
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edmund Kozieł | -- | 2004 | 2023-11-27 10:52:24 | | | |
| 2 | Peter Tang | + 2 word(s) | 2006 | 2023-11-28 01:50:39 | | |
Video Upload Options
Biological plant protection presents a promising and exciting alternative to chemical methods for safeguarding plants against the increasing threats posed by plant diseases. This approach revolves around the utilization of biological control agents (BCAs) to suppress the activity of significant plant pathogens. Microbial BCAs have the potential to effectively manage crop disease development by interacting with pathogens or plant hosts, thereby increasing their resistance.
1. Introduction
|
Active Substance |
Trade Name |
Distributor |
Country |
Form. |
Target Crops |
Target Disease |
|---|---|---|---|---|---|---|
|
Aureobasidium pullulans DSM 14940 + DSM 14941 |
BLOSSOM PROTECT; BONI PROTECT; BOTECTOR |
Bio-ferm Biotechnologische Entwicklung und Produktion GmbH |
US; CA; EU; SK; TN; GB; NI; BE; DE; EL; ES; FR; HU; IT; LU; NL; PT; PL; RO; SI; SK |
WP |
Apple, medlar, pear, quince |
Fire blight Erwinia amylovora |
|
Trichoderma virens G-41 + T. harzianum Rifai T-22 |
RootShield® PLUS WP |
BioWorks, Inc. |
US; CA |
WG |
Greenhouse and nursery vegetables, herbs, ornamentals, fruits, conifer tree seedlings, various trees, legumes, oil seeds, and peanuts |
Phytophthora, Rhizoctonia, Pythium, Fusarium, Thielaviopsis, Cylindrocladium |
|
