| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaison Jeevanandam | -- | 1390 | 2023-11-25 23:16:06 | | | |
| 2 | Jessie Wu | Meta information modification | 1390 | 2023-11-27 03:51:25 | | |
Video Upload Options
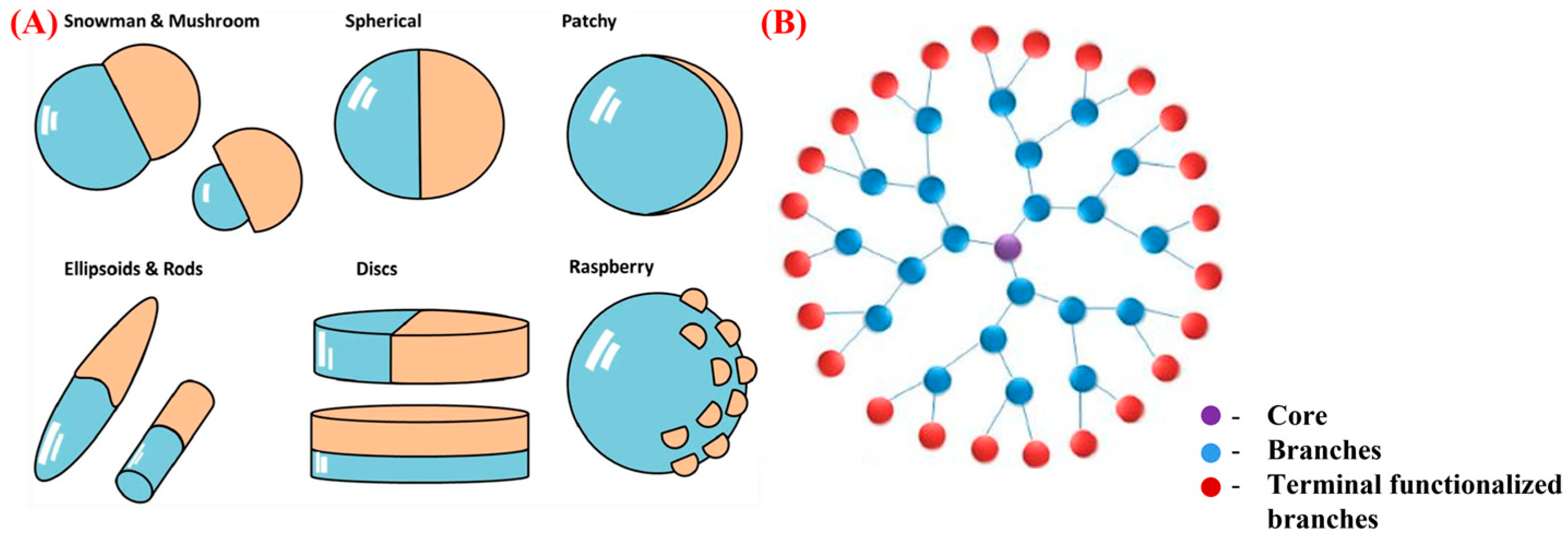
Nanosized Janus and dendrimer particles have emerged as promising nanocarriers for the target-specific delivery and improved bioavailability of pharmaceuticals. Janus particles, with two distinct regions exhibiting different physical and chemical properties, provide a unique platform for the simultaneous delivery of multiple drugs or tissue-specific targeting. Conversely, dendrimers are branched, nanoscale polymers with well-defined surface functionalities that can be designed for improved drug targeting and release. Both Janus particles and dendrimers have demonstrated their potential to improve the solubility and stability of poorly water-soluble drugs, increase the intracellular uptake of drugs, and reduce their toxicity by controlling the release rate.
1. Introduction
2. Janus Nanoparticles
3. Dendrimers
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596.
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615.
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880.
- Gorantla, S.; Wadhwa, G.; Jain, S.; Sankar, S.; Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent advances in nanocarriers for nutrient delivery. Drug Deliv. Transl. Res. 2021, 12, 2359–2384.
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429.
- Alven, S.; Aderibigbe, B.A. The therapeutic efficacy of dendrimer and micelle formulations for breast cancer treatment. Pharmaceutics 2020, 12, 1212.
