
Video Upload Options
After the Fukushima nuclear accident, the development of new accident-tolerant fuel cladding materials has become a research hotspot around the world. Due to its outstanding corrosion resistance, radiation resistance, and creep properties at elevated temperatures, the oxide dispersion strengthened (ODS) FeCrAl alloy, as one of the most promising candidate materials for accident-tolerant fuel cladding, has been extensively studied during the past decade. In particular, the reasonable/optimized content of Cr is explained from the aspects of oxidation resistance, radiation resistance, and thermal stability. The essential role of the Al element in oxidation resistance, high-temperature stability, and workability was reviewed in detail. The roles of oxide-forming elements, i.e., Y (Y2O3), Ti, and Zr, and the solid solution strengthening element, i.e., W.
1. Introduction
- (1)
-
High thermal conductivity, low coefficient of thermal expansion;
- (2)
-
Small neutron absorption cross-section, low induced radioactivity, short radioactive half-life, and good radiation resistance;
- (3)
-
Good compatibility between the fuel and the coolant (strong corrosion resistance);
- (4)
-
High strength, good plasticity, and toughness at elevated temperatures.
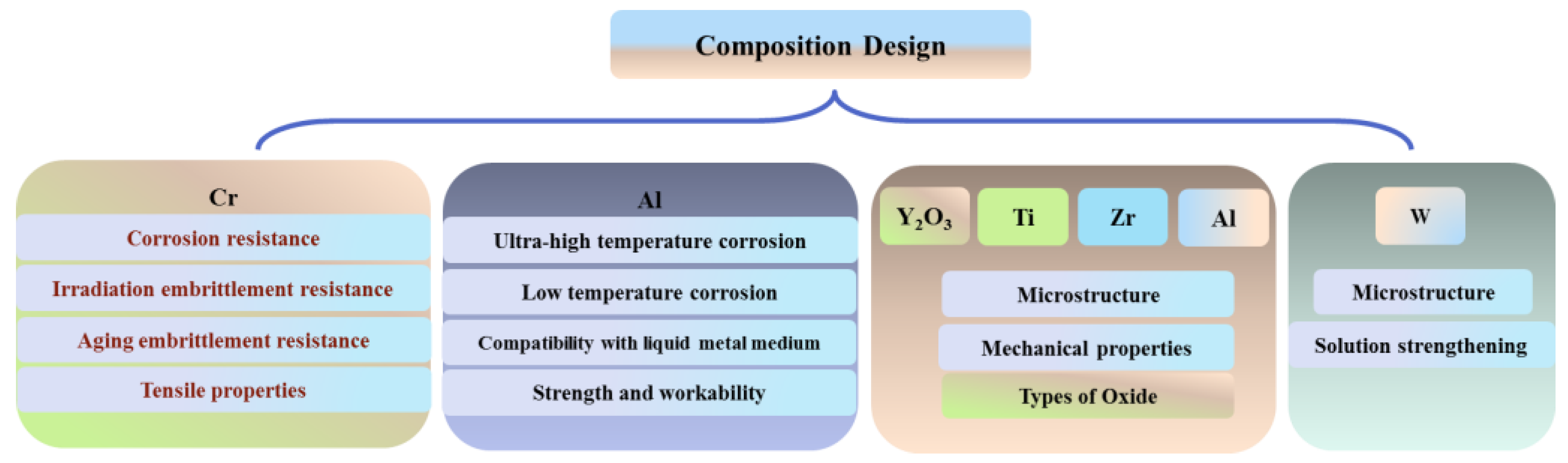
2. Effect of Chemical Elements on the Properties and Microstructure of ODS FeCrAl
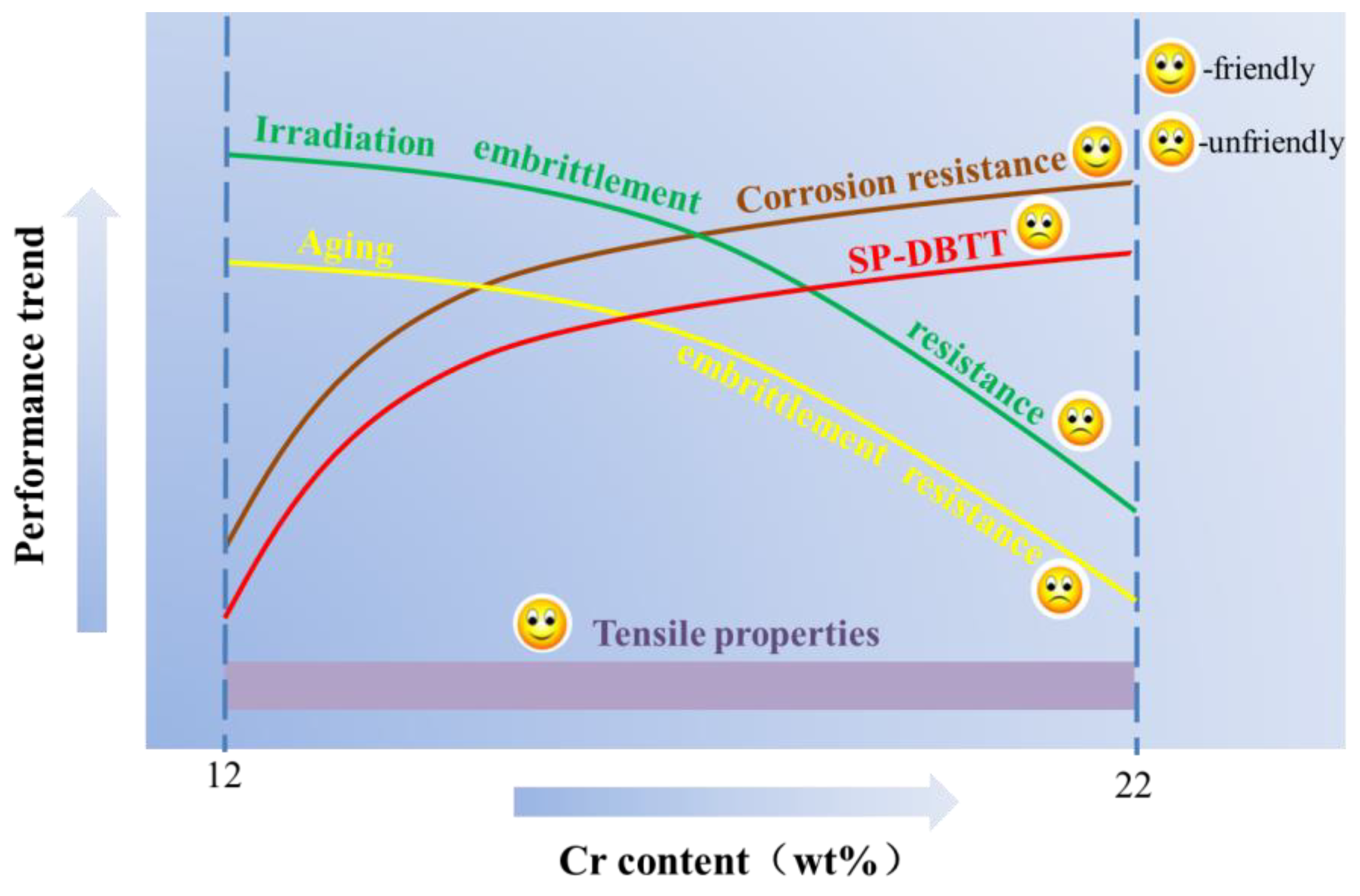
2.1. The Influence of Cr
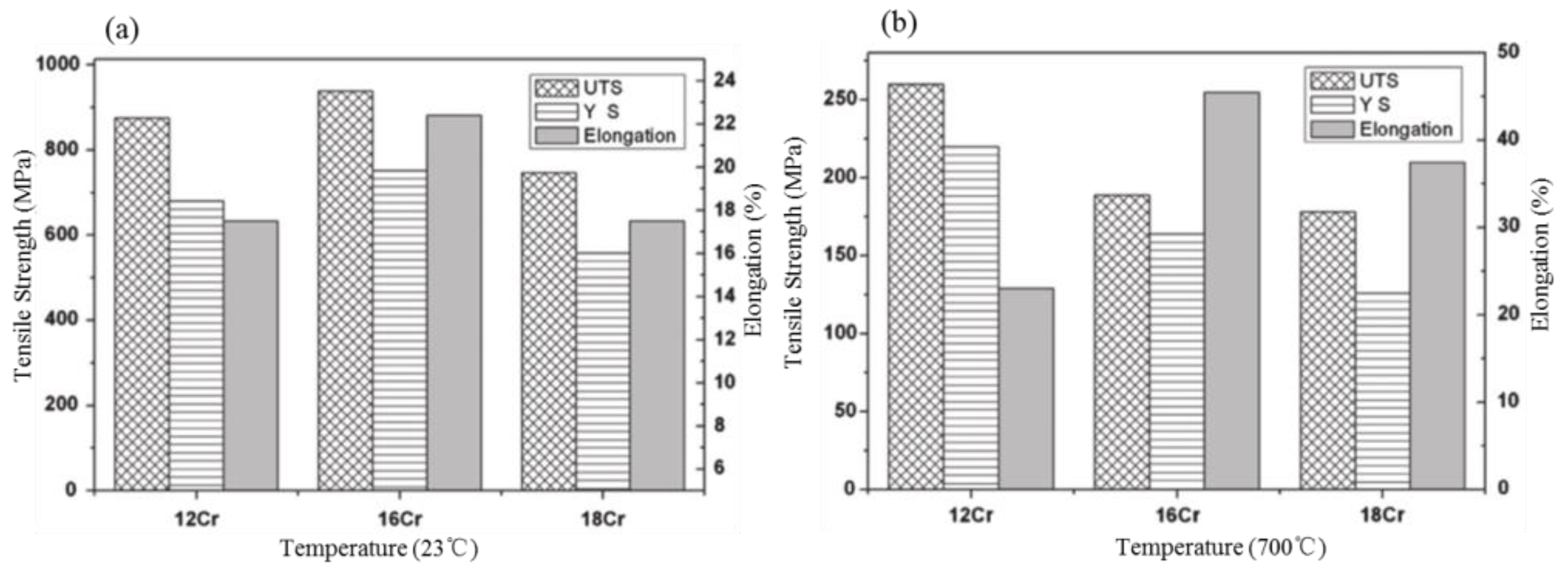
2.2. The Influence of Al
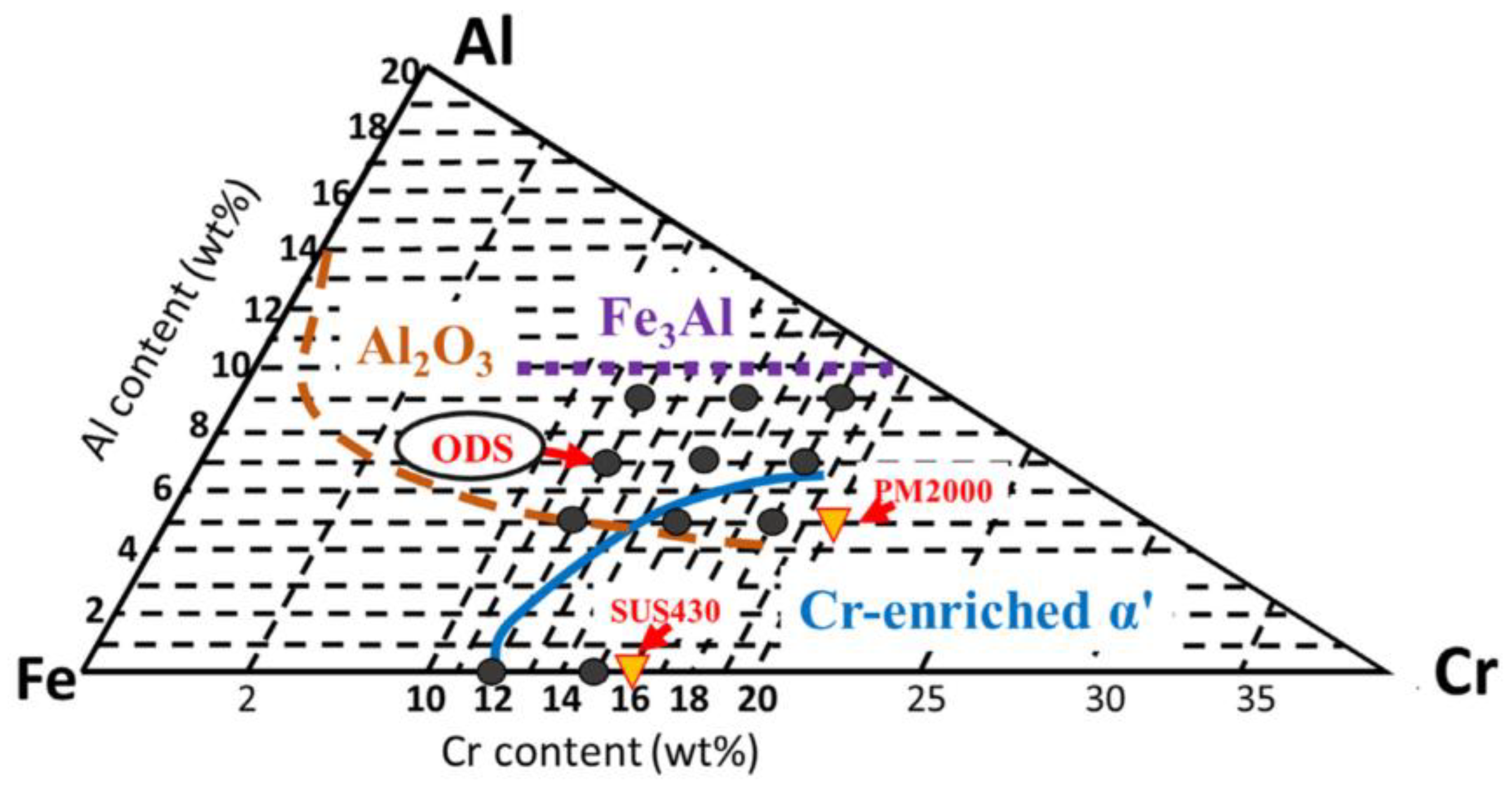
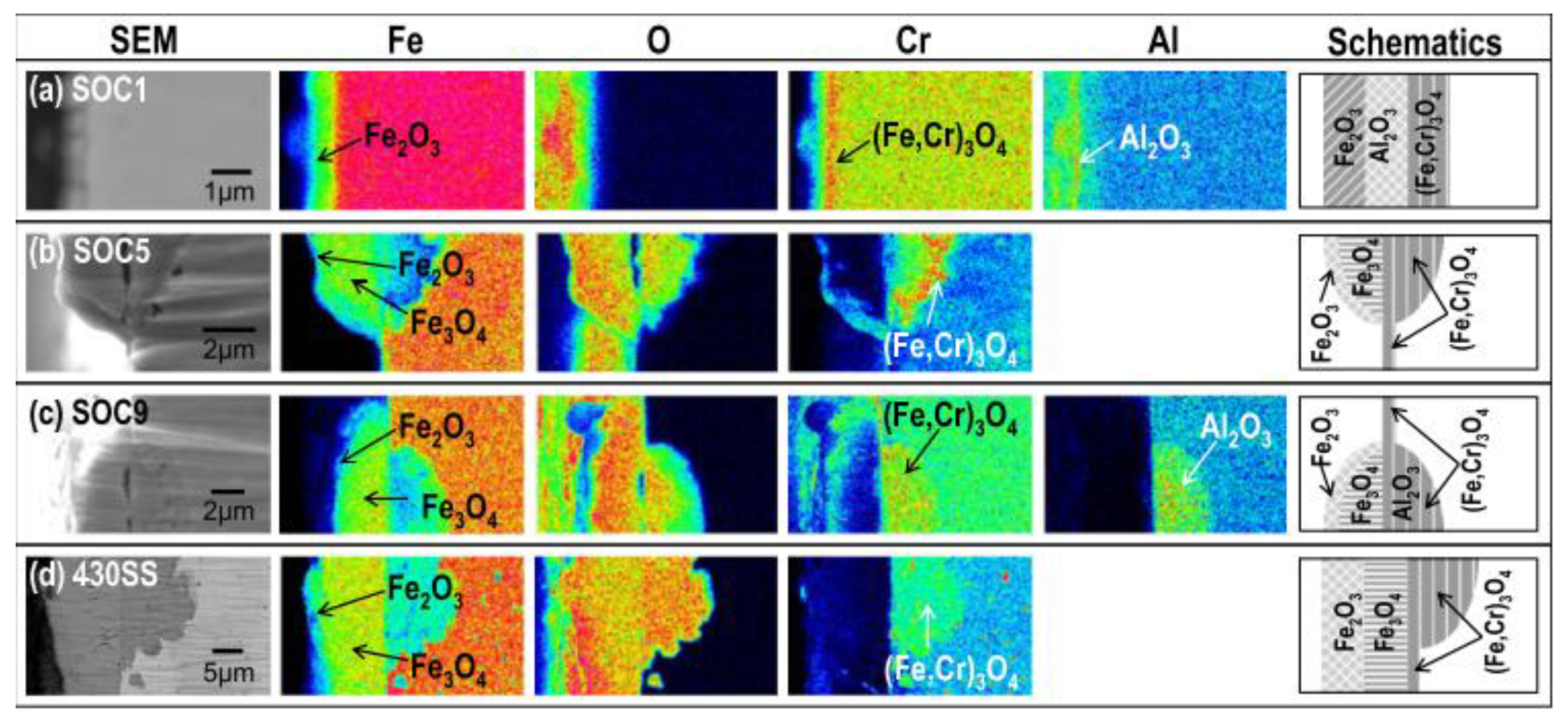
2.2.1. Ultra-High Temperature Corrosion (T > 1000 °C)
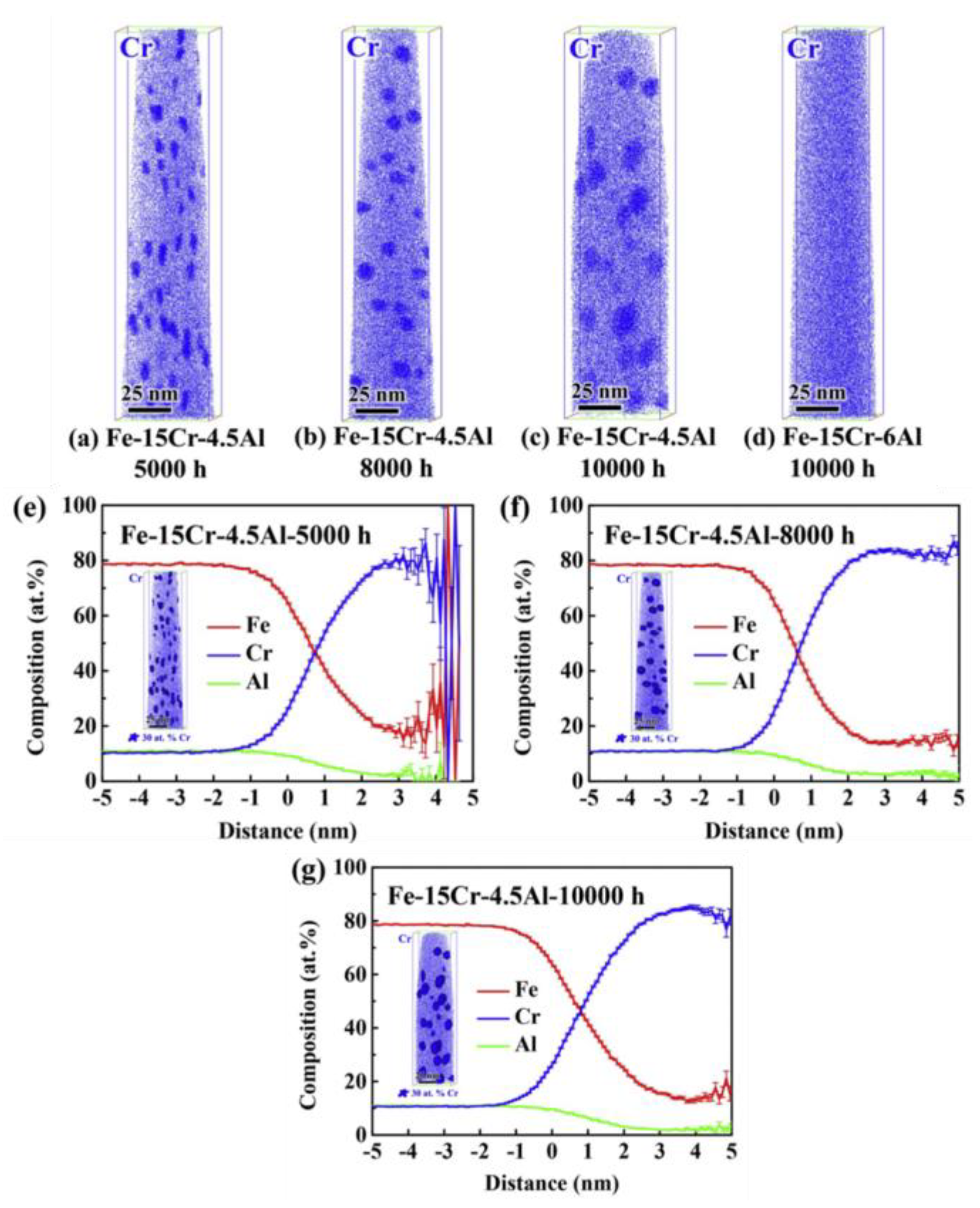
