Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, X.; Wang, S.; Liu, N.; Wei, B.; An, T. Progress of Dispersants for Coal Water Slurry. Encyclopedia. Available online: https://encyclopedia.pub/entry/52239 (accessed on 07 February 2026).
Liu X, Wang S, Liu N, Wei B, An T. Progress of Dispersants for Coal Water Slurry. Encyclopedia. Available at: https://encyclopedia.pub/entry/52239. Accessed February 07, 2026.
Liu, Xiaotian, Shan Wang, Ning Liu, Bo Wei, Tian An. "Progress of Dispersants for Coal Water Slurry" Encyclopedia, https://encyclopedia.pub/entry/52239 (accessed February 07, 2026).
Liu, X., Wang, S., Liu, N., Wei, B., & An, T. (2023, November 30). Progress of Dispersants for Coal Water Slurry. In Encyclopedia. https://encyclopedia.pub/entry/52239
Liu, Xiaotian, et al. "Progress of Dispersants for Coal Water Slurry." Encyclopedia. Web. 30 November, 2023.
Copy Citation
This article provides a comprehensive review of existing coal water slurry dispersants, and points out the existing problems and possible future development directions.
coal water slurry dispersant
dispersant type
three-dimensional structure dispersant
adsorption performance
1. Classification and Characteristics of CWS Dispersants
Dispersants denote the surfactants added to CWS that enable stable dispersion of coal particles in water, preventing stratification and precipitation over extended periods [1]. As significant additives in CWS preparation, dispersants can adhere to the coal surface, altering its properties and thereby enhancing CWS performance [2].
Dispersants possess unique structural characteristics. One-dimensional dispersants encompass a linear hydrophobic end, while two-dimensional comb-like dispersants incorporate numerous hydrophilic and hydrophobic groups. Both types adhere to the coal surface via their hydrophobic ends. To enhance the adsorption capacity, a third type of dispersant, known as three-dimensional structure dispersants, has been developed. These typically comprise linear and comb structures to cater to the polar groups of coal [3][4]. The hydrophobic end and the hydrophilic end of the one-dimensional linear structure dispersant are in a straight line, such as naphthalene sulfonate formaldehyde condensate (NSF) [5][6], sodium dodecyl benzene sulfonate, and sodium dodecyl sulfate (SDS) [7], while the two-dimensional structure dispersant contains a large number of hydrophobic and polar groups, such as comb polymer sodium polystyrene sulfonate (PSS) [8]. The latter is formed by copolymerization of polymer monomer, polyethylene glycol acrylate monoester, sodium p-Phenylethane sulfonate, and acrylamide [9][10]. Preparation of these comb-like polymers involves initiation and copolymerization between diverse unsaturated monomers, which can facilitate a high coal content and low apparent viscosity via the use of super-performance one-dimensional or two-dimensional dispersants [11][12]. However, apart from the coal surface’s abundance of hydrophobic groups, it also hosts polar groups, including substances containing O, S, and N [13][14]. Therefore, the three-dimensional structure dispersant developed for this situation has more polar groups. Zhang [15] utilized renewable resources, such as tannic acid and acrylic acid, to formulate an eco-friendly polymer dispersant that possesses a jellyfish-like three-dimensional structure. Tannic acid, which comprises both hydrophobic aromatic rings and polar hydroxyl groups, closely resembles the surface properties of coal. Hence, long side chains were grafted onto the tannic acid’s plane structure, and under electrostatic repulsion and steric hindrance, aligned in parallel. This led to the formation of a hydration film on the surface of the coal particles, substantially improving the stability and reducing the viscosity of CWS. Consequently, these studies significantly reduced the production cost of dispersants, and achieved environmental sustainability during the production and use of dispersants.
Dispersants can be obtained by modifying or synthesizing natural products. Natural products such as lignin, starch, and cellulose are widely available and are indeed very low-cost and environmentally friendly. Synthetic dispersants such as naphthalene sulfonate dispersants have good performance but relatively higher costs [16]. Starch, owing to its abundance and eco-friendliness, is a frequently employed natural raw material for dispersants. However, due to its high molecular weight, starch is not chemically stable and requires degradation. This necessitates the introduction of certain groups to elevate starch’s efficacy as a dispersant, fulfilling the pulping requirements of CWS [17]. Generally, the dispersants obtained by modification are starch sulfonate, starch xanthin compound, and starch phosphate [18]. For instance, a comb-like CWS dispersant can be synthesized by polymerizing starch, acrylic acid, and styrene (SAS), which boasts numerous hydroxyl groups contributing to the hydrophilic segment [19]. These hydroxyl groups interact with water via hydrogen bonding. The hydrophobic component, the phenyl from the grafted polystyrene chains, influences the dispersant’s adsorption on coal. The molecular structure of the SAS dispersant facilitates connections between the hydrophobic groups on the coal surface and the hydrophilic groups in the water, enabling the even dispersion of coal particles in water. Natural products like starch and cellulose, which are sourced widely, are advantageous due to their low cost, renewability, eco-friendly nature, natural biodegradability, chemical stability, and biocompatibility [20][21]. Dispersants produced from these raw materials are cost-effective, cause minimal environmental pollution, and demonstrate exceptional performance. This expands the selection of dispersants, making them a prime candidate for extensive industrial application in future research [22].
