1. Introduction
Progress in food processing and the continuing high demand for convenient and functional foods have resulted in increased use of allowed food additives in food processing. The use of these substances in accordance with current health and legal recommendations enables food producers to achieve the intended technological, quality, economic, and health effects. Due to their useful and functional values, food additives are essential components of many food products. Although they constantly raise health concerns among some consumers due to the increase in public awareness of the role and the legally regulated conditions of use of food additives, they are becoming more and more widely accepted
[1].
Allowed additives are substances not consumed separately as food and are not typical food ingredients; allowed additives are intentionally used in the process of production, processing, preparation, packaging, transport, and storage to provide the intended or expected results in a food or in semi-finished products that are components of the food. Allowed additives may be used only when their use is compliant with current legal regulations and technologically justified and does not pose a threat to the health or life of the consumer. The basic legal acts in the European area regulating the use of food additives are Regulation (EC) No. 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives
[2] and Commission Regulation (EU) No. 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives
[3].
2. Modified Starches as Food Additives—Production and Characteristics
2.1. Single Modified Starches
2.1.1. Oxidized Starch (E 1404)
Oxidized starch is formed in the reaction of native starch with a suitable oxidizing agent. During starch oxidation, two types of reaction occur. The first reaction is conversion of hydroxyl groups of the starch monomers, primarily at C-2, C-3, and C-6 positions, into carbonyl groups followed by carboxyl groups
[4], and the second reaction is starch depolymerization. Therefore, the carboxyl and carbonyl contents and the degree of depolymerization in oxidized starch are indicators of the degree of oxidation
[5]. The oxidation process mainly occurs in amorphous regions of starch granules, especially on their peripheries
[4]. The high content of amylose hinders oxidation of amylopectin chains in the crystalline areas since the co-crystallization of amylose with amylopectin affects the packing of double helices, which consequently limits the access of oxidizing agents to hydroxyl groups
[6][7]. Moderate alkalinity of the oxidation reaction environment favours the formation of carboxyl groups. The share of carbonyl and/or carboxyl groups in the oxidized starches as well as the degree of depolymerization affects the physicochemical properties of the starch, such as swelling capacity and solubility, gelatinization time and temperature, rheological characteristics of pastes and gels, and susceptibility to retrogradation
[8][9][10][11]. Oxidation causes weakening of the molecular structure of starch granules, including, destruction of amylopectin double helices, which in turn manifests in reduced temperature and reduced gelatinization enthalpy as well as in a lower swelling power of the oxidized starch
[12]. Compared to native starch, the oxidized starch is lighter in colour. As a consequence of partial depolymerization, it is more soluble in water, forms pastes of lower viscosity, and produces weaker gels. Moreover, it shows a lower tendency for retrogradation, which means that oxidized starch gels are less susceptible to syneresis
[4][9][13]. Limited retrogradation is due to both partial depolymerization of starch polymers and the presence of anionic groups. The presence of carboxyl groups in the oxidized starch structure makes it prone to interact with metal ions, amines, polyols, or other substances.
Although starches can be oxidized with various oxidizing agents, only sodium hypochlorite is allowed for oxidation of starch for food purposes, and the carboxyl group content in the finished preparation must not be greater than 1.1%, dwb. Factors affecting the degree of oxidation with sodium hypochlorite include reagent concentration, pH, reaction temperature and time, the molecular structure of the starch, and its botanical origin
[4][11][13]. Oxidized starches approved as food additive is used as a stabilizer, gelling agent, or thickener in confectionery products (e.g., cake fillings, powdered cake mixes), pudding desserts, or whipped cream. Moreover, this starch is used in the production of sauces, thickened soups, frozen lunch dishes, or baby food, in which it performs texturizing (binding, thickening) and stabilizing functions
[8][14][15][16].