Trichoderma asperellum ICC012 + T25 + TV1 |
XEDAVIR; PATRIOT GOLD; BIOTRIX; XEDAVIR PFNPE |
Xeda International S.A.; Timac AGRO Espańa SA |
IT; PT; FR; EU; |
WP, WG |
Greenhouse and open field vegetables |
Pythium spp., Phytophthora capsici, Rhizoctonia solani |
|
Trichoderma atroviride IMI 206040 + T11 |
Binab TF WP; Binab T Vector; |
Borregaard Bioplant |
SE; EU |
WP |
Tomatoes, strawberries, ornamental trees |
Botrytis cinerea, Chondrostereum purpureum |
|
Trichoderma asperellum ICC012 + T. gamsii ICC080 |
Tellus; Foretryx; Bio-Tam2.0; DonJon; Bioten WP; Blindar; Remedier |
Syngenta; Isagro S.p.A.; Bayer; Gowan |
NL; CA; PL; US; PT; FR; TN; CY |
WP |
Tomatoes, horticultural flowers, ornamental and tree crops |
Verticillium dahliae, Rhizoctonia solani, Sclerotinia sclerotiorum, Thielaviopsis basicola, Phytophthora capsici |
|
Trichoderma asperellum T25 + T. atroviride T11 |
Tusal |
Newbiotechnic S.A. |
FR; EL; GB; EU |
WG |
Strawberry, tomato, eggplant, pepper, cucumber, courgetti, melon, watermelon, pumpkin, cut flowers, lettuce, escarole, similars, trees, and shrubs |
Phytophthoracactorum, Rhizoctonia solani, Sclerotinia sclerotiorum, Phytophthora spp., Fusarium spp., Pythium spp., Phomopsis sp., |
2. Ecological Interactions: Mechanisms of Plant Disease Control
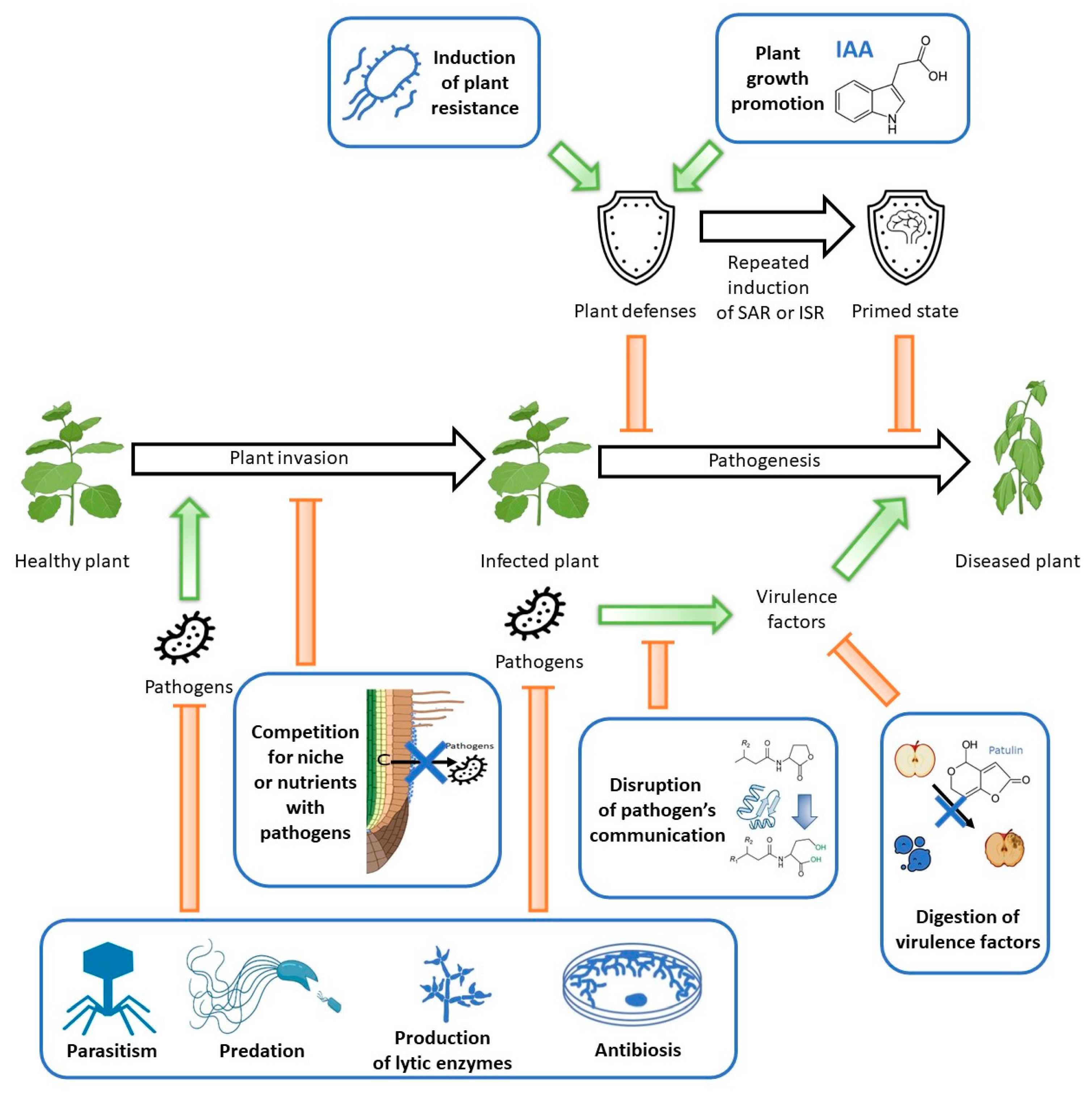
3. Interactions between Components: Menace or a New Hope
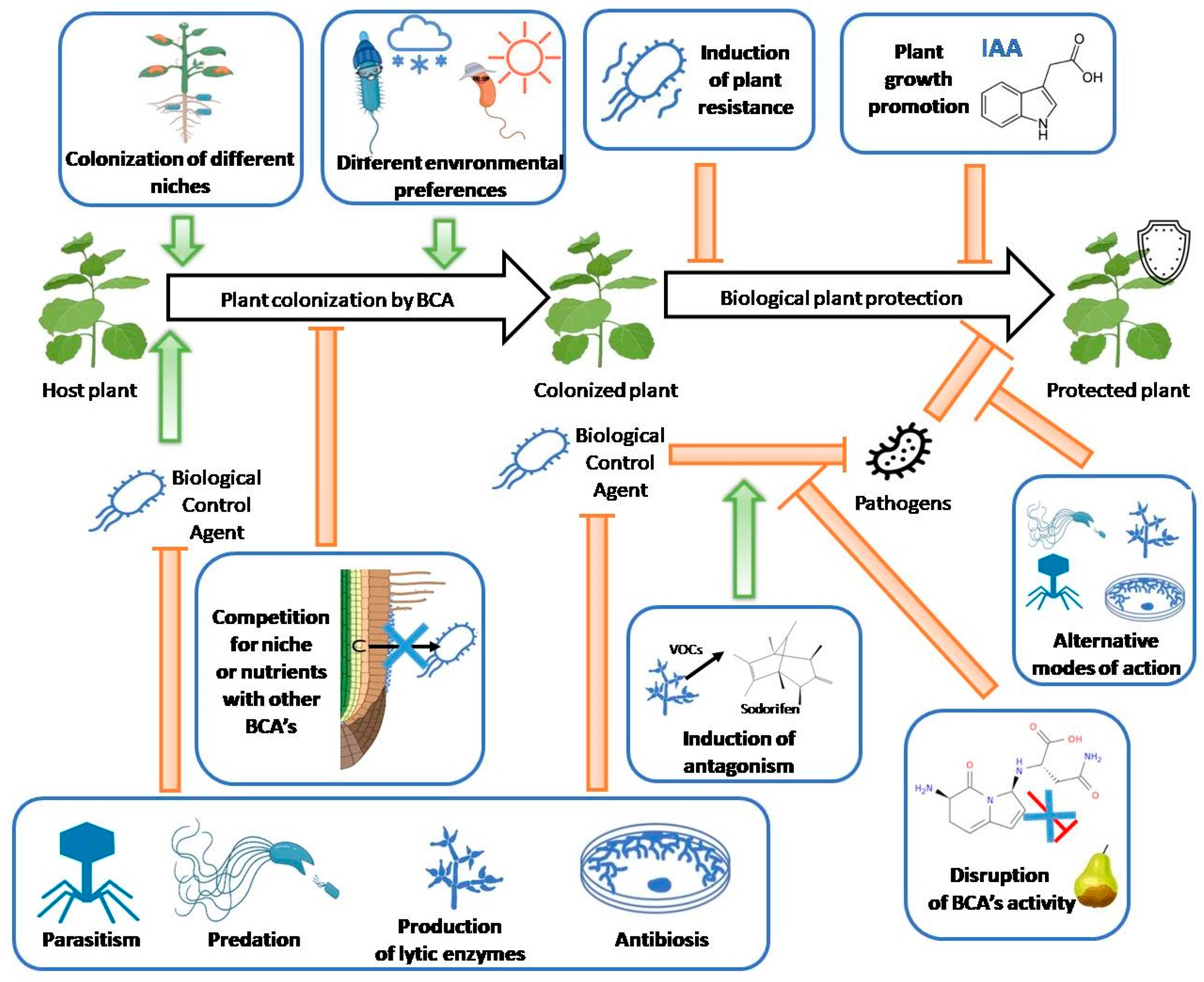
4. Successful Solutions
References
