Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Banu Metin | -- | 4245 | 2023-11-24 10:38:26 | | | |
| 2 | Rita Xu | Meta information modification | 4245 | 2023-11-24 11:06:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Metin, B. Penicillium roqueforti Secondary Metabolites. Encyclopedia. Available online: https://encyclopedia.pub/entry/52030 (accessed on 07 February 2026).
Metin B. Penicillium roqueforti Secondary Metabolites. Encyclopedia. Available at: https://encyclopedia.pub/entry/52030. Accessed February 07, 2026.
Metin, Banu. "Penicillium roqueforti Secondary Metabolites" Encyclopedia, https://encyclopedia.pub/entry/52030 (accessed February 07, 2026).
Metin, B. (2023, November 24). Penicillium roqueforti Secondary Metabolites. In Encyclopedia. https://encyclopedia.pub/entry/52030
Metin, Banu. "Penicillium roqueforti Secondary Metabolites." Encyclopedia. Web. 24 November, 2023.
Copy Citation
Penicillium roqueforti is a fungal starter culture used for the production of blue-veined cheeses, such as Roquefort, Gorgonzola, Stilton, Cabrales, and Danablue. During ripening, this species grows in the veins of the cheese, forming the emblematic blue-green color and establishing the characteristic flavor owin to its biochemical activities. P. roqueforti synthesizes a diverse array of secondary metabolites, including the well-known compounds roquefortine C, clavine alkaloids, such as isofumigaclavine A and B, mycophenolic acid, andrastin A, and PR-toxin.
bioactivity
biosynthetic gene clusters
blue cheese
isofumigaclavine
1. Introduction
Fungal secondary metabolites are chemically diverse natural products with low molecular weights [1]. Because of their biological activities, these compounds have a wide range of pharmacological applications. They are used as antibiotics, such as penicillin and cephalosporins; antifungals, like griseofulvin; immunosuppressants, including mycophenolic acid and cyclosporins; cholesterol-lowering drugs, such as lovastatin; and anti-migraine agents, e.g., ergotamine [1][2][3][4]. They are also important in the food and feed industry mostly because of their toxic properties [5]. Toxic fungal secondary metabolites, mycotoxins, were first described when large numbers of turkeys fed by the Brazilian groundnut meal died in 1960 [6][7]. The responsible contaminating fungus was identified as Aspergillus flavus, and the toxin it produces was named aflatoxin [7]. On the other hand, there are also beneficial secondary metabolites used in the food industry. For example, Monascus spp. pigments have been employed as food colorants in Southeast Asia for a long time [8].
Secondary metabolites serve a wide range of functions for the producing organism [9][10]. In nature, different organisms share the same environment and live in communities. These compounds have roles in the interaction of the producing fungus with the surrounding organisms [2]. The fungus might use these compounds to defend itself, compete with the other organisms, as communication signals, and even regulate symbiotic relationships [9][10]. These compounds might also serve as virulence factors for animal and plant pathogens [9]. Moreover, secondary metabolites have roles in fungal development; for example, proper formation of the fruiting bodies or spore germination depends on the produced metabolites [2].
Secondary metabolites are generally grouped into three major categories: acyl-CoA-derived polyketides, acyl-CoA-derived terpenes, and amino acid-derived peptides [2]. A combination of these is also commonly observed, such as alkaloids, meroterpenoids, and peptide–polyketide hybrids [11]. The genes involved in the biosynthesis of secondary metabolites are generally present as clusters in the genomes and are often co-regulated [2]. A secondary metabolite biosynthetic gene cluster primarily harbors a core gene encoding an enzyme synthesizing the backbone of the secondary metabolite, such as a polyketide synthase, a non-ribosomal peptide synthetase, or a terpene synthase or a terpene cyclase, in addition to genes, products of which, are used in further modification of the backbone [2]. The contiguous configuration of the biosynthetic genes allows for the regulation of the secondary metabolite biosynthesis epigenetically. Epigenetic regulation involves tightly or loosely packed chromatin by histone modifications that change the chromatin’s availability to the transcriptional machinery [12]. Some clusters also harbor a transcription factor with binding sites located at the promoters of the cluster genes [2]. Environmental signals, including temperature, light, pH, solid/liquid medium, heat and oxidative stress, and nutritional factors, regulate the production of secondary metabolites [13].