2.1.2. Stabilized Starches
Starch Esters
During starch acetylation, the starch slurry is treated with acetic anhydride or vinyl acetate, which results in the substitution of hydroxyl groups of glucose monomers with hydrophobic acetyl groups
[9][16]. The substituent groups are introduced in the amorphous domain of the starch granules
[16]. Depending on the amount of reactant and the environmental conditions, a low, medium, or high degree of starch substitution can be obtained. Acetylated starch intended for food purposes may not contain more than 2.5% of acetyl groups, dwb. The physical and chemical properties of acetylated starch preparations depend on the type of the modified starch, the type and amount of the reagent used, the reaction medium (aqueous or anhydrous), the pH, the reaction temperature and duration, and the presence of catalysts or other factors influencing the course of the reaction, and, consequently, the degree of substitution
[8][16]. During the acetylation process, partial depolymerization of starch may also occur, which is manifested by changes in the average molecular weight of starch and a decrease in the viscosity of its pastes. Morphological changes in the structure of starch granules were also observed and included the erosion of the granule surface, the formation of pits and pores, and the tendency of granules to aggregate
[9][16]. The presence of acetyl groups in the chemical structure of starch creates a steric hindrance, which loosens the starch structure and limits interactions between starch polymer chains. As a consequence, water absorption by starch granules as well as their swelling and water solubility are increased compared to native starch
[16][17][18]. These changes are manifested by a lower gelatinization temperature of the modified starch
[12][16]. In addition, the hindered association of starch polymer chains is manifested by restricted starch retrogradation in gels
[9][16][17]. The partial leaching of amylose resulting from starch acetylation contributes to a reduction in the degree of starch crystallinity.
Acetylated starches are used in the food industry as food additives that regulate consistency and increase food stability
[16][19]. The pastes formed by acetylated starch are clear and stable during heat treatment. In addition, acetylated starches have the ability to form gels, but they are not resistant to elevated temperatures, acidic environments, and mechanical forces. Therefore, acetylated starches are not dedicated to products preserved by sterilization. The main application of acetylated starches includes the production of frozen cakes, fruit confectionery fillings, fruit cakes and bakery products, protective coatings, sauces, soups, dessert concentrates, flavoured yoghurts, and thermized curds
[14][15][16][20].
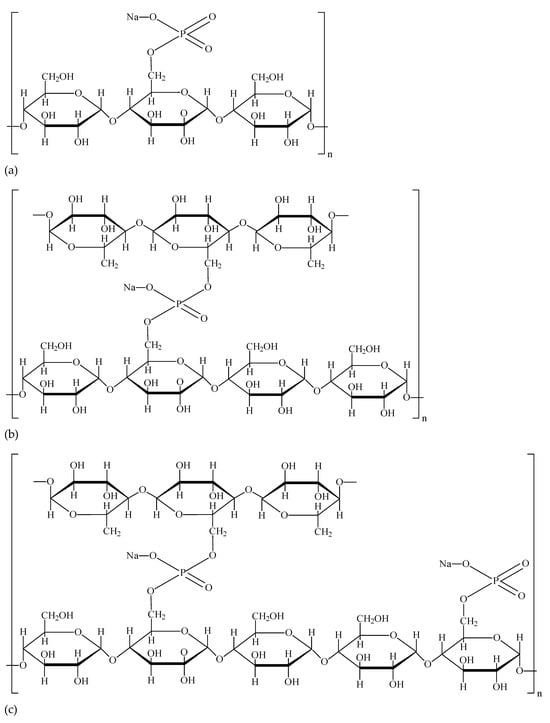
Monostarch phosphates are formed in the reaction of starch with phosphoric acid or salts of this acid. The chemical structure of such modified starch is shown in
Figure 1a. These starches are characterized by a high degree of water binding and swelling, as well as greater solubility in cold water compared to native starch
[8][21]. They form transparent pastes, which are very stable during freezing and thawing given that the modified starch practically exhibits no retrogradation tendency
[21]. Monostarch phosphates do not change their structure-forming properties even after prolonged exposure to mechanical forces. However, they are sensitive to a low pH environment. To a limited extent, they have the ability to stabilize emulsion systems. Monostarch phosphates are used as food thickeners, especially in cold gelling desserts. In addition, they are used in the production of soups, vegetable sauces, ketchups, sauces for canned vegetables and meat, dressings, frozen preserves, confectionery fillings and creams, instant desserts, flavoured and thermized yoghurts
[14][15].
Figure 1. Chemical structures of starches modified by esterification: (a) monostarch phosphate (E 1410)—stabilized starch; (b) distarch phosphate (E 1412)—cross-linked starch; (c) phosphorylated distarch phosphate (E 1413)—cross-linked and stabilized starch
As an alternative to the traditional method of starch phosphorylation, the use of a microwave field as a source of thermal energy was proposed
[22]. It was found that the exposure to microwave radiation did not affect the type of chemical groups substituted in starch and the size distribution of molecular weights of starch esters obtained in this way. There were also no differences in the chemical and crystal structure of starch esters obtained using microwave and conventional technologies. At the same time, it was found that the modification of starch with the use of microwave radiation occurs in a much shorter time than the modification obtained with the conventional technique, which has a positive effect on the cost-effectiveness of the process
[22]. In other studies, high-voltage electrical discharges have been proposed as a source of energy used during phosphorylation. It was found that such starch treatment has a positive effect on the modification efficiency, phosphorus content, and functional properties, including water absorption, solubility, and rheological characteristics of the finished preparation
[23].