2.2.2. Low Temperature Corrosion in a Light Water Nuclear Reactor (T < 1000 °C)
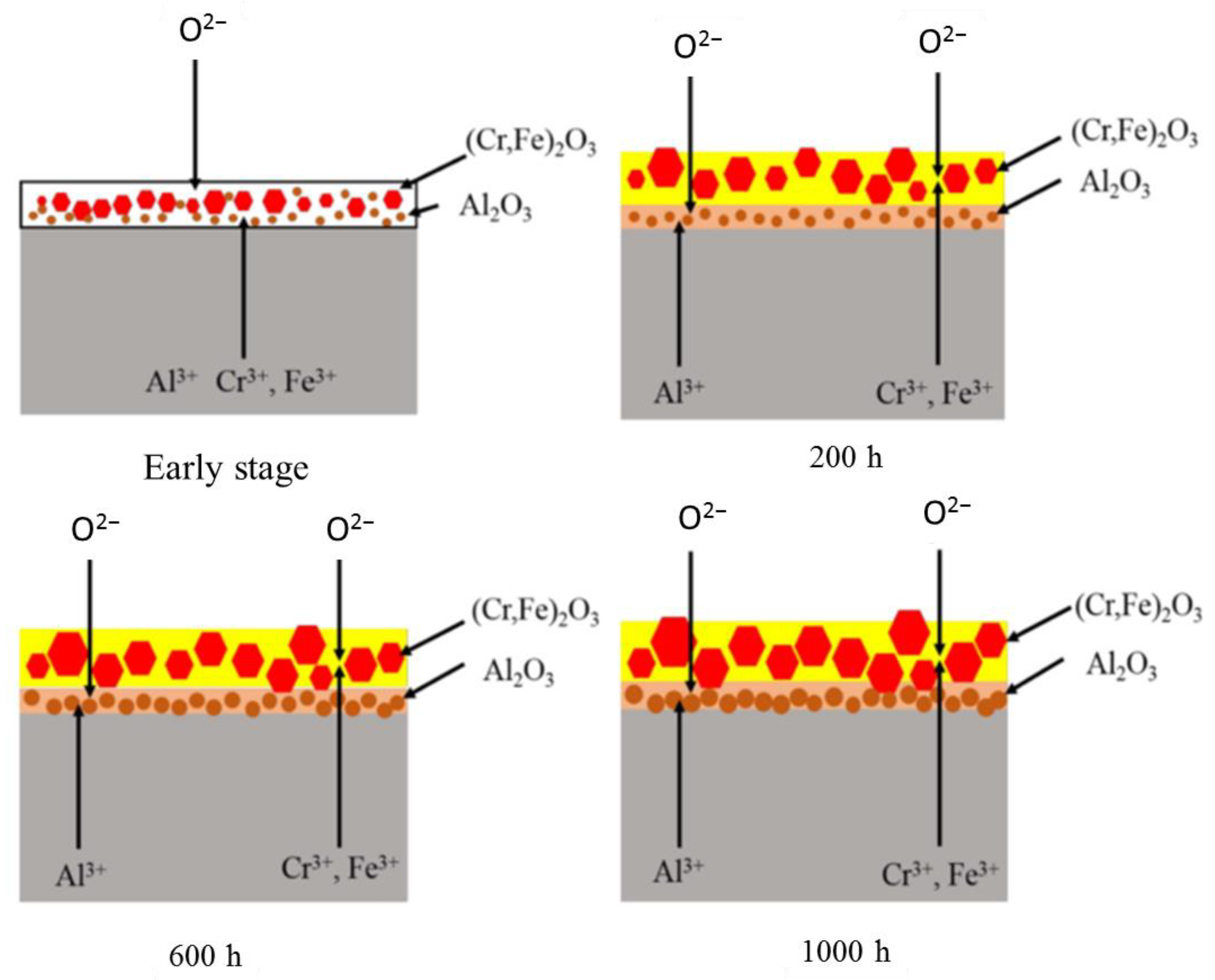
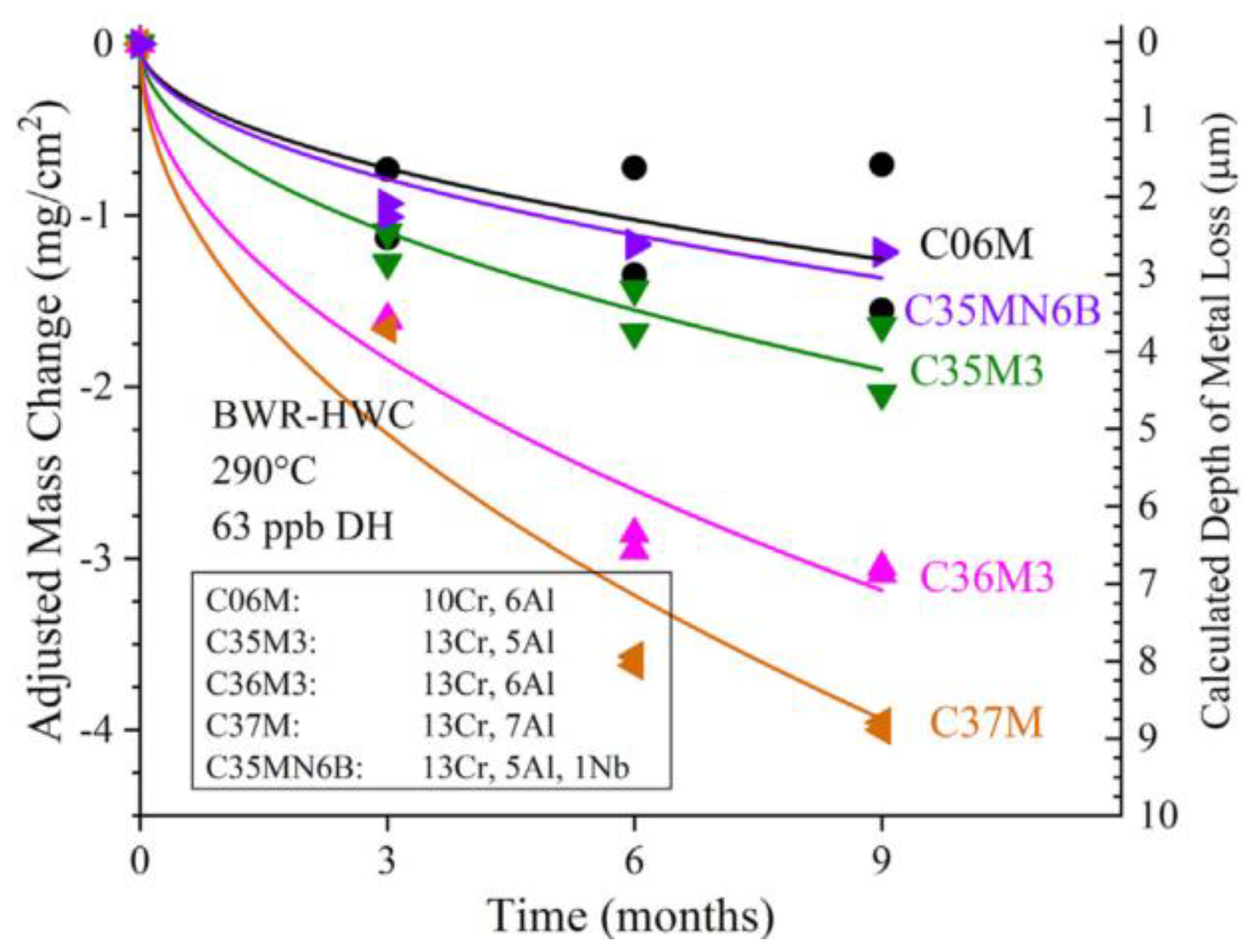
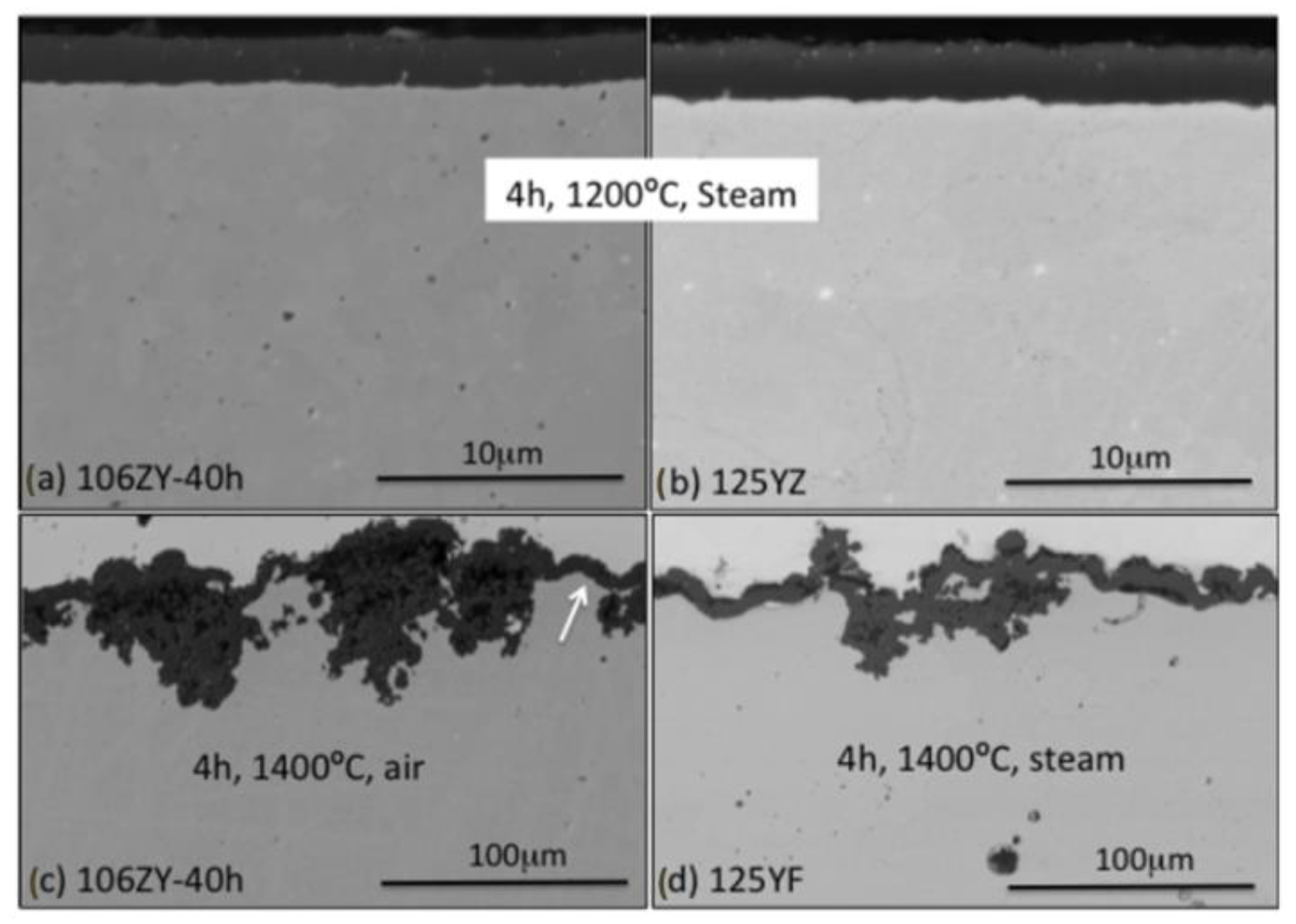
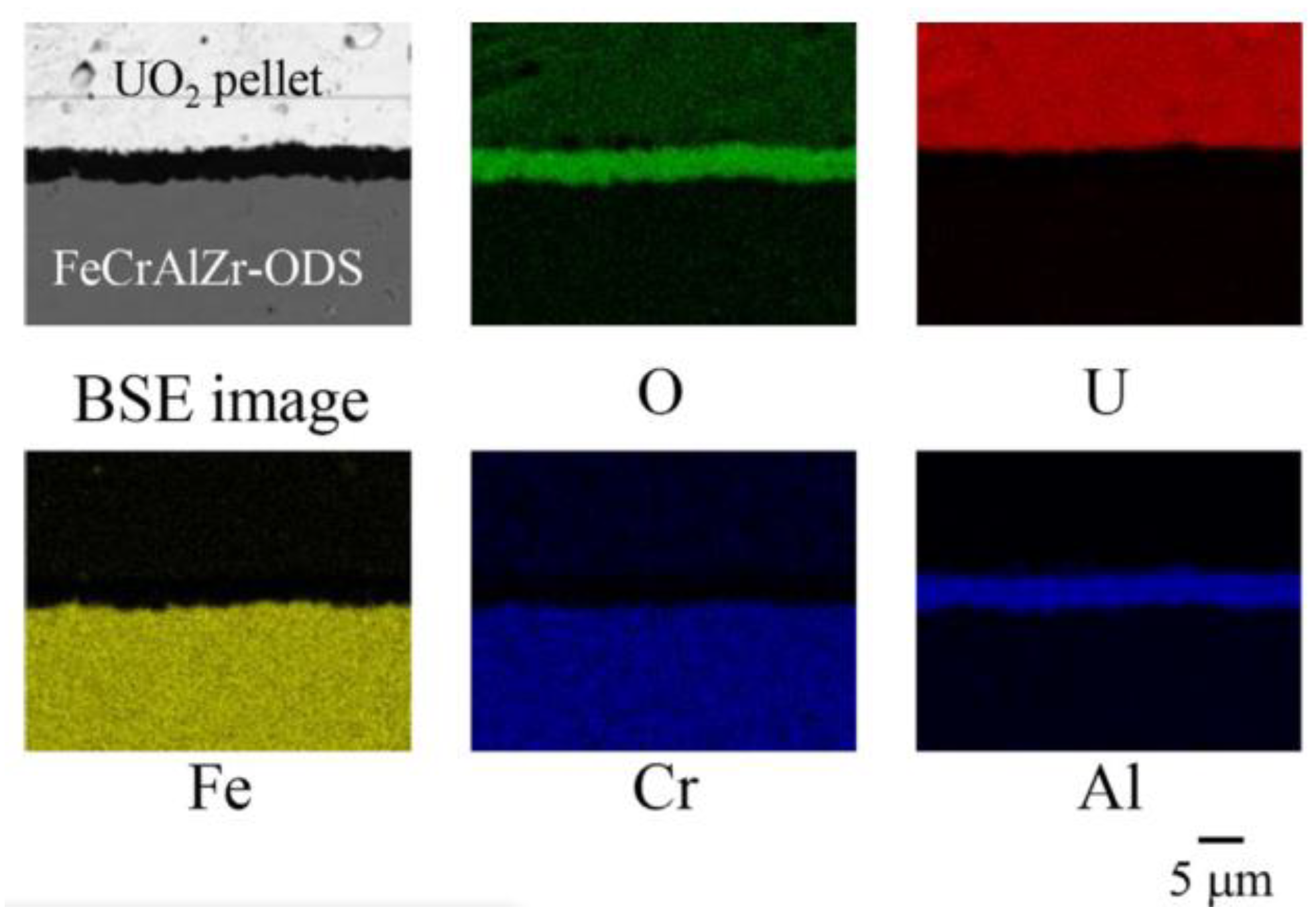
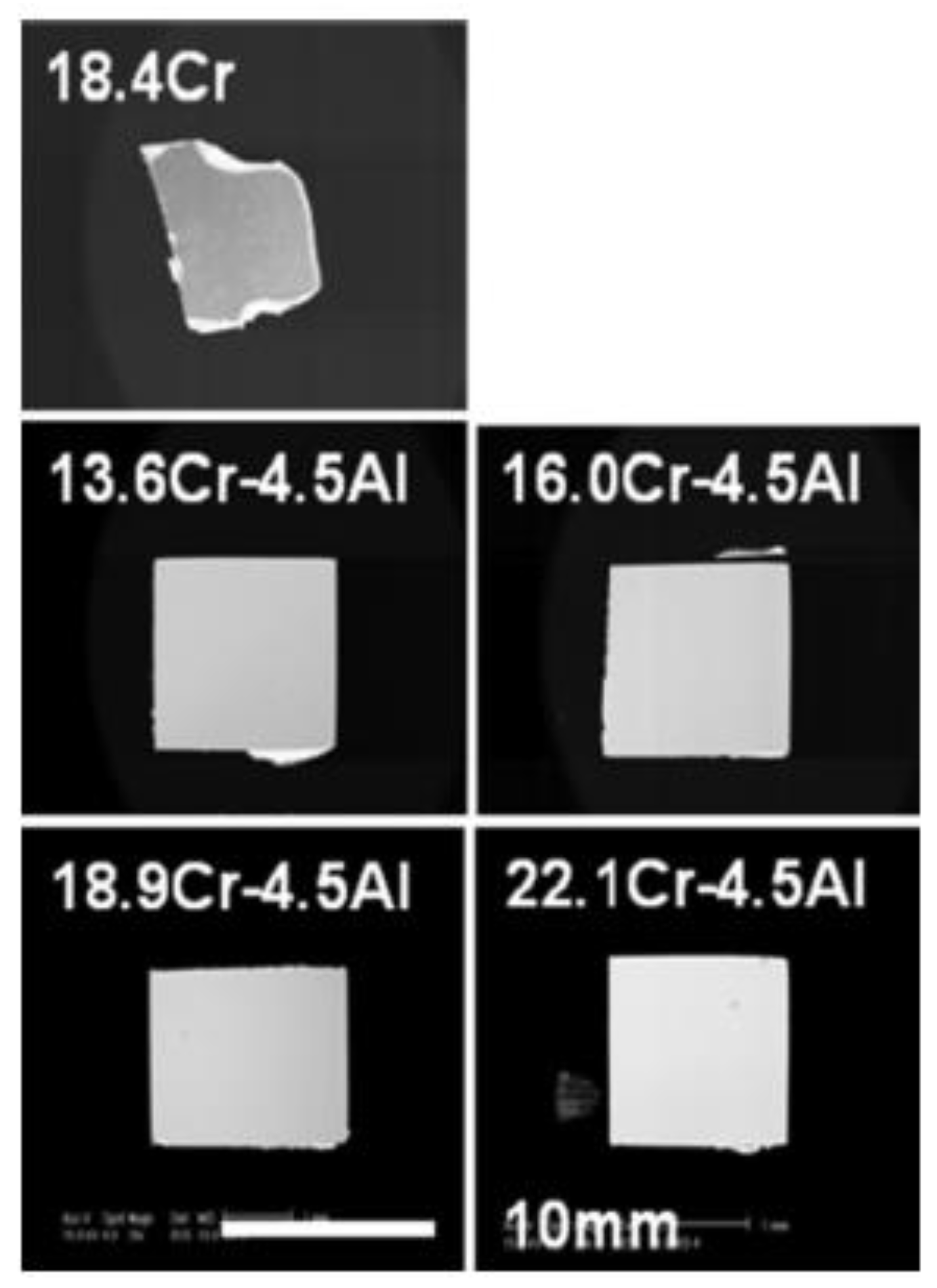
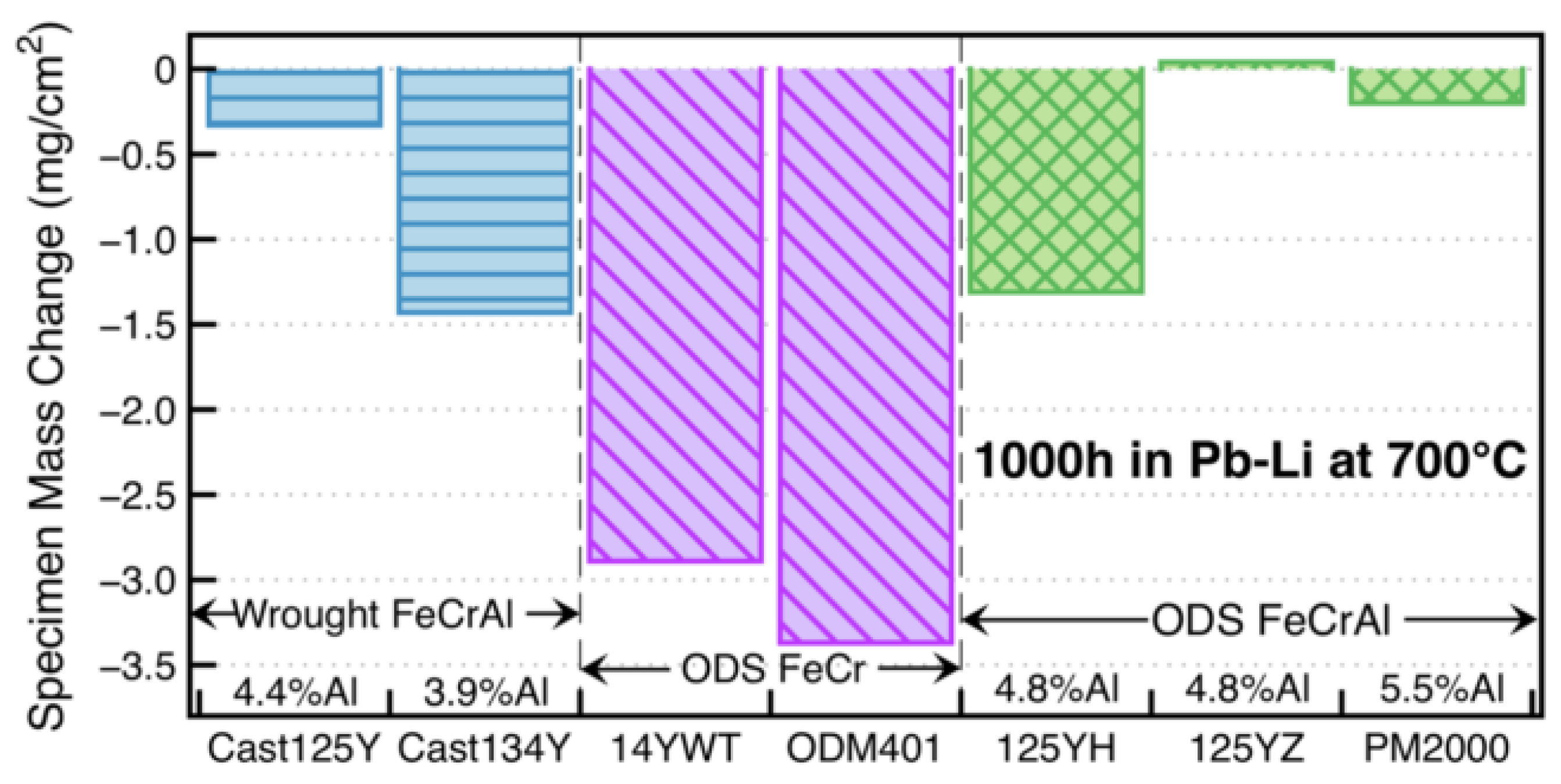
2.2.3. Compatibility with Liquid Metal Medium
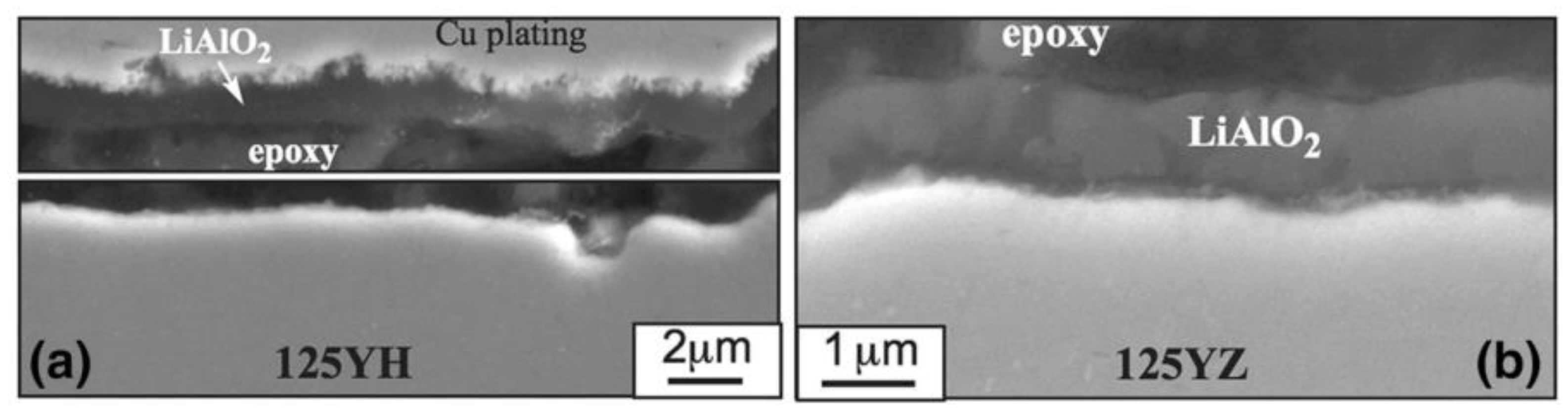
2.2.4. Effect of Al on Strength and Workability
References
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379.
- Department of Energy. Development of Light Water Reactor Fuels with Enhanced Accident Tolerance. In Report to Congress; Department of Energy: Washington, DC, USA, 2015.
- Rebak, R.B.; Andresen, P.L.; Kim, Y.J.; Dolley, E.J. Characterization of Advanced Steels as Accident Tolerant Fuel Cladding for Light Water Reactors; Technical Report IAEA-TECDOC-1797; International Atomic Energy Agency: Vienna, Austria, 2014.