Penicillium roqueforti is the filamentous fungal species responsible for the characteristic blue-green color of blue cheeses, produced under different names in different countries, such as Roquefort (France), Gorgonzola (Italy), Stilton (UK), Cabrales (Spain), and Danablue (Denmark) [14]. All of these cheeses were given Protected Designation of Origin/Protected Geographical Indication (PDO/PGI) status [15]. In the production of blue cheeses, P. roqueforti spores are either intentionally added to the milk or curd as a secondary starter or ripening culture or grow on the cheese spontaneously in the humid and cool atmosphere of the ripening cellars or caves [16]. After 2–3 weeks, the blue color appears in the cheese veins [17]. P. roqueforti not only gives the characteristic color of blue cheeses but also takes a significant role in aroma development with its biochemical activities [18]. While this species is a starter culture in blue cheeses, it is also a food contaminant in dairy products and rye bread and has been observed in other environments, such as silage and wood [19][20]. In addition, P. roqueforti has been proposed to have a cell factory potential for producing high-value-added molecules with its convenient fermentation properties, such as a wide pH tolerance and ability to metabolize both pentoses and hexoses [21].
P. roqueforti has been reported to produce a variety of secondary metabolites, e.g., roquefortine C, isofumigaclavines, mycophenolic acid, andrastin A, PR-toxin, citreoisocoumarin, and orsellinic acid [22][23][24]. The metabolites of cheese-associated fungi, including P. roqueforti, have been reviewed previously [18][25][26]. There are also reviews specifically on the secondary metabolites of P. roqueforti [27][28]. Garcia-Estrada and Martin [27] concentrated on biosynthesis and the corresponding gene clusters of roquefortine C, PR toxin, and mycophenolic acid. Chavez et al. [28] encompassed a broader spectrum of compounds and, notably, offered valuable insights into regulatory mechanisms.
2. Secondary Metabolites of P. roqueforti
2.1. Roquefortine C
Roquefortine C, first described to be isolated from P. roqueforti in 1975 [29][30], is produced by at least 30 Penicillium species associated with different environments [31]. This compound belongs to a group of secondary metabolites called indole alkaloids [32]. Roquefortine C has neurotoxic properties, as observed in mice and cockerels [30][33]. The compound was also detected in intoxication cases in dogs and cattle [34][35]. While an LD50 value (the dose causing the death of 50% of the tested animals) of 15–20 mg/kg was reported in mice in one study [30], another study reported 169–189 mg/kg in two different mice strains [36]. Cytotoxicity of roquefortine C on Caco-2 cells determined a 50% inhibition concentration (IC50) of 48 µg/mL in one study [37] and >100 µM in another study [38]. The mechanism of toxicity is not precisely known, but the inhibition of rat and human cytochrome P450 enzymes by this compound was reported [39].
In addition to the neurotoxic effects, roquefortine C has antimicrobial properties against Gram-positive bacteria, such as Staphylococcus aureus and some Bacillus species [40]. The mechanism of the antibacterial effect could be the impairment of RNA synthesis [41]. On the other hand, the growth of lactic acid bacteria was only insignificantly impaired in the presence of roquefortine C [40]. During cheese ripening, the production of this metabolite might be useful for controlling unwanted bacterial growth while lactic acid bacteria remain unaffected.
Roquefortine C is produced by almost all of the studied P. roqueforti strains [42][43]. Production on solid substrates is much higher than in cheese [26]. The roquefortine C concentration in cheeses varies considerably; the average concentration was 858 ± 1670 µg/kg, determined using 83 blue cheese samples [44]. Based on this value and the average blue cheese consumption levels in France, a 21-day exposure representing chronic conditions was investigated on Caco-2 cells [38]. Chronic exposure did not change the intestinal barrier, suggesting that the amounts taken with blue cheese consumption do not cause significant toxicological effects [38].