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757.
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63.
- Koyyada, A.; Orsu, P. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431.
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18.
- Tomás, H.; Rodrigues, J. Chapter 2—Dendrimers and dendrimer-based nano-objects for oncology applications. In New Trends in Smart Nanostructured Biomaterials in Health Sciences; Gonçalves, G., Marques, P., Mano, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 41–78.
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532.
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982.
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science 2010, 328, 1009–1014.
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-type dendrimers: Synthesis, properties, and applications. J. Mol. Liq. 2022, 347, 118396.
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research advances in the synthesis, application, assembly, and calculation of Janus materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095.
- Kirillova, A.; Marschelke, C.; Synytska, A. Hybrid Janus particles: Challenges and opportunities for the design of active functional interfaces and surfaces. ACS Appl. Mater. Interfaces 2019, 11, 9643–9671.
- Rosati, M.; Acocella, A.; Pizzi, A.; Turtù, G.; Neri, G.; Demitri, N.; Nonappa; Raffaini, G.; Donnio, B.; Zerbetto, F.; et al. Janus-Type Dendrimers Based on Highly Branched Fluorinated Chains with Tunable Self-Assembly and 19F Nuclear Magnetic Resonance Properties. Macromolecules 2022, 55, 2486–2496.
- de Gennes, P.-G. Soft Matter (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1992, 31, 842–845.
- Song, Y.; Chen, S. Janus Nanoparticles: Preparation, Characterization, and Applications. Chem. Asian J. 2014, 9, 418–430.
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus Nanoparticles: From Fabrication to (Bio)Applications. ACS Nano 2021, 15, 6147–6191.
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308.
- Zhang, Y.; Huang, K.; Lin, J.; Huang, P. Janus nanoparticles in cancer diagnosis, therapy and theranostics. Biomater. Sci. 2019, 7, 1262–1275.
- Yan, J.; Chaudhary, K.; Chul Bae, S.; Lewis, J.A.; Granick, S. Colloidal ribbons and rings from Janus magnetic rods. Nat. Commun. 2013, 4, 1516.
- Wang, C.; Xu, C.; Zeng, H.; Sun, S. Recent Progress in Syntheses and Applications of Dumbbell-like Nanoparticles. Adv. Mater. 2009, 21, 3045–3052.
- Link, J.R.; Sailor, M.J. Smart dust: Self-assembling, self-orienting photonic crystals of porous Si. Proc. Natl. Acad. Sci. USA 2003, 100, 10607–10610.
- Zhao, R.; Yu, X.; Sun, D.; Huang, L.; Liang, F.; Liu, Z. Functional Janus Particles Modified with Ionic Liquids for Dye Degradation. ACS Appl. Nano Mater. 2019, 2, 2127–2132.
- Mou, F.; Chen, C.; Guan, J.; Chen, D.-R.; Jing, H. Oppositely charged twin-head electrospray: A general strategy for building Janus particles with controlled structures. Nanoscale 2013, 5, 2055–2064.
- Bradley, L.C.; Chen, W.-H.; Stebe, K.J.; Lee, D. Janus and patchy colloids at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2017, 30, 25–33.
- Su, H.; Hurd Price, C.A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus particles: Design, preparation, and biomedical applications. Mater. Today Bio. 2019, 4, 100033.
- Li, X.; Chen, L.; Cui, D.; Jiang, W.; Han, L.; Niu, N. Preparation and application of Janus nanoparticles: Recent development and prospects. Coord. Chem. Rev. 2022, 454, 214318.
- Kim, M.; Jeon, K.; Kim, W.H.; Lee, J.W.; Hwang, Y.-H.; Lee, H. Biocompatible amphiphilic Janus nanoparticles with enhanced interfacial properties for colloidal surfactants. J. Colloid Interface Sci. 2022, 616, 488–498.
- Jia, R.; Jiang, H.; Jin, M.; Wang, X.; Huang, J. Silver/chitosan-based Janus particles: Synthesis, characterization, and assessment of antimicrobial activity in vivo and vitro. Food Res. Int. 2015, 78, 433–441.