2. Anionic Dispersants
Anionic dispersants are the most researched and applied CWS dispersants at present owing to their outstanding dispersing ability, wide sources, and low price. Common anionic dispersants include lignin dispersants, humic acid-based dispersants, naphthalene-based dispersants, and polycarboxylic acid-based dispersants.
2.1. Lignin Dispersants
Lignin dispersants are mainly obtained by modifying alkali lignin or lignosulfonate, a by-product of the paper industry. In addition to having a wide range of sources and economy, lignin dispersants can be employed to prepare coal water slurry with relatively superior stability than naphthalene-based dispersants [23]. However, the CWS viscosity prepared by such dispersants is relatively high, and the performance of lignin dispersants can be improved by modification methods such as sulfonation, polycondensation modification, and graft copolymerization modification [24][25][26].
Sulfonation modification is used to improve the hydrophilic properties of dispersants and the stability of CWS by replacing the benzene ring of lignin sulfonate molecules or the hydrogen, hydroxyl, and methoxy groups on the side chain of a benzene ring with the sulfonic group [27]. The sulfonation reaction can endow alkali lignin with good water solubility, surface activity, and reactivity. Sulfated lignin can be modified by oxidation and sulfomethylation to prepare sulfomethylated lignin [28]. Oxidation and sulfomethylation can raise the carboxyl and sulfonate contents of lignin, leading to an increase in the anion charge density. Sulfomethylated lignin can be adsorbed on coal particles and improve the fluidity of slurry more effectively.
Polycondensation modification is aimed at the phenol hydroxyl, alcohol hydroxyl, and aldehyde groups, and other unit structures of lignin sulfonate molecules that are prone to polycondensation reactions of aldehydes, phenols, lipids, and other molecules. The dispersion and adsorption of lignin dispersants can be strengthened through polycondensation modification [29]. The polycondensation reaction can endow the resulting products with certain relative molecular weights and adsorption and dispersion properties [30]. Chen et al. modified horsetail pine alkali lignin into an efficient coal water slurry dispersant, ALB, by sulfomethylation polycondensation reactions using sulfomethylated alkali lignin and sulfonated acetone formaldehyde as raw materials [24]. The experiment revealed that ALB dispersants displayed a comparatively stable viscosity reduction effect, surpassing the performance of conventional lignin dispersants. Furthermore, the molecular weight and sulfonic acid group content were identified as key factors influencing the dispersion and viscosity reduction capability of CWS. Graft copolymerization leverages the properties of lignin and its derivatives. Under the initiation process, lignin molecules can react with acrylic acid, acrylamide, styrene, hydroxyethyl methacrylate, vinyl acetate, and other functional branches, thereby enhancing their dispersion and stability. Maryam et al. [31] extracted kraft lignin and sulfonated lignin from papermaking wastewater as the source of lignin and chemical structure for acrylamide graft radical copolymerization modification initiated by thermal or redox initiators. Aliphatic hydroxyl groups were identified as the active sites of graft copolymerization, and the number of these functional groups in the lignin chain caused an important influence on the progress of graft copolymerization.
Lu et al. [32] copolymerized β-cyclodextrin (β-CD) and chlorotrione epoxide into alkali lignin to synthesize a modified alkali lignin dispersant (β-CD-AL), which dramatically increased the stability of the lignin dispersant pulping and reduced the viscosity of the slurry in their study. The synthesis scheme of the dispersant is described in Figure 1. The effects of the β-CD content on the dispersibility, zeta potential, and adsorption properties of β-CD-AL were also investigated. It was found that the stability was gradually enhanced with the increase in the copolymerized β-CD dosage under the synergistic effect of electrostatic repulsion and spatial site resistance. In addition, the amount of copolymerized β-CD reached a peak value for the optimal viscosity reduction effect.