Roquefortine C biosynthesis begins with the condensation of L-tryptophane and L-histidine to form a cyclodipeptide with a diketopiperazine ring by the action of a nonribosomal peptide synthetase (NRPS), named roquefortine dipeptide synthetase (RDS) (Figure 1A) [45]. After the formation of the cyclodipeptide, there is a grid in the biochemical pathway. The resulting dipeptide might first be prenylated to produce roquefortine D and dehydrogenated, or the pathway proceeds with dehydrogenation first and then prenylation to yield roquefortine C [31][46]. The relative amounts of the metabolites in the deletion strains in P. chrysogenum (renamed as P. rubens) indicate that the former path is the predominant one [46]. Some fungal species can convert roquefortine C to metabolites, such as meleagrin, oxaline, and neoxaline [31]. For example, P. chrysogenum produces meleagrin, and the formation of this compound requires three additional steps after roquefortine C is formed [31]. In parallel, the biosynthetic gene clusters responsible for the production of roquefortine C in P. roqueforti and meleagrin in P. chrysogenum are very similar (Figure 1B). However, while the cluster in P. chrysogenum harbors seven genes [46], the P. roqueforti cluster is a shorter version [47]. It has been suggested that the biosynthetic gene cluster for roquefortine C (roq) in P. roqueforti harbors four genes [47]. These genes include gmt (roqN, PROQFM164S01g003515), represented as a white arrow in Figure 1B, rds (roqA), which encodes a nonribosomal peptide synthetase, rdh (roqR), responsible for encoding a dehydrogenase, and rpt (roqD), which codes for a roquefortine prenyltransferase. However, between P. roqueforti FM164 and P. chrysogenum Wisconsin 54-1255 genomes, three stretches of high identity, >85%, are observed; one corresponds to the rpt (roqD) gene, while the other long stretch harbors rdh (roqR) and rds (roqA) (Figure 1B). There is also a tiny stretch at the end of the analyzed regions. The roq clusters in the two P. roqueforti genomes, FM164 and CECT 2905, are 99.2% identical, as indicated by the bright red color of the Artemis Comparison Tool (ACT) in the entire region analyzed (Figure 1B). PROQFM164_S01g003515 (old name: Proq01g022760), shown with a white arrow in Figure 1B, was mentioned to be part of the P. roqueforti cluster as a remnant homolog of the methyltransferase gene, gmt (roqN), in P. chrysogenum [47]. However, the low sequence identity (22%) of this gene with gmt (roqN, Pc21g15440) and its higher sequence identity (79%) with another gene (Pc21g15540) located ~29 kb downstream in the P. chrysogenum genome (Figure S1), suggest that it is not part of the cluster. Therefore, it is likely that the P. roqueforti roquefortine C cluster comprises only three genes. Silencing of rds and rpt genes in P. roqueforti reduced roquefortine C production, indicating these genes’ involvement in biosynthesis [47]. In P. chrysogenum, deletions of the genes in the cluster identified their functions in the pathway. The three genes that are absent in the P. roqueforti gene cluster, nox (roqM), sro (roqO), and gmt (roqN) in P. chrysogenum take part in the formation of meleagrin from roquefortine C [46]. An MFS transporter gene, roqT, in P. chrysogenum is also absent in P. roqueforti. RoqT deletion in P. chrysogenum did not inhibit the production and secretion of meleagrin; however, the concentrations of the intermediate compounds were altered [46]. RoqT in P. chrysogenum might facilitate the transport of the intermediates within the cell or the secretion of the final product, which would otherwise occur by passive transport or by an alternative transporter.