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118.
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518.
- O’Brien, P.A. Biological Control of Plant Diseases. Australa. Plant Pathol. 2017, 46, 293–304.
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340.
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 2452.
- Dinis, M.; Vicente, J.R.; César de Sá, N.; López-Núñez, F.A.; Marchante, E.; Marchante, H. Can Niche Dynamics and Distribution Modeling Predict the Success of Invasive Species Management Using Biocontrol? Insights From Acacia longifolia in Portugal. Front. Ecol. Evol. 2020, 8, 576667.
- Kozieł, E.; Bujarski, J.J.; Otulak-Kozieł, K. Plant cell apoplast and symplast dynamic association with plant-RNA virus interactions as a vital effect of host response. In Plant RNA Viruses Molecular Pathogenesis and Management, 1st ed.; Gaur, R.K., Patil, B.E., Selvarajan, R., Eds.; Academic Press: London, UK, 2023; Volume 16, pp. 311–328.
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. Appl. Soil Ecol. 2023, 190, 105011.
- Ram, R.M.; Debnath, A.; Negi, S.; Singh, H.B. Use of microbial consortia for broad spectrum protection of plant pathogens. Biopesticides 2021, 2, 319–335.
- Kredics, L.; Manczinger, L.; Antal, Z.; Pénzes, Z.; Szekeres, A.; Kevei, F.; Nagy, E. In vitro water activity and pH dependence of mycelial growth and extracellular enzyme activities of Trichoderma strains with biocontrol potential. J. Appl. Microbiol. 2004, 96, 491–498.