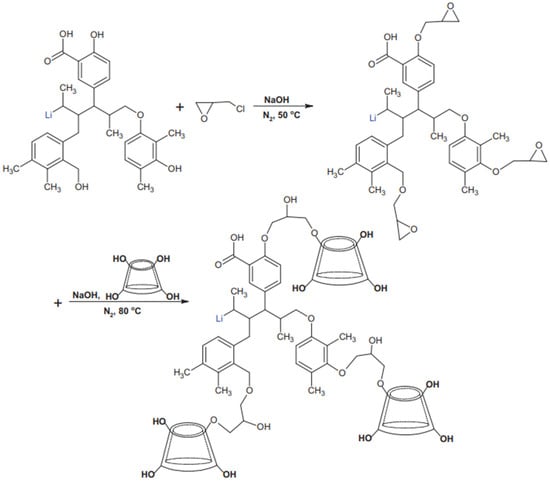
Figure 1. Synthetic schematic diagram of β-CD-AL.
2.2. Humic Acid-Based Dispersants
Low-rank coal, such as lignite, contains a large amount of humic acid. Humic acid dispersants, extracted from low-rank coal such as lignite, present good dispersion performance and can be used alone [33]. It has been confirmed that the lower the maturity of raw coal, the better the viscosity reduction effect of the prepared dispersants for CWS. However, the disadvantages are that these dispersants are sensitive to metal ions, propensity form precipitates, lead to prepared slurry with poor stability, and propose correspondingly higher requirements for pulping water quality [34][35].
Humic acid is rich in condensed aromatic units, which are similar to the structure of coal, and thus can be tightly adsorbed on the coal surface [36][37]. In addition, humic acid encompasses active groups, such as hydroxyl and carboxyl groups, providing the possibility for chemical modification of humic acid [38]. Currently, studies on the modification of humic acid as a dispersant focus on the sulfonation, nitration, sulfomethylation, and graft copolymerization of humic acid molecules. The purpose is to introduce functional groups with strong hydrophilicity into humic acid molecules [39][40]. Zhang et al. [35] fabricated a novel humic acid-based dispersant, humic acid-grafted sodium polystyrene sulfonate (HA-g-pssNa). This dispersant possesses a hydrophobic humic acid core and a hydrophilic sodium polystyrene sulfonate side chain, synthesized through a surface acylation reaction and atom transfer radical polymerization of humic acid. Various properties of HA-g-pssNa, pssNa, and naphthalene sulfonate formaldehyde condensate (NSF) as dispersants for the preparation of CWS were compared. The results revealed that the pulping properties of HA-g-pssNa were reinforced with the increase in pssNa side chain length. In the case of the appropriate chain length of HA-g-pssNa, the CWS prepared with this dispersant achieved good apparent viscosity and static stability, with superior performance than pssNa and NSF.
Kang et al. [34] modified sodium humate solution by sulfomethylation to prepare sulfomethylated humic acid dispersant (LSHA dispersant) and determined the optimal process conditions for modification through orthogonal experiments. Compared with the slurry-forming performance of commercial sodium naphthalenesulfonate dispersants, both of them can meet the requirements of CWS gasification, but the LSHA dispersant is better in terms of stability.
A humic acid-based polycarboxylate dispersant for CWS can be synthesized by copolymerizing humic acid, acrylic acid, and maleic acid, and its dispersion performance is much better than that of humic acid before copolymerization modification. The synthesis scheme of the dispersant is described in Figure 2 [41]. When the dosage of the dispersant reached 0.5 wt%, the apparent viscosity of the CWS was 505 mPa·s, while the permeability reached 85.45% after 96 h. The stability of the CWS was 12.87% higher than that of CWS prepared directly with humic acid as a dispersant. And the maximum concentration of the coal water slurry could reach up to 70 wt%. Overall, the aforementioned studies have greatly broadened the application scope of humic acid dispersants.

Figure 2. Humic acid-based dispersant polymerization reaction scheme.
2.3. Naphthalene-Based Dispersants
Naphthalene-based dispersants, primarily comprised of naphthalene sulfonic acid polymers, are the most prevalent dispersants on the market. The structure of typical naphthalene dispersants is depicted in Figure 3. These dispersants offer superior dispersion performance, viscosity reduction, and slurry fluidity when compared with lignin-based dispersants. However, they also have notable disadvantages including high cost, suboptimal slurry stability, and a propensity for precipitation [23][42].