Octenyl succinic starches (OSA starches) are produced by esterification of starch in its granular form with octenyl succinic anhydride (OSA), usually in an aqueous mild alkaline medium (pH of 7.0–8.0) and at a temperature of 30 to 40 °C
[24][25][26]. One of the alternative methods of producing the OSA preparations is the esterification of starch in aqueous medium catalysed by lipase at a temperature of 65 °C. The increased temperature contributes to a sufficient loosening of the starch granules and thus to a more effective penetration of the modifying agent
[25]. Chemical esterification of starch with OSA can be assisted by ultrasound; microwaves; intensive stirring; or hydrothermal, mechanical, chemical, or enzymatic pre-treatment
[25][26].
The introduction of hydrophobic octenyl succinate groups into the hydrophilic starch molecule gives the starch surface activity, i.e., amphiphilic properties
[26], and additionally anionic properties. As a result, OSA starch becomes cold water soluble and resistant to retrogradation; it supports emulsification and perfectly stabilizes emulsions and foams
[24][25][27]. Moreover, as a result of the presence of octenyl succinic groups and concomitant changes produced in the granules’ structure, OSA starches usually show a lower temperature and gelatinization enthalpy and a higher swelling power, and they form pastes of higher viscosity that are clearer compared to a native starch
[18][24][25][28]. The above-mentioned properties of OSA starches imply their use as emulsion stabilizers, e.g., in milk drinks, mayonnaises, and dressings as texturizing agents (thickeners, binding agents), and in gluten-free bread as carriers and matrices for encapsulating bioactive compounds as well as raw materials for producing biodegradable films and coatings
[14][25][27][29][30][31][32]. Due to instant properties, OSA starches are widely used in cold-prepared products. Starch octenyl succinates have also been shown to reduce the glycaemic response and exhibit some resistance to digestion, indicating their potential use in functional foods.
Starch Ether—Hydroxypropyl Starch (E 1440)
Hydroxypropyl starch (E 1440) is obtained by reacting starch with propylene oxide in the presence of a strongly alkaline catalyst, usually at 40 °C. The reaction of replacing hydroxyl groups with hydroxypropyl groups has a nucleophilic substitution mechanism
[19][20]. The content of hydroxypropyl groups in hydroxypropyl starch intended for food purposes may be up to 7.0%, dwb. The presence of hydrophilic ether groups limits the number of hydrogen bonds that join starch polymers, which weakens the internal structure of starch granules. Changes in the ordering of the structure of polymers result in their greater availability to water molecules; thus, starch becomes more hydrophilic. This results in an increase in swelling degree and viscosity of the gels formed, as well as a reduced gelatinization temperature of starch compared to native starch. In addition, it significantly reduces the ability of amylose to recrystallize, which reduces the phenomenon of retrogradation
[9][19][20][33]. Hydroxypropyl starch is also stable at high temperatures and in a low pH environment. Due to the above-mentioned properties, preparations of this starch are used as thickeners in products based on water or milk that are frozen and stored in refrigerated conditions
[14][15].
2.1.3. Cross-Linked Starch—Distarch Phosphate (E 1412)
Starch cross-linking consists of introducing additional, stiffening cross-links to the chemical structure of starch
[21]. Starch cross-linking reactions leading to distarch phosphate are carried out in an alkaline environment at a temperature of 20 to 50 °C, treating starch in suspension with phosphorus oxychloride or sodium trimetaphosphate
[8][21]. The amount of phosphate in terms of phosphorus may not exceed 0.5% for potato or wheat starch and 0.4% in other cases. In contrast to monostarch phosphate (E 1410), two hydroxyl groups of glucose units from two adjacent starch chains are esterified with one phosphate group in distarch phosphate (
Figure 1b). A special feature of this type of starch is the reduced ability of starch granules to swell, therefore producing a higher gelatinization temperature, a lower tendency of starch polymers to undergo retrogradation, and a reduced susceptibility to enzymatic hydrolysis compared to the native counterpart. Depending on the degree of substitution, the viscosity of distarch phosphate pastes may be higher or lower than that of natural unmodified starch pastes
[6][9][21]. Moreover, unlike monostarch phosphate, the pastes formed by distarch phosphate are not transparent. Distarch phosphates with a high degree of cross-linking form gels characterized by high rheological stability even at high temperatures, low pH, and under intense mechanical forces
[33].