Figure 1. Roquefortine C biosynthesis and the associated gene clusters. (A) Roquefortine C biosynthetic pathway. The thicker arrows indicate the predominant path in the metabolic grid in P. chrysogenum. While the pathway stops at roquefortine C in P. roqueforti, the end product is meleagrin in P. chrysogenum, as indicated in the blue rectangle. (B) Roquefortine C biosynthesis gene clusters in P. roqueforti CECT 2905 and FM164, and P. chrysogenum (P. rubens) Wisconsin 54-1255. The sequence comparison file for the gene clusters was generated using BLAST and used in the Artemis Comparison Tool (ACT) Release 18.2.0 to obtain a visual image. ACT indicates high sequence similarity in red and blue colors representing forward and reverse matches, respectively, with the color intensity proportional to the percent sequence identity. P. roqueforti CECT 2905 roquefortine cluster is in contig 377. P. roqueforti FM164 gene IDs are as follows: rpt (roqD): PROQFM164_S01g003515, rdh (roqR): PROQFM164_S01g003516, rds (roqA): PROQFM164_S01g003517. P. chrysogenum Wisconsin 54-1255 gene IDs: roqT: Pc21g15420, rpt (roqD): Pc21g15430, gmt (roqN): Pc21g15440, sro (roqO): Pc21g15450, nox (roqM): Pc21g15460, rdh (roqR): Pc21g15470, rds (roqA): Pc21g15480. DMAPP: dimethylpyrophosphate.
2.2. Clavine Alkaloids
P. roqueforti was reported to produce clavine-type indole alkaloids, isofumigaclavine A [23][42][43][48][49][50], isofumigaclavine B [43][48], festuclavine [24][29], and agroclavine [24][51]. Because of the structural resemblance of clavine alkaloids to the neurotransmitters dopamine, serotonin, and adrenaline, they