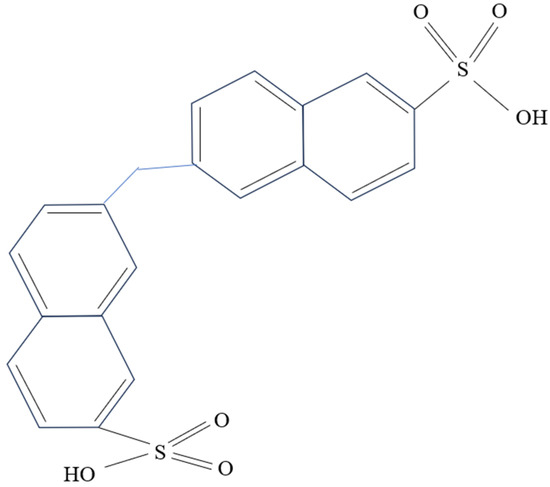
Figure 3. Structural formula of sodium methylene naphthalene sulfonate.
The dispersion performance of naphthalene-based dispersants can primarily be adjusted by varying the degree of condensation and sulfonation [43]. An increased degree of condensation correlates positively with the binding strength and slurry effect of coal. However, for coal molecules with medium and low degrees of metamorphism, there exists a significant steric hindrance effect. This results in a weakened bond between the dispersant and the coal, despite an increase in the molecular chain length of the dispersants concurrent with the condensation degree. This suggests an optimal value for the coagulability of coal water slurries derived from medium and low-grade coals exists [44][45]. Another strategy for improving the performance of naphthalene dispersants is graft modification. Modifying the branch chain length through graft copolymerization can produce modified naphthalene dispersants with varying chain lengths. This can be achieved by adjusting the ratio of ethylene oxide to aromatic monomer.
Currently, naphthalene sulfonate formaldehyde condensate (NSFC) has emerged as a widely used dispersant in CWS applications due to its effective viscosity reduction properties. At present, scholars mainly study the effects of the reaction conditions, sulfonation quality, and degree of polymerization on its dispersion performance [16]. Upon the addition of NSFCs to CWS, the coal surface’s hydrophobicity decreases while the conversion of weakly bound water to free water is facilitated, thereby enhancing water fluidity. Consequently, the hydrophilicity of the coal surface is increased and the viscosity is significantly reduced [46]. Due to its cost-effectiveness and superior viscosity control characteristics, NSFC holds potential for broader industrial applications compared to other dispersants [47].
2.4. Polycarboxylic Acid Dispersants
The molecular structure of polycarboxylate-based additives consists of comb-shaped surfactants with graft copolymers. The main chain is polymerized by active monomers comprising functional groups, and the side chain is grafted onto active monomers containing functional groups and the main chain [9][48]. The structure is easy to design and can be modified according to different needs. Therefore, the viscosity reduction effect of polycarboxylate-based dispersants is stronger than that of traditional lignin dispersants and naphthalene dispersants. Combined with the advantage of low pollution, such dispersants enjoy a wider range of applications [49][50]. The structure of common polycarboxylate dispersant is shown in Figure 4 [51].
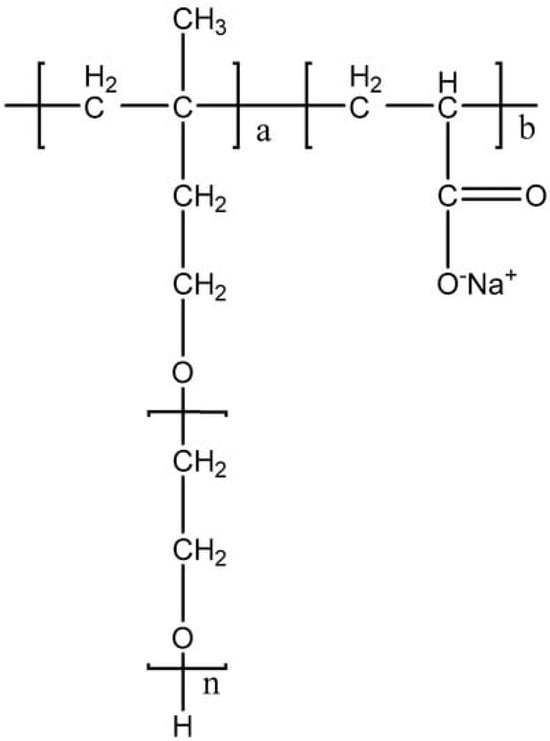
Figure 4. Structure of polycarboxylate dispersant.
