzati A. K. Farzana; Joanne L. Fothergill; Nattiya Hirankarn; Barry J. Campbell; Krit Pongpirul; Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis. Biol. 2023, 12, 280.
- Cristina Torres-Fuentes; Harriët Schellekens; Timothy G Dinan; John F Cryan; The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747-756.
- Maria Guirro; Andrea Costa; Andreu Gual-Grau; Pol Herrero; Helena Torrell; Núria Canela; Lluis Arola; Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLOS ONE 2019, 14, e0218143.
- Tiffany M. Newman; Mara Z. Vitolins; Katherine L. Cook; From the Table to the Tumor: The Role of Mediterranean and Western Dietary Patterns in Shifting Microbial-Mediated Signaling to Impact Breast Cancer Risk. Nutr. 2019, 11, 2565.
- Lécuyer L, Dalle C, Lefevre-Arbogast S, Micheau P, Lyan B, Rossary A, Demidem A, Petera M, Lagree M, Centeno D, Galan P, Hercberg S, Samieri C, Assi N, Ferrari P, Viallon V, Deschasaux M, Partula V, Srour B, Latino-Martel P, Kesse-Guyot E, Druesne-Pecollo N, Vasson MP, Durand S, Pujos-Guillot E, Manach C, Touvier M. Diet-Related Metabolomic Signature of Long-Term Breast Cancer Risk Using Penalized Regression: An Exploratory Study in the SU.VI.MAX Cohort.. Cancer Epidemiol Biomarkers Prev. 2020, 29, 396–405.
- Rasoul Mirzaei; Behnaz Bouzari; Seyed Reza Hosseini-Fard; Maryam Mazaheri; Yaghoub Ahmadyousefi; Milad Abdi; Saba Jalalifar; Zahra Karimitabar; Ali Teimoori; Hossein Keyvani; et al.Farhad ZamaniRasoul YousefimashoufSajad Karampoor Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661.
- Balazs I Bodai; Therese E Nakata; Breast Cancer: Lifestyle, the Human Gut Microbiota/Microbiome, and Survivorship. Perm. J. 2020, 24, 19.129.