have diverse pharmacological properties [52]. Agroclavine was reported to enhance natural killer cell activity, an immunostimulatory effect [53]. In blue cheeses, limited amounts of isofumigaclavine A (<5 ppm) and only traces of isofumigaclavine B were reported [48]. The relatively low amounts might be attributed to the low oxygen conditions in cheese that do not allow the high number of oxidation steps in the biosynthetic pathway (Figure 2A) [18].
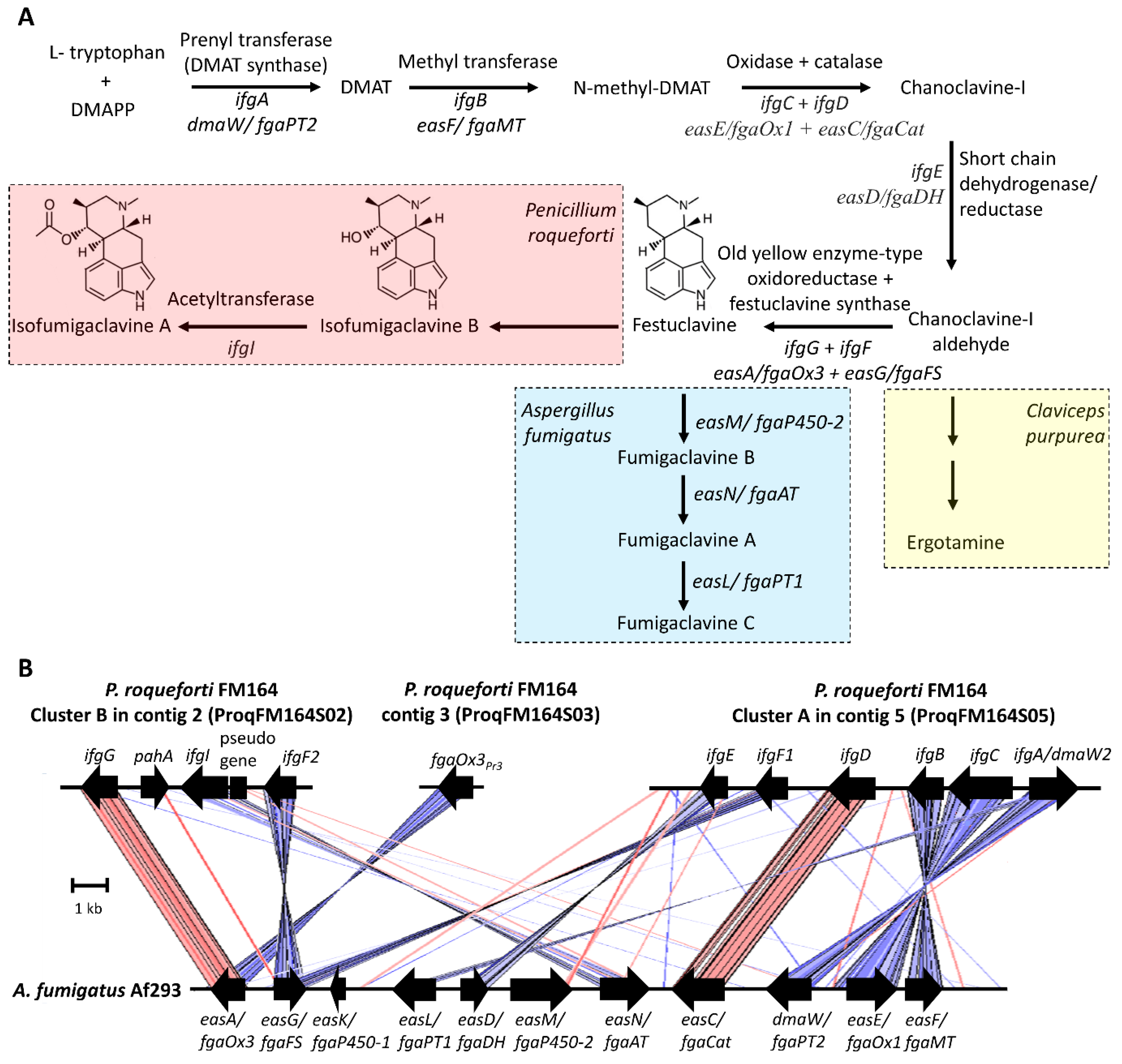
Figure 2. Biosynthesis of clavine alkaloids. (A) Biosynthetic pathway for the production of clavine alkaloids in Penicillium roqueforti and Aspergillus fumigatus and ergotamine in Claviceps purpurea. (B) Gene clusters for the production of clavine alkaloids in P. roqueforti and A. fumigatus. The gene IDs in the P. roqueforti FM164 genome are as follows; cluster A genes: ifgA/dmaW2 (PROQFM164_S05g000511), ifgC (PROQFM164_S05g000510), ifgB (PROQFM164_S05g000509), ifgD (PROQFM164_S05g000508), ifgF1 (PROQFM164_S05g000507), ifgE (PROQFM164_S05g000506); cluster B genes: ifgG (PROQFM164_S02g000300), pahA (PROQFM164_S02g000301), ifgI (PROQFM164_S02g000302), pseudogene (PROQFM164_S02g000303), ifgF2 (PROQFM164_S02g000304). The old yellow enzyme gene homolog fgaOx3Pr3 unlinked to cluster A or B: PROQFM164_S03g000127 (CDM33403). The A. fumigatus Af293 cluster was redrawn based on Bilovol et al. and Wallwey et al. The color intensity created by ACT is proportional to the percent sequence identity. Red and blue colors represent forward and reverse matches, respectively. DMAPP—dimethylpyrophosphate, DMAT—dimethylallyltryptophan.