Polycarboxylate dispersants are easy to prepare because they can regulate the adsorption capacity on coal surfaces and improve the chemical properties of coal surfaces by introducing polycarboxylate. Zhu [49] and others adopted ammonium persulfate sodium bisulfite as a redox catalyst to synthesize a new amphoteric polycarboxylate dispersant for CWS by using sodium p-phenylethylsulfonate, polyethylene glycol acrylate monoester, and ethyl trimethyl ammonium methacrylate. When the dosage of the dispersant was 0.3 wt%, the maximum concentration of CWS could reach 65.0 wt%. Polycarboxylate dispersants containing anionic and cationic groups lead to a better anchoring effect on coal through ion adsorption.
In recent years, it is a research hotspot to adjust the synthesis scheme of polycarboxylate dispersants to obtain better-performing dispersants, such as controlling the ratio of acrylic acid to sodium phenylene sulfonate, initiator composition, and temperature. The most suitable PC dispersants for pulping were selected according to the viscosity of each type of CWS at 100 s−1, shear thinning behavior, static stability (>14), and maximum solid loads (>55 wt%). Polycarboxylate dispersants are effectively adsorbed on the surface of coal particles through horizontal multipoint adsorption, mainly through the interaction between the hydrophobic groups of dispersant molecules and the hydrophobic regions of coal particles, finally intensifying the hydrophilicity of coal particles and the stability of the hydration membrane [52].
3. Cationic Dispersants
The molecular structure of cationic dispersants typically comprises two components: positively charged non-polar hydrophilic groups and lipophilic hydrocarbon chains. Unlike anionic types, cationic dispersants facilitate the dispersion of particles in water into a colloidal solution via interaction between the positively charged molecular groups and the negatively charged particle surface. Currently, quaternary ammonium salts, heterocycles, and octadecenylamine acetate represent some of the widely used cationic dispersants on the market [53]. Figure 5 presents a typical molecular structure diagram of a quaternary ammonium salt dispersant.
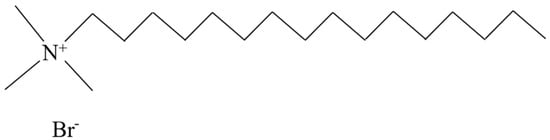
Figure 5. Structure diagram of cetyltrimethylammonium bromide.
4. Non-Ionic Dispersants
The primary types of non-ionic CWS dispersants are polyoxyethylene ether and polyoxyethylene block polyether surfactants, with the former attracting more attention. Characterized by their non-ionization in water, non-ionic dispersants can control both hydrophilic and hydrophobic groups, making them less susceptible to the influence of water quality and the substances in coal on the dispersion effect compared to other dispersants. At the same time, there is no need for a stabilizer when using non-ionic dispersants. They are also the most expensive dispersants for CWS [54]. A CWS dispersant prepared by non-ionic surfactants extracted from natural plants is a relatively low-cost, environment-friendly, and strong non-ionic dispersant. Figure 6 illustrates the structure of a typical non-ionic dispersant.
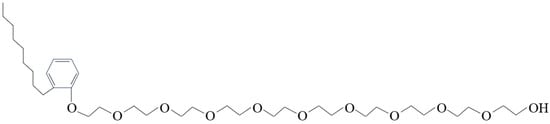
Figure 6. Molecular structure of alkylphenol polyoxyethylene ether.
The minimum apparent viscosity that CWS can achieve with polyoxyethylene ether as a dispersant is closely related to the polyoxyethylene adduct number [55]. Alkyl polyoxyethylene ethers with more alkyl carbon atoms have an optimal addition number. Li [56] utilized two non-ionic dispersants, polyoxyethylene dodecyl phenol ether (PDPE) and polyoxyethylene lauryl ether (PLE), as CWS dispersants and compared their slurry forming performance. Both dispersants contained 30% ethylene oxide. At a CWS viscosity of 1000 mPa·s, the maximum concentrations of PDPE and PLE were 67.60% and 62.95%, respectively. PDPE displayed easier adsorption onto coal than PLE and formed more stable bonds due to the P-P stacking effect, leading to more uniform coal dispersion in the solution.
References
- LiZhuang, Z.O.U.; Shuquan, Z.H.U.; Xiaoling, W.; Xiangkun, G.U.O.; Guangwen, C.U.I. Interaction between different CWS dispersants and coal—X Adsorptive characteristics of dispersant on coal surface. J. Fuel Chem. Technol. 2006, 34, 10–14.
- Wang, S.; Liu, J.; Wu, H.; Wang, Y.; Li, N. Research status of compound dispersant of coal water slurry. Appl. Chem. Ind. 2017, 46, 1616.