- Mascher, F.; Hase, C.; Bouffaud, M.L.; Défago, G.; Moënne-Loccoz, Y. Cell culturability of Pseudomonas protegens CHA0 depends on soil pH. FEMS Microbiol. Ecol. 2014, 87, 441–450.
- Hoitink, H.A.J.; Boehm, M.J. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446.
- Pierson, E.A.; Weller, D.M. To suppress Take-all and improve the growth of wheat. Phytopathology 1994, 84, 940–947.
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845.
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596.
- Zhu, X.; Chen, W.J.; Bhatt, K.; Zhou, Z.; Huang, Y.; Zhang, L.H.; Chen, S.; Wang, J. Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Front. Plant Sci. 2022, 13, 1063393.
- Someya, N.; Nakajimal, M.; Hirayae, K.; Hibi, T.; Akutsu, K. Synergistic Antifungal Activity of Chitinolytic Enzymes and Prodigiosin Produced by Biocontrol Bacterium, Serratia marcescens Strain B2 against Gray Mold Pathogen, Botrytis cinerea. J. Gen. Plant Pathol. 2001, 67, 312–317.
- Li, Y.; Feng, X.; Wang, X.; Zheng, L.; Liu, H. Inhibitory effects of Bacillus licheniformis BL06 on Phytophthora capsici in pepper by multiple modes of action. Biol. Control 2020, 144, 104210.
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2017, 10, 19–21.
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Inoculants: An advantage in PGPM-assisted tomato production? Agronomy 2019, 9, 105.
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361.
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219.
- Czajkowski, R.; Maciag, T.; Krzyzanowska, D.M.; Jafra, S. Biological Control Based on Microbial Consortia—From Theory to Commercial Products. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; Springer: Cham, Switzerland, 2020; pp. 183–202.
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801.
- Pallavi Mittal, P.M.; Madhu Kamle, M.K.; Shubhangini Sharma, S.S.; Pooja Choudhary, P.C.; Rao, D.P.; Pradeep Kumar, P.K. Plant Growth-Promoting Rhizobacteria (PGPR): Mechanism, Role in Crop Improvement and Sustainable Agriculture. In Advances in PGPR Research; CABI: Wallingford, UK, 2017; pp. 386–397.
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic Production by Bacterial Biocontrol Agents. Antonie Van Leeuwenhoek 2002, 81, 531–547.
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 2011, 101, 113–123.
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Valos, M.; Guzmán-Trampe, S.; et al. Carbon source regulation of antibiotic production. J. Antibiot. 2010, 63, 442–459.
- Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M.; Blum, B. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol. Control 2011, 57, 1–12.
- Sharma, P. Biocontrol strategies—Retrospect and prospects. Indian Phytopathol. 2023, 76, 47–59.
- Guzmán-Guzmán, P.; Kumar, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432.
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329.
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786.
- Liu, Y.; He, P.; He, P.; Munir, S.; Ahmed, A.; Wu, Y.; Yang, Y.; Lu, J.; Wang, J.; Yang, J.; et al. Potential biocontrol efficiency of Trichoderma species against oomycete pathogens. Front. Microbiol. 2022, 13, 974024.
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90.
- Silva, L.G.; Camargo, R.C.; Mascarin, G.M.; Nunes, P.S.d.O.; Dunlap, C.; Bettiol, W. Dual functionality of Trichoderma: Biocontrol of Sclerotinia sclerotiorum and biostimulant of cotton plants. Front. Plant Sci. 2022, 13, 983127.
- del Carmen, H.; Rodríguez, M.; Evans, H.C.; de Abreu, L.M.; de Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671.
- Naamala, J.; Smith, D.L. Relevance of plant growth promoting microorganisms and their derived compounds, in the face of climate change. Agronomy 2020, 10, 1179.
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369.
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 2022, 11, 2656.
- Muñoz-Carvajal, E.; Araya-Angel, J.P.; Garrido-Sáez, N.; González, M.; Stoll, A. Challenges for Plant Growth Promoting Microorganism Transfer from Science to Industry: A Case Study from Chile. Microorganisms 2023, 11, 1061.
- OEPP EPPO Databases of Registered PPPs. Available online: https://www.eppo.int/ACTIVITIES/plant_protection_products/registered_products (accessed on 5 May 2023).