Clavine alkaloid biosynthesis has been largely elucidated in Aspergillus fumigatus (Neosartorya fumigata), which produces festuclavine and fumigaclavines [54]. The biosynthesis of clavine alkaloids starts with the condensation of tryptophan and dimethylpyrophosphate (DMAPP) by a prenyltransferase enzyme called dimethylallyltryptophan (DMAT) synthase (DmaW/FgaPT2) (Figure 2) [55]. The produced DMAT is methylated by a methyltransferase (EasF/FgaMT) [56], and by the action of at least two enzymes, an oxidase (EasE/fgaOx1), and a catalase (EasC/fgaCat), chanoclavine I, is produced [57][58]. After that, a short-chain dehydrogenase/reductase (EasD/FgaDH) converts chanoclavine I to chanoclavine I aldehyde [59]. From this point on, different fungi produce different metabolites using the intermediate chanoclavine I aldehyde. For example, while Claviceps purpurea produces the ergot alkaloid ergotamine, A. fumigatus produces fumigaclavines via festuclavine (Figure 2A) [60]. P. roqueforti, on the other hand, produces festuclavine similar to A. fumigatus; however, festuclavine is converted into isofumigaclavines. In the production of festuclavine from chanoclavine I aldehyde, two enzymes, one, a member of the old yellow enzyme family of oxidoreductases (EasA/FgaOx3), and the other, festuclavine synthase (EasG/FgaFS), are involved [60][61][62]. In A. fumigatus, festuclavine is hydroxylated by EasM/fgaP450-2) to fumigaclavine B [63], which is later acetylated by EasN/FgaAT to produce fumigaclavine A [64]. This compound is prenylated once more by easL/fgaPT1 and yields fumigaclavine C [65].
The discovery of the clavine alkaloids biosynthetic cluster in P. roqueforti started with the finding of a second prenyltransferase gene (ifgA/dmaW2, proq05g069320 [new ID: PROQFM164_S05g000511]), different from rpt involved in roquefortine biosynthesis [66]. The silencing of ifgA resulted in the suppression of isofumigaclavine A and three other intermediate products in P. roqueforti [67]. Interestingly, ifgA silencing leads to an increase in roquefortine C production [67], which might be the result of the unused precursors, tryptophan and DMAPP, directed to this pathway. IfgA was found in a cluster of six genes (cluster A) (Figure 2B). There is a methyltransferase gene in the cluster, ifgB, the product of which presumably methylates DMAT to produce N-methyl-DMAT [67]. The FAD-binding oxidoreductase (ifgC) and the catalase (ifgD) gene products are the putative enzymes that produce chanoclavine I [67]. The gene ifgE is a short-chain dehydrogenase/reductase that corresponds to the enzyme catalyzing the production of chanoclavine I aldehyde from chanoclavine [67]. The final gene in this cluster is a festuclavine synthase ortholog, named efgF1. In cluster A, only the function of the ifgA/dmaW2 gene in clavine alkaloid biosynthesis has been demonstrated experimentally [67].
Because cluster A in contig 2 did not involve the gene for the old yellow enzyme family necessary for festuclavine production, the authors looked for this gene (ifgG) in the genome. They found one in a second cluster in contig 5 (Figure 2B) [67]. This cluster, cluster B, has three additional genes, another copy of the festuclavine synthase gene, efgF2, and an acetyltransferase gene, ifgI, which putatively catalyzes the acetylation of isofumigaclavine B to isofumigaclavine A, similar to the corresponding enzyme EasN/FgaAT in A. fumigatus that acetylates fumigaclavine B to fumigaclavine A [64]. There is also a phytanoyl-CoA hydroxylase gene, pahA, in cluster B [54][67]. While this gene is not present in the A. fumigatus cluster, it is found in some ergot alkaloid and clavine alkaloid gene clusters in different fungi [54]. For example, in C. purpurea, EasH, a phytanoyl-CoA hydroxylase gene product, functions as a cyclolizing dioxygenase in ergopeptine synthesis [68]. Whether this gene in P. roqueforti has a function in clavine biosynthesis is unknown. In addition, the roles of the cluster B genes in the biosynthesis of clavine alkaloids in P. roqueforti have yet to be shown experimentally.