- Li, L.; Zhao, L.Y.; Wang, Y.X.; Wu, J.N.; Meng, G.H.; Liu, Z.Y.; Zhang, J.S.; Hu, B.X.; He, Q.H.; Guo, X.H. Novel Dispersant with a Three-Dimensional Reticulated Structure for a Coal-Water Slurry. Energy Fuels 2018, 32, 8310–8317.
- Zhu, J.F.; Li, J.L.; Liu, R.Q.; Wang, J.Q.; Tang, Y.W.; Zhang, W.B.; Zhang, G.H. Synthesis and evaluation of a multi-block polycarboxylic acid for improving low-rank coal to make the slurry. Colloids Surf. A—Physicochem. Eng. Asp. 2022, 646, 128966.
- Chen, Y.; Song, Z.; Sun, Q.; Liu, K.; Hu, S.; Li, J. Insights into the Dispersion Mechanism of Microfine Coal Particles Modified with Naphthalene Sulfonate Formaldehyde Based on EDLVO Theory. Energy Fuels 2023, 37, 7777–7787.
- Xu, R.; He, Q.; Cai, J.; Pan, Y.; Shen, J.; Hu, B. Effects of chemicals and blending petroleum coke on the properties of low-rank Indonesian coal water mixtures. Fuel Process. Technol. 2008, 89, 249–253.
- Zhang, M.; Hu, T.; Ren, G.; Zhu, Z.; Yang, Y. Research on the Effect of Surfactants on the Biodesulfurization of Coal. Energy Fuels 2017, 31, 8116–8119.
- Yi, F.; Gopan, A.; Axelbaum, R.L. Characterization of coal water slurry prepared for PRB coal. J. Fuel Chem. Technol. 2014, 42, 1167–1171.
- Zhou, M.; Huang, K.; Yang, D.; Qiu, X. Development and evaluation of polycarboxylic acid hyper-dispersant used to prepare high-concentrated coal–water slurry. Powder Technol. 2012, 229, 185–190.
- Zhu, J.; Zhang, G.; Liu, G.; Qu, Q.; Li, Y. Investigation on the rheological and stability characteristics of coal–water slurry with long side-chain polycarboxylate dispersant. Fuel Process. Technol. 2014, 118, 187–191.
- Yu, Y.J.; Liu, J.Z.; Hu, Y.X.; Gao, F.Y.; Zhou, J.H.; Cen, K.F. The properties of Chinese typical brown coal water slurries. Energy Sources Part A—Recovery Util. Environ. Eff. 2016, 38, 1176–1182.
- Li, J.; Zhang, G.; Shang, T.; Zhu, J. Synthesis, characterization and application of a dispersant based on rosin for coal-water slurry. Int. J. Min. Sci. Technol. 2014, 24, 695–699.
- Silva, L.F.O.; Oliveira, M.L.S.; Sampaio, C.H.; de Brum, I.A.S.; Hower, J.C. Vanadium and Nickel Speciation in Pulverized Coal and Petroleum Coke Co-combustion. Energy Fuels 2013, 27, 1194–1203.
- Cutruneo, C.M.N.L.; Oliveira, M.L.S.; Ward, C.R.; Hower, J.C.; de Brum, I.A.S.; Sampaio, C.H.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F.O. A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. Int. J. Coal Geol. 2014, 130, 33–52.
- Zhang, K.; Zhang, X.; Jin, L.E.; Cao, Q.; Li, P. Synthesis and evaluation of a novel dispersant with jellyfish-like 3D structure for preparing coal–water slury. Fuel 2017, 200, 458–466.
- Hu, S.; Li, J.; Liu, K.; Chen, Y. Comparative study on distribution characteristics of anionic dispersants in coal water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129176.
- Zhu, N.; Zhang, G. Synthesis of Benzylated Hydrophobic Modified Starch and Dispersion Property of Coal Water Slurry. Coal Convers. 2020, 43, 81–88.
- Zhu, J.; Wang, P.; Li, Y.; Li, J.; Zhang, G. Dispersion performance and mechanism of polycarboxylates bearing side chains of moderate length in coal-water slurries. Fuel 2017, 190, 221–228.
- Zhu, J.; Zhang, G.; Li, J.; Zhao, F. Synthesis, adsorption and dispersion of a dispersant based on starch for coal–water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 165–171.
- Shen, J.; Fatehi, P.; Ni, Y.H. Biopolymers for surface engineering of paper-based products. Cellulose 2014, 21, 3145–3160.
- Kontturi, E.; Vuorinen, T. Indirect evidence of supramolecular changes within cellulose microfibrils of chemical pulp fibers upon drying. Cellulose 2009, 16, 65–74.