- Chun, H.J.; Kim, S.; Han, Y.D.; Kim, D.W.; Kim, K.R.; Kim, H.-S.; Kim, J.-H.; Yoon, H.C. Water-soluble mercury ion sensing based on the thymine-Hg2+-thymine base pair using retroreflective Janus particle as an optical signaling probe. Biosens. Bioelectron. 2018, 104, 138–144.
- Wang, Y.; Shang, M.; Wang, Y.; Xu, Z. Droplet-based microfluidic synthesis of (Au nanorod@Ag)–polyaniline Janus nanoparticles and their application as a surface-enhanced Raman scattering nanosensor for mercury detection. Anal. Methods 2019, 11, 3966–3973.
- Zheng, F.; Ke, W.; Shi, L.; Liu, H.; Zhao, Y. Plasmonic Au–Ag Janus Nanoparticle Engineered Ratiometric Surface-Enhanced Raman Scattering Aptasensor for Ochratoxin A Detection. Anal. Chem. 2019, 91, 11812–11820.
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. I. Gelation1. J. Am. Chem. Soc. 1941, 63, 3083–3090.
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091–3096.
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A—R—Bf-1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723.
- Kim, Y.H.; Webster, O.W. Hyperbranched polyphenylenes. Macromolecules 1992, 25, 5561–5572.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132.
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A -arborol. J. Org. Chem. 1985, 50, 2003–2004.
- Augustus, E.N.; Allen, E.T.; Nimibofa, A.; Donbebe, W. A review of synthesis, characterization and applications of functionalized dendrimers. Am. J. Polym. Sci. 2017, 7, 8–14.
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of dendrimers in nanomedicine and drug delivery: A review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261.
- Akki, R.; Ramya, M.G.; Sadhika, C.; Spandana, D. A novel approach in drug delivery system using dendrimers. Pharm. Innov. J. 2019, 8, 166–174.
- Frechet, J.M.J. Functional polymers and dendrimers: Reactivity, molecular architecture, and interfacial energy. Science 1994, 263, 1710–1715.
- Prakash, P.; Kunjal, K.K.; Shabaraya, A. Dendrimer architecture: A comprehensive review. World J. Pharm. Res. 2021, 10, 638–659.
- England, R.M.; Sonzini, S.; Buttar, D.; Treacher, K.E.; Ashford, M.B. Investigating the properties of l-lysine dendrimers through physico-chemical characterisation techniques and atomistic molecular dynamics simulations. Polym. Chem. 2022, 13, 2626–2636.
- Munavalli, B.B.; Naik, S.R.; Torvi, A.I.; Kariduraganavar, M.Y. Dendrimers. In Functional Polymers; Springer: Berlin/Heidelberg, Germany, 2019; pp. 289–345.
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, M.; Rahman, M. Dendrimers: A new race of pharmaceutical nanocarriers. BioMed Res. Int. 2021, 2021, 8844030.
- Smith, R.J.; Gorman, C.; Menegatti, S. Synthesis, structure, and function of internally functionalized dendrimers. J. Polym. Sci. 2021, 59, 1028.
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Nouri Khorasani, S.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954.
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and applications. Acta Biochim. Pol. 2001, 48, 199–208.
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330.
- Gupta, A.; Dubey, S.; Mishra, M. Unique structures, properties and applications of dendrimers. J. Drug Deliv. Ther. 2018, 8, 328–339.
- Mignani, S.; Shi, X.; Rodrigues, J.; Tomas, H.; Karpus, A.; Majoral, J.-P. First-in-class and best-in-class dendrimer nanoplatforms from concept to clinic: Lessons learned moving forward. Eur. J. Med. Chem. 2021, 219, 113456.
- Mignani, S.; Rodrigues, J.; Tomas, H.; Roy, R.; Shi, X.; Majoral, J.-P. Bench-to-bedside translation of dendrimers: Reality or utopia? A concise analysis. Adv. Drug Deliv. Rev. 2018, 136, 73–81.
- Honciuc, A. Amphiphilic Janus Particles at Interfaces. In Flowing Matter; Toschi, F., Sega, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–136.
- Araújo, R.V.; Santos, S.D.; Ferreira, E.I.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849.