- Rebak, R.B. Characterization of Advanced Steels as Accident Tolerant Cladding for Light Water Reactor Nuclear Fuel. In Proceedings of the ASME 2015 Pressure Vessels and Piping Conference, Boston, MA, USA, 19–23 July 2015.
- Rebak, R.B. Alloy selection for accident tolerant fuel cladding in commercial light water reactors. Metall. Mater. Trans. E 2015, 2, 197–207.
- Rebak, R.B.; Terrani, K.A.; Fawcett, R.M. FeCrAl Alloys for Accident Tolerant Fuel Cladding in Light Water Reactors. In Proceedings of the ASME 2016 Pressure Vessel & Piping Conference, Vancouver, BC, Canada, 17–21 July 2016.
- Rebak, R.B.; Larsen, M.; Kim, Y.J. Characterization of oxides formed on iron-chromium-aluminum alloy in simulated light water reactor environments. Corros. Rev. 2017, 35, 177–188.
- Rebak, R.B.; Terrani, K.A.; Gassmann, W.P.; Williams, J.B.; Ledford, K.L. Improving nuclear power plant safety with FeCrAl alloy fuel cladding. MRS Adv. 2017, 2, 1217–1224.
- Dolley, E.J.; Schuster, M.; Crawford, C.; Rebak, R.B. Mechanical behavior of FeCrAl and other alloys following exposure to LOCA conditions plus quenching. In Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Portland, OR, USA, 13–17 August 2017; Springer: Cham, Switzerland, 2018; pp. 185–200.
- Tortorelli, P.F.; Brady, M.P. Alloy design approaches for high-temperature oxidation resistance. JOM 2000, 52, 15.
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30.
- Opila, E.J.; Jacobson, N.S.; Myers, D.L.; Copland, E.H. Predicting oxide stability in high-temperature water vapor. JOM 2006, 58, 22–28.
- Cheng, T.; Keiser, J.R.; Brady, M.P.; Terrani, K.A.; Pint, B.A. Oxidation of fuel cladding candidate materials in steam environments at high temperature and pressure. J. Nucl. Mater. 2012, 427, 396–400.
- Miller, M.K.; Russell, K.F.; Hoelzer, D.T. Characterization of precipitates in MA/ODS ferritic alloys. J. Nucl. Mater. 2006, 351, 261–268.
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Acta Mater. 2009, 57, 392–406.
- Miller, M.K.; Reinhard, D.; Larson, D.J. Detection and quantification of solute clusters in a nanostructured ferritic alloy. J. Nucl. Mater. 2015, 462, 428–432.
- Ohtsuka, S.; Ukai, S.; Fujiwara, M. Nano-mesoscopic structural control in 9CrODS ferritic/martensitic steels. J. Nucl. Mater. 2006, 351, 241–246.
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent Developments in Irradiation-Resistant Steels. Annu. Rev. Mater. Res. 2008, 38, 471–503.