- Ding, C.; Zhu, X.; Ma, X.; Yang, H. Synthesis and Performance of a Novel Cotton Linter Based Cellulose Derivatives Dispersant for Coal-Water Slurries. Polymers 2022, 14, 1103.
- Li, P.-W.; Yang, D.-J.; Lou, H.-M.; Qiu, X.-Q. Study on the stability of coal water slurry using dispersion-stability analyzer. J. Fuel Chem. Technol. 2008, 36, 524–529.
- Chen, X.; Liu, M.; Liu, Y.; Chen, Z. Study on Performance of Masson Pine Alkali Lignin Prepared as a Dispersant of Coal Water Slurry. J. Fujian Norm. Univ. Nat. Sci. Ed. 2013, 29, 63–67.
- Yang, D.; Guo, W.; Li, X.; Wang, Y.; Qiu, X. Effects of molecular weight of grafted sulfonated lignin on its dispersion and adsorption properties as a dispersant for coal water slurries. J. Fuel Chem. Technol. 2013, 41, 20–25.
- Yang, D.; Li, X.; Li, H.; Jiang, H.; Li, Q. Application of High-Efficiency Bamboo Lignin-Based Dispersant to Coal Phenol-Water Slurry. J. South China Univ. Technology. Nat. Sci. Ed. 2014, 42, 1–7.
- Li, P.; Yang, D.; Qiu, X.; Feng, W. Study on Enhancing the Slurry Performance of Coal–Water Slurry Prepared with Low-Rank Coal. J. Dispers. Sci. Technol. 2014, 36, 1247–1256.
- He, W.; Fatehi, P. Preparation of sulfomethylated softwood kraft lignin as a dispersant for cement admixture. RSC Adv. 2015, 5, 47031–47039.
- Wenxin, J.I.; Liqiong, W. Preparation and Study of High Concentration Coal-water-slurry of Ningdong Coal Suited to Texco Gasification Process. J. Henan Norm. Univ. Nat. Sci. 2011, 39, 92–94.
- Gong, Z.; Shuai, L. Lignin condensation, an unsolved mystery. Trends Chem. 2023, 5, 163–166.
- Pourmahdi, M.; Abdollahi, M.; Nasiri, A. Effect of lignin source and initiation conditions on graft copolymerization of lignin with acrylamide and performance of graft copolymer as additive in water- based drilling fluid. J. Pet. Sci. Eng. 2023, 220, 111253.
- Lu, H.Y.; Li, X.F.; Zhang, C.Q.; Li, W.H.; Xu, D.P. beta-Cyclodextrin grafted on alkali lignin as a dispersant for coal water slurry. Energy Sources Part A–Recovery Util. Environ. Eff. 2019, 41, 1716–1724.
- Zhang, G.; Liu, L.; Li, J.; Shang, T.; Xu, H.; Qiang, Y. Synthesis And properties research of sulfonated humic acid grafted copolymer. Coal Convers. 2013, 36, 92–96.
- Zhang, K.; Tian, Y.; Zhang, R.; Shi, G.; Zhou, B.; Zhang, G.; Niu, Y.; Tian, Z.; Gong, J. Interactions of amphiphilic humic acid-based polymers with coal and effect on preparation of coal-water slurry. Powder Technol. 2020, 376, 652–660.
- Zhang, W.B.; Luo, J.; Huang, Y.; Zhang, C.; Du, L.; Guo, J.; Wu, J.; Zhang, X.; Zhu, J.F.; Zhang, G.H. Synthesis of a novel dispersant with topological structure by using humic acid as raw material and its application in coal water slurry preparation. Fuel 2020, 262, 116576.
- Doskočil, L.; Grasset, L.; Válková, D.; Pekař, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413.
- Wang, C.-F.; Fan, X.; Zhang, F.; Wang, S.-Z.; Zhao, Y.-P.; Zhao, X.-Y.; Zhao, W.; Zhu, T.-G.; Lu, J.-L.; Wei, X.-Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677–20684.
- Ge, L.; Li, J. Study on the Influence of Pulping Process on Rheological Properties of Coal Water Slurry. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012107.
- Zhang, G.; Wei, Y.; Li, J.; Niu, Y.; Qu, Q. Synthesis and properties of sulfomethlated humic acid dispersant for coal-water-slurry. Mod. Chem. Ind. 2014, 34, 72.
- Wu, J.; Zhang, G.; Zhang, W.; Du, L.; Li, J.; Duan, Q.; Liu, J. New humic acid dispersants and their dispersion to coal water slurries. Appl. Chem. Ind. 2018, 47, 2638–2642.