This modified starch is used in the production of products subjected to thermal treatment in the technological process, in particular pasteurization or sterilization. It shows a special ability to prevent thermal leakage occurring during the thermal processing of meat as well as meat and vegetable products
[15]. The use of distarch phosphates in the production of meat products contributes to the improvement of the cohesiveness and consistency of products and increased yield while maintaining satisfactory sensory characteristics. Distarch phosphates also improve the stability of defrosted products, which allows maintenance of the desired consistency of ready meals. In addition, they are used as stabilizers, thickeners, binders, or carriers in products such as baby food, salad dressings, mayonnaises, soups, frozen dishes, puddings, fruit confectionery fillings, and flavoured and thermized yoghurts
[14][15][21].
2.2. Dually Modified Starches
Dual chemical modification is a type of homogeneous dual modification, as it is a combination of two modifications of the same type. In turn, heterogeneous dual modification is a modification carried out by combining two processes of a different nature, e.g., physical and chemical processes or enzymatic and chemical processes
[34]. Heterogeneous dual modification of starch is described in
Section 2.3.
2.2.1. Oxidized and Stabilized Starch—Acetylated Oxidized Starch (E 1451)
Acetylated oxidized starch is obtained in two stages: oxidation of starch with sodium hypochlorite at low temperature (21–38 °C) in an alkaline environment, followed by esterification with acetic anhydride under slightly alkaline conditions. Acetylation of oxidized starch improves the clarity, stability, and rheological characteristics of the gels it forms. The properties of acetylated oxidized starch are affected, apart from the botanical origin of the starch itself, by the degree of oxidation and acetylation
[35]. Acetylated oxidized starch has a lower gelatinization temperature than native starch. It creates clear and highly viscous pastes stable at high temperatures and gels resistant to retrogradation. It acts as a thickening and binding agent, especially in products kept at low temperatures. In the confectionery industry, it can be used as a substitute for gelatine and acacia gum
[14][35].
2.2.2. Cross-Linked and Stabilized Starches
Phosphated Distarch Phosphate (E 1413)
Phosphated distarch phosphate is a double chemically modified starch: cross-linked and stabilized with phosphoric acid. Through cross-linking, cross-links are formed between the various chains of the starch polymers, while stabilizing phosphate functional groups are incorporated as a result of simple esterification (
Figure 1c). The modified starch may contain phosphate residues (calculated as phosphorus) at levels less than 0.5%, dwb, for wheat and potato starch or less than 0.4%, dwb, for starches of other origins
[36].
The discussed starch derivative forms transparent pastes and gels with high thickening abilities. Cross-linking reduces the sensitivity of starch to changes in temperature and pH and to mechanical factors
[17], while stabilisation reduces starch retrogradation. For this reason, this modified starch is used in products that are required to be resistant to high temperatures and stable in freezing and thawing conditions. This starch is used, among others, in the production of fruit confectionery fillings, vegetable sauces, dressings, yoghurts, and desserts
[14][15][37].
Acetylated Distarch Phosphate (E 1414)
Acetylated distarch phosphate is produced by the cross-linking of starch with phosphorus oxychloride, followed by stabilization by esterification with acetic anhydride. Phosphate content, calculated as phosphorus, must not exceed 0.14%, dwb, in potato or wheat starch derivative and 0.04%, dwb, in other starches, while the content of acetyl groups cannot be greater than 2.5%, dwb
[36]. The E 1414 starch pastes and gels are transparent and resistant to high temperatures, acidic environments, and shear forces
[38][39]. Their advantage is that they do not undergo retrogradation processes during long-term storage. Acetylated distarch phosphate is a universal binder and thickener with good texturizing and stabilizing properties
[34]. Therefore, it is used in the production of mayonnaises, dressings, ketchups, vegetable and vegetable-meat sauces, and fruit concentrates. Acetylated distarch phosphate is also used in the production of fermented milk drinks, thermized curds, and low-calorie margarine
[15][38][40].