- Ukai, S.; Mizuta, S.; Fujiwara, M.; Okuda, T.; Kobayashi, T. Development of 9Cr-ODS martensitic steel claddings for fuel pins by means of Ferrite to Austenite phase transformation. J. Nucl. Sci. Technol. 2002, 39, 778–788.
- Ohtsuka, S.; Shizukawa, Y.; Tanno, T.; Imagawa, Y.; Hashidate, R.; Yano, Y.; Onizawa, T.; Kaito, T.; Ohnuma, M.; Mitsuhara, M.; et al. High-temperature creep properties of 9Cr- ODS tempered martensitic steel and quantitative correlation with its nanometer- scale structure. J. Nucl. Sci. Technol. 2023, 60, 288–298.
- Zinkle, S.J.; Boutard, J.L.; Hoelzer, D.T.; Kimura, A.; Lindau, R.; Odette, G.R.; Rieth, M.; Tan, L.; Tanigawa, H. Development of next generation tempered and ODS reduced activation ferritic/martensitic steels for fusion energy applications. Nucl. Fusion 2017, 57, 092005.
- Odette, G.R. On the status and prospects for nanostructured ferritic alloys for nuclear fission and fusion application with emphasis on the underlying science. Scr. Mater. 2018, 143, 142–148.
- Wharry, J.P.; Swenson, M.J.; Yano, K.H. A review of the irradiation evolution of dispersed oxide nanoparticles in the b. c. c. Fe-Cr system: Current understanding and future directions. J. Nucl. Mater. 2017, 486, 11–20.
- Azeem, M.M.; Li, Z.; Wang, Q.; Zubair, M. Molecular dynamics studies and irradiation effects in ODSS alloys. Int. J. Nucl. Energy Sci. Technol. 2019, 12, 381–399.
- Mustafa Azeem, M.; Wang, Q.Y.; Li, Z.Y.; Zhang, Y. Dislocation-oxide interaction in Y2O3 embedded Fe: A molecular dynamics simulation study. Nucl. Eng. Technol. 2020, 52, 337–343.
- Locatelli, G.; Mancini, M.; Todeschini, N. Generation IV nuclear reactors: Current status and future prospects. Energy Policy 2013, 61, 1503–1520.
- Abram, T.; Ion, S. Generation–IV nuclear power: A review of the state of the science. Energy Policy 2008, 36, 4323–4330.
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high—Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179.
- Pint, B.A.; Wright, I.G. Long-term high temperature oxidation behavior of ODS ferritics. J. Nucl. Mater. 2002, 307–311, 763–768.
- Pint, B.A.; Wright, I.G. Oxidation behavior of ODS Fe-Cr alloys. Oxid. Met. 2005, 63, 193–213.
- Kimura, A.; Cho, H.S.; Toda, N.; Kasada, R.; Yutani, K.; Kishimoto, H.; Iwata, N.; Ukai, S.; Fujiwara, M. High Burnup Fuel Cladding Materials R&D for Advanced Nuclear Systems: Nano-sized oxide dispersion strengthening steels. J. Nucl. Sci. Technol. 2007, 44, 323–328.
- Cho, H.S.; Kimura, A. Corrosion resistance of high-Cr oxide dispersion strengthened ferritic steels in super-critical pressurized water. J. Nucl. Mater. 2007, 367–370, 1180–1184.
- Capdevila, C.; Miller, M.K.; Chao, J. Phase separation kinetics in a Fe-Cr-Al alloy. Acta Mater. 2012, 60, 4673–4684.
- Capdevila, C.; Miller, M.K.; Toda, I.; Chao, J. Influence of the a-a’phase separation on the tensile properties of Fe-base ODS PM2000 alloy. Mater. Sci. Eng. 2010, 527, 7931–7938.
- Terada, M.; Hupalo, M.F.; Costa, I.; Padilha, A.F. Effect of alpha prime due to 475 °C aging on fracture behavior and corrosion resistance of DIN 1.4575 and MA 956 high performance ferritic stainless steels. J. Mater. Sci. 2008, 43, 425–433.
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of low-Cr ODS FeCrAl alloys for accident-tolerant fuel. J. Nucl. Mater. 2018, 501, 59–71.
- Lee, J.S.; Jang, C.H.; Kim, I.S.; Kimura, A. Embrittlement and hardening during thermal aging of high Cr oxide dispersion strengthened alloys. J. Nucl. Mater. 2007, 367–370, 229–233.
- Field, K.G.; Hu, X.; Littrell, K.C.; Yamamoto, Y.; Snead, L.L. Radiation tolerance of neutron-irradiated model Fe-Cr-Al alloys. J. Nucl. Mater. 2015, 465, 746–755.
- Briggs, S.A.; Edmondson, P.D.; Littrell, K.C.; Yamamoto, Y.; Howard, R.H.; Daily, C.R.; Terrani, K.A.; Sridharan, K.; Field, K.G. A combined APT and SANS investigation of a’ phase precipitation in neutron-irradiated model FeCrAl alloys. Acta Mater. 2017, 129, 217–228.
- Li, S.; Zhou, Z.; Jang, J.; Wang, M.; Hu, H.; Sun, H.; Zou, L.; Zhang, G.; Zhang, L. The influence of Cr content on the mechanical properties of ODS ferritic steels. J. Nucl. Mater. 2014, 455, 194–200.
- Noh, S.; Choi, J.E.; Choi, B.K.; Kang, S.H.; Kim, T.K. Effects of Cr, Mo, Al, Zr, Y2O3 on the microstructures and tensile properties of ODS ferritic/martensitic alloys. J. Met. Mater. 2014, 52, 705–712.
- Bachhav, M.; Odette, G.R.; Marquis, E.A. Microstructural Changes in a Neutron-Irradiated Fe-15 at. % Cr Alloy. J. Nucl. Mater. 2014, 454, 381–386.
- Pawel, J.E.; Rowcliffe, A.F.; Lucas, G.E.; Zinkle, S.J. Irradiation Performance of Stainless Steels for ITER Application. J. Nucl. Mater. 1996, 239, 126–131.
- Tanigawa, H.; Shiba, K.; Möslang, A.; Stoller, R.E.; Lindau, R.; Sokolov, M.A.; Odette, G.R.; Kurtz, R.J.; Jitsukawa, S. Status and key issues of reduced activation ferritic/martensitic steels as the structural material for a DEMO blanket. J. Nucl. Mater. 2011, 417, 9–15.
- Niu, Y.; Wang, S.; Gao, F.; Zhang, Z.G.; Gesmundo, F. The nature of the third- element effect in the oxidation of Fe-xCr-3at.%Al alloys in 1 atm O2 at 1000 °C. Corros. Sci. 2008, 50, 345–356.
- Stott, F.H.; Wood, G.C.; Stringer, J. The Influence of Alloying Elements on the Development and Maintenance of Protective Scales. Oxid. Met. 1995, 44, 113–145.
- Kobayashi, S.; Takasugi, T. Mapping of 475 °C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107.
- Li, W.; Lu, S.; Hu, Q.M.; Mao, H.H.; Johansson, B.; Vitos, L. The effect of Al on the 475 °C embrittlement of Fe-Cr alloys. Comp. Mater. Sci. 2013, 74, 101–106.
- Han, W.T.; Yabuuchi, K.; Kimura, A.; Ukai, S.; Oono, N.; Kaito, T.; Torimaru, T.; Hayashi, S. Effect of Cr/Al contents on the 475 °C age-hardening in oxide dispersion strengthened ferritic steels. Nucl. Mater. Energy 2016, 9, 610–615.
- Field, K.G.; Littrell, K.C.; Briggs, S.A. Precipitation of α′ in neutron irradiated commercial FeCrAl alloys. Scr. Mater. 2018, 142, 41–45.
- Yang, Z.; Wang, Z.X.; Xia, C.H.; Ouyang, M.H.; Peng, J.C.; Zhang, H.W.; Xiao, X.S. Aluminum suppression of α′ precipitate in model Fe–Cr–Al alloys during long-term aging at 475 °C. Mater. Sci. Eng. A 2020, 772, 138714.
- Maeda, T.; Ukai, S.; Hayashi, S.; Oono, N.; Shizukawa, Y.; Sakamoto, K. Effects of zirconium and oxygen on the oxidation of FeCrAl-ODS alloys under air and steam conditions up to 1500 °C. J. Nucl. Mater. 2019, 516, 317–326.