- Zhang, K.; Deng, S.; Li, P.; Jin, L.E.; Cao, Q. Synthesis of a novel humic acid-based polycarboxylic dispersant for coal water slurry. Int. J. Green Energy 2016, 14, 205–211.
- Ra, H.W.; Yoon, S.M.; Mun, T.Y.; Seo, M.W.; Moon, J.H.; Yoon, S.J.; Kim, J.H.; Lee, J.G.; Jung, M.Z.; Lee, J.D. Influence of surfactants and experimental variables on the viscosity characteristics of coal water mixtures. Korean J. Chem. Eng. 2018, 35, 1219–1224.
- Xiong, W.; Zhang, G.; Zhu, J. Performance of sulfonated naphthol formaldehyde/naphthalene composites dispersant for coal water slurry. Coal Convers. 2013, 36, 40–43.
- Shang, Y.; Wang, X.G.; Lei, X.; Shang, L. Performance of Sodium Ligninsulfonate/naphthalene Composites Dispersant. Coal Convers. 2018, 41, 36.
- Qiu, X.Q.; Zhou, M.S.; Yang, D.J.; Lou, H.M.; Ouyang, X.P.; Pang, Y.X. Evaluation of sulphonated acetone-formaldehyde (SAF) used in coal water slurries prepared from different coals. Fuel 2007, 86, 1439–1445.
- Lu, H.-Y.; Li, X.-F.; Zhang, C.-Q.; Chen, J.-Y.; Ma, L.-G.; Li, W.-H.; Xu, D.-P. Experiments and molecular dynamics simulations on the adsorption of naphthalenesulfonic formaldehyde condensates at the coal-water interface. Fuel 2020, 264, 116838.
- Hu, S.; Jiang, F.; Li, J.; Wu, C.; Liu, K.; Chen, Y. Understanding the adsorption behaviors of naphthalene sulfonate formaldehyde in coal water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127245.
- Wang, C.; Hou, Y.; Cao, H.; Wang, T.; Zhou, L.; Zhang, K. Preparation and properties of amphoteric polycarboxylate coal tar pitch water slurry dispersant. Appl. Chem. Ind. 2022, 51, 2215.
- Zhu, J.F.; Zhang, G.H.; Miao, Z.; Shang, T. Synthesis and performance of a comblike amphoteric polycarboxylate dispersant for coal-water slurry. Colloids Surf. A-Physicochem. Eng. Asp. 2012, 412, 101–107.
- Hong, N.L.; Zhang, S.W.; Yi, C.H.; Qiu, X.Q. Effect of Polycarboxylic Acid Used as High-Performance Dispersant on Low-Rank Coal-Water Slurry. J. Dispers. Sci. Technol. 2016, 37, 415–422.
- Xun, W.; Wu, C.; Li, J.; Yang, C.; Leng, X.; Xin, D.; Li, Y. Effect of Functional Polycarboxylic Acid Superplasticizers on Mechanical and Rheological Properties of Cement Paste and Mortar. Appl. Sci. 2020, 10, 5418.
- Wu, H.; Lv, D.; Nie, Y.; Jiang, T.; Li, J.; Gong, S.; Yan, H. Experimental and computational studies of polycarboxylate dispersant effect on the properties of low-rank coal water slurries. Asia-Pac. J. Chem. Eng. 2022, 17, e2837.
- Ionkin, A.S.; Fish, B.M.; Li, Z.R.; Liang, L.; Lewittes, M.E.; Cheng, L.K.; Westphal, C.; Pepin, J.G.; Gao, F. Quaternary phosphonium salts as cationic selective dispersants in silver conductive pastes for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2014, 124, 39–47.
- Slaczka, A.; Piszczynski, Z. Effect of selected additives on the stability and rheology of Coal-Water Slurry Fuels (CWSF). Gospod. Surowcami Miner. -Miner. Resour. Manag. 2008, 24, 363–373.
- Yi, S.U.; Shuquan, Z.H.U. Slurryability of single chain alkylphenol polyoxyethylene as coal water slurry dispersant. J. China Coal Soc. 2011, 36, 1396–1400.
- Li, L.; Ma, C.; Hu, S.; He, M.; Yu, H.; Wang, Q.; Cao, X.; You, X. Effect of the benzene ring of the dispersant on the rheological characteristics of coal-water slurry: Experiments and theoretical calculations. Int. J. Min. Sci. Technol. 2021, 31, 515–521.
More
Information
Subjects:
Chemistry, Applied; Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
664
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