Acetylated Distarch Adipate (E 1422)
Acetylated distarch adipate is produced by cross-linking of starch with adipic acid and stabilizing with acetyl groups derived from acetic anhydride
[39]. The content of adipic groups in the obtained derivative must not exceed 0.135%, dwb, while the content of acetyl groups cannot exceed 2.5%, dwb
[36]. Pastes and gels of the discussed starch are characterized not only by resistance to elevated temperatures and shear forces, but also resistance to weakly acidic environments
[34][39]. Thus, acetylated distarch adipate is used to thicken and stabilize a wide range of food products that are subjected to thermal treatment at temperatures above 70 °C. These include, among others, ketchups, vegetable and vegetable-meat sauces, lunch concentrates, dessert powder concentrates, and baking fillings. E 1422 starch is also used in the production of mayonnaise, dressings, or fermented milk drinks
[14][15][40].
Distarch Hydroxypropyl Phosphate (E 1442)
Stabilized cross-linked starches also include distarch hydroxypropyl phosphate, which is a starch modified by cross-linking with sodium trimetaphosphate or phosphorus oxychloride and by etherification with propylene oxide. In E 1442 starch, the content of hydroxypropyl groups must not exceed 7.0%, dwb, while the residual phosphates, calculated as phosphorus, must not exceed 0.14%, dwb, in wheat and potato starch and 0.04%, dwb, in other starches
[36]. This starch is characterized by a reduced pasting temperature compared to the unmodified starch. It forms transparent, stable gels, resistant to mechanical processing and not subject to syneresis
[39]. Distarch hydroxypropyl phosphate preparations are used in particular for the production of fruit preparations, as they give them a relatively low viscosity during thermal processing. Moreover, after cooling the finished product, they contribute to a significant increase in viscosity. In addition, they give these products a short, creamy texture that does not change during freezing and thawing. E 1442 starch is also used in the production of dairy products, including pasteurized and sterilized cream, flavoured yoghurts, and baby foods, and is also a functional additive in powdered soups or vegetable sauces
[15][38][40].
2.3. Heterogeneously Dually Modified Starches
Due to the possibility of using very different physical processes or enzymatic reactions and many chemical reactions, the methods of producing dually modified starch preparations are practically unlimited
[19]. If one of the two starch modifications used is a chemical modification and the quality criteria for chemically modified starches constituting food additives are simultaneously met, the obtained starch preparations have the status of food additives. Examples of physical processes that can be used in combination with chemical modification include pregelatinization, high hydrostatic pressure treatment, microwave treatment, sonication, gamma radiation, pulsed electric field, extrusion and others
[6][19][41]. In heterogeneous dual modifications, a very important factor influencing the functional properties of the resulting starch preparation is the sequence of applied modification processes. The first modification is aimed at increasing the effectiveness of the second modification and most often leads to the disruption of the starch granule surface and the weakening of the bonding forces of starch chains. Thus, the properties of starches modified in this way depend largely on the second modification. For example, in sonicated acetylated starch, sonication causes morphological damage to the starch granule surfaces (cracks, pores, channels), which facilitates the subsequent penetration of reactants during acetylation and more efficient substitution of starch with acetyl groups
[34]. An example of a heterogeneous modification is the enzymatic hydrolysis of chemically modified starch
[42][43]. The preparations produced in this manner are characterized by a reduced molecular weight of starch polymers, higher solubility, lower gelling capacity, and a reduced tendency for retrogradation compared to starch not subjected to enzymatic treatment. They form pastes of lower viscosity, but these pastes are rheologically stable
[44]. The presence of stabilizing and/or cross-linking groups in the structure of the modified starch hydrolysates results in different functional properties in relation to unmodified starch hydrolysates
[44][45]. For example, enzymatically hydrolysed starch octenyl succinate has the ability to reduce interfacial tension in foams and emulsion systems
[21]. Far-reaching enzymatic hydrolysis of chemically modified starches leads to the production of chemically modified maltodextrins
[44]. Maltodextrins obtained by enzymatic hydrolysis of chemically modified starches may be interesting stabilizers of food emulsions and modifiers of the rheological properties of the latter
[45].
The term “triple starch modification” is also used. In triple modification, the first and dual modifications prepare the starch chains, structures, and polymeric components of the starch granules for the penetration of the modifying agents during the third modification. Triple modification can be used for the synthesis of resistant starches, porous starches, and preparations with high adsorption capacities
[41].