- Sakamoto, K.; Miura, Y.; Ukai, S.; Oono, N.H.; Kimura, A.; Yamaji, A.; Kusagaya, K.; Takano, S.; Kondo, T.; Ikegawa, T.; et al. Development of accident tolerant FeCrAl-ODS fuel cladding for BWRs in Japan. J. Nucl. Mater. 2021, 557, 153276.
- Liu, T.; Wang, C.X.; Shen, H.L.; Chou, W.S.; Iwata, N.Y.; Kimura, A. The effects of Cr and Al concentrations on the oxidation behavior of oxide dispersion strengthened ferritic alloys. Corros. Sci. 2013, 76, 310–316.
- Liu, T.; Wang, L.B.; Wang, C.X.; Shen, H.L. Effect of Al content on the oxidation behavior of Y2Ti2O7-dispersed Fe-14Cr ferritic alloys. Corros Sci. 2016, 104, 17–25.
- Weisenburger, A.; Jianu, A.; Doyle, S.; Bruns, M.; Fetzer, R.; Heinzel, A.; Del Giacco, M.; An, W.; Muller, G. Oxide scales formed on Fe—Cr—Al—Based model alloys exposed to oxygen containing molten lead. J. Nucl. Mater. 2013, 437, 282–292.
- Tomaszewicz, P.; Wallwork, G.R. The oxidation of high-purity iron-chromium-aluminum alloys at 800 °C. Oxid. Met. 1983, 20, 75–109.
- Pint, B.A.; Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T. Development of ODS FeCrAl for compatibility in fusion and fission energy applications. JOM 2014, 66, 2458–2466.
- Unocic, K.A.; Hoelzer, D.T.; Pint, B.A. Microstructure and environmental resistance of low Cr ODS FeCrAl. Mater. High Temp. 2015, 32, 123–132.
- Dryepondt, S.; Massey, C.; Edmonson, P.D. 2nd Generation ODS FeCrAl Alloy Development for Accident-Tolerant Fuel Cladding; ORNL/TM—2016/456; National Technical Information Service: Springfield, VA, USA, 2016.
- Qiao, Y.J.; Wang, P.; Qi, W.; Du, S.Y.; Liu, Z.; Meng, F.P.; Zhang, X.H.; Wang, K.; Li, Q.W.; Yao, Z.D.; et al. Mechanism of Al on FeCrAl steam oxidation behavior and molecular dynamics simulations. J. Alloys Compd. 2020, 828, 154310.
- Hofmann, P. Current knowledge on core degradation phenomena, a review. J. Nucl. Mater. 1999, 270, 194–211.
- Isselin, J.; Kasada, R.; Kimura, A. Corrosion behaviour of 16% Cr—4% Al and 16% Cr ODS ferritic steels under different metallurgical conditions in a supercritical water environment. Corros. Sci. 2010, 52, 3266–3270.
- Lee, J.H.; Kasada, R.; Kimura, A.; Okudab, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abef, F. Influence of alloy composition and temperature on corrosion behavior of ODS ferritic steels. J. Nucl. Mater. 2011, 417, 1225–1228.
- Ren, J.; Yu, L.M.; Liu, Y.C.; Ma, Z.Q.; Liu, C.X.; Li, H.J.; Wu, J.F. Corrosion behavior of an Al added high-Cr ODS steel in supercritical water at 600 °C. Appl. Surf. Sci. 2019, 480, 969–978.
- Huttunen-Saarivirta, E.; Kuokkala, V.T.; Pohjanne, P. Thermally grown oxide films and corrosion performance of ferritic stainless steels under simulated exhaust gas condensate conditions. Corros. Sci. 2014, 87, 344–365.
- Liu, Y.C.; Chen, S.M.; Ouyang, F.Y.; Kai, J.J. Corrosion behavior of pre-oxidized HR—224 superalloy in supercritical water environment at 700 °C. J. Nucl. Mater. 2018, 505, 7–14.
- Terrani, K.A.; Pint, B.A.; Kim, Y.J.; Unocic, K.A.; Yang, Y.; Silva, C.M.; Meyer, H.M.; Rebak, R.B. Uniform corrosion of FeCrAl alloys in LWR coolant environments. J. Nucl. Mater. 2016, 479, 36–47.
- Raiman, S.S.; Field, K.G.; Rebak, R.B.; Yamamoto, Y.; Terrani, K.A. Hydrothermal corrosion of 2nd generation FeCrAl alloys for accident tolerant fuel cladding. J. Nucl. Mater. 2020, 536, 152221.
- Hosemann, P.; Thau, H.T.; Johnson, A.L.; Maloy, S.A.; Li, N. Corrosion of ODS steels in lead—Bismuth eutectic. J. Nucl. Mater. 2008, 373, 246–253.
- Takaya, S.; Furukawa, T.; Aoto, K.; Müller, G.; Weisenburger, A.; Heinzel, A.; Inoue, M.; Okudac, T.; Abed, F.; Ohnuki, S.; et al. Corrosion behavior of Al—Alloying high Cr—ODS steels in lead-bismuth eutectic. J. Nucl. Mater. 2009, 386–388, 507–510.
- Unocic, K.A.; Pint, B.A. Alloying and coating strategies for improved Pb—Li compatibility in DEMO—Type fusion reactors. J. Nucl. Mater. 2014, 455, 330.
- Unocic, K.A.; Hoelzer, D.T. Evaluation of Pb—17Li compatibility of ODS Fe-12Cr-5Al alloys. J. Nucl. Mater. 2016, 479, 357–364.
- Klueh, R.L.; Shingledecker, J.P.; Swindeman, R.W.; Hoelzer, D.T. Oxide dispersion-strengthened steels: A comparison of some commercial and experimental alloys. J. Nucl. Mater. 2005, 341, 103–114.
- Kasada, R.; Toda, N.; Yutani, K.; Cho, H.S.; Kishimoto, H.; Kimura, A. Pre- and post-deformation microstructures of oxide dispersion strengthened ferritic steels. J. Nucl. Mater. 2007, 367–370, 222–228.
- Gong, M.; Zhou, Z.; Hu, H.; Zhang, G.; Li, S.; Wang, M. Effects of Aluminum on Microstructure and Mechanical Behavior of 14Cr—ODS Steels. J. Nucl. Mater. 2015, 462, 502–507.
- Lee, J.H. Development of oxide dispersion strengthened ferritic steels with and without aluminum. Front. Energy 2012, 6, 29–34.
- Zhang, G.; Zhou, Z.; Mo, K.; Miao, Y.; Li, S.; Liu, X.; Wang, M.; Park, J.S.; Almer, J.; Stubbins, J.F. The comparison of microstructures and mechanical properties between 14Cr—Al and 14Cr—Ti ferritic ODS alloys. Mater. Des. 2016, 98, 61–67.
- Gussev, M.N.; Field, K.G.; Yamamoto, Y. Design, properties and weldability of advanced oxidation-resistant FeCrAl alloys. Mater. Des. 2017, 129, 227–238.
- Capdevila, C.; Miller, M.K.; Russell, K.F. Aluminum partitioning during phase separation in Fe—20%Cr-6%Al ODS alloy. J. Mater. Sci. 2008, 43, 3889–3893.
- Vicente, A.D.A.; Moreno, J.R.S.; Espinosa, D.C.R.; Santos, T.F.D.A.; Tenorio, J.A.S. Study of the high temperature oxidation and Kirkendall porosity in dissimilar welding joints between Fe—Cr—Al alloy and stainless steel AISI 310 after isothermal heat treatment at 1150 °C in air. J. Mater. Res. Technol. 2019, 8, 1636–1644.
- Wukusick, C.S.; Collis, J.F. An Iron—Chromium—Aluminum Alloy Containing Yttrium. Mater. Res. Stand. 1964, 12, 637–646.
- Xu, S.; Zhou, Z.J.; Long, F.; Jia, H.D.; Guo, N.; Yao, Z.W.; Daymond, M.R. Combination of back stress strengthening and Orowan strengthening in bimodal structured Fe—9Cr—Al ODS steel with high Al addition. Mater. Sci. Eng. A 2019, 739, 45–52.
- Maji, B.C.; Ukai, S.; Oono, N. Microstructural stability and intermetallic embrittlement in high Al containing FeCrAl—ODS alloys. Mater. Sci. Eng. A 2021, 807, 140858.
- Maréchal, L.; Lesage, B.; Huntz, A.M.; Molins, R. Oxidation Behavior of ODS Fe—Cr—Al Alloys: Aluminum Depletion and Lifetime. Oxid. Met. 2003, 60, 1–28.