Meanwhile, Gerhards and Li [60] reported an additional old yellow enzyme gene homolog (CDM33403, named FgaOx3PR3) in contig 3 of the P. roqueforti genome, unlinked to cluster A or B (Figure 2B). They cloned this gene, overexpressed the corresponding protein, and demonstrated the activity of converting chanoclavine I aldehyde to festuclavine in the presence of festuclavine synthase of A. fumigatus. The enzyme also increased the conversion yield of chanoclavine I to chanoclavine aldehyde, the previous step in the biosynthetic pathway, catalyzed by a short-chain dehydrogenase/reductase. The authors reported an amino acid-level sequence identity of 51% between FgaOx3PR3 and IfgG, the old yellow enzyme in cluster B [60]. Meanwhile, A. fumigatus EasA/FgaOx3 amino acid sequence identity is 67.4% and 53.9% to IfgG and FgaOx3PR3, respectively. The higher sequence identity between EasA/FgaOx3 and IfgG suggests that the enzyme responsible for festuclavine synthesis might be IfgG with a better activity towards chanoclavine-I aldehyde, which remains to be explored.
While in A. fumigatus, the genes (n = 11) responsible for the clavin biosynthesis pathway are grouped contiguously in a single cluster, the biosynthesis in P. roqueforti, is apparently associated with more than one gene cluster (Figure 2B) [54]. Generally, for ergot alkaloid biosynthesis in ascomycetes, this is not an uncommon configuration; a single cluster or two unlinked clusters can be involved in biosynthesis [69]. The conservation of the genes responsible for chanoclavine synthesis (ifgA/dmaW2, ifgC, ifgB, and ifgD) as a block between P. roqueforti and A. fumigatus except a single inversion is remarkable (Figure 2B). Eight of the eleven genes in the A. fumigatus cluster have homologs in P. roqueforti cluster A or B. One of the three missing genes is easL/fgaPT1, the product of which prenylates fumigaclavine A to yield fumigaclavine C (Figure 2B). Because in P. roqueforti, the pathway stops at isofumigaclavine A, this gene is not expected. The second missing gene is easK/fgaP450-1, whose function, if any, is unknown in A. fumigatus. The third gene is easM, the product of which is responsible for hydroxylating festuclavine. A homologous gene is not present in the P. roqueforti cluster A or B and might be elsewhere in the genome. When the A. fumigatus EasM amino acid sequence (Genbank ID: Q4WZ65.1) was used to search the genomes of P. roqueforti strains FM164 and LCP96 04111, different hits were obtained with a percent identity between 45–23%. How the hydroxylation reaction is catalyzed in P. roqueforti awaits further research.
2.3. Mycophenolic Acid
Mycophenolic acid is one of the main secondary metabolites P. roqueforti produces [22]. The compound was discovered by an Italian physician, Bartolomeo Gosio, in 1893 while he was studying spoiled corn to find an agent causing pellagra [70]. At those times, pellagra was believed to be associated with a toxic substance rather than a vitamin deficiency as we know it today. Gosio isolated a fungus from spoiled corn and purified a compound from the culture filtrate of this fungus. This compound had phenolic properties and stopped the growth of “anthrax bacillus” [71][72]. Later, in 1913, Alsberg and Black [73] isolated an acidic compound from a corn-spoilage Penicillium and named it mycophenolic acid. When Clutterbuck et al. conducted extensive studies on the metabolic products of Penicillium brevicompactum in 1932, they concluded that the compounds Gosio, Alsberg, and Black isolated was one and the same [74]. Because of Gosio’s work, mycophenolic acid was prized later as the first fungal antibiotic crystallized [75].
After the first report on its activity against Bacillus anthracis by Gosio [70], mycophenolic acid was later shown to have antibacterial [75][76], antifungal [75][77], antiviral [78], antipsoriasis [79][80], and antitumor [81] properties. However, mycophenolic acid is used in medicine today primarily because of its immunosuppressive activity [72]. Two prodrugs of mycophenolic acid, mycophenolate mofetil (CellCept®, Roche Pharma, Basel, Switzerland) and mycophenolic acid sodium salt (Myfortic®, Novartis Pharma, Basel, Switzerland), are used as immunosuppressants in transplantation to prevent organ rejection and to treat autoimmune diseases [82]. Mycophenolic acid acts as an inhibitor of the inosine 5′-monophosphate dehydrogenase (IMPDH), an enzyme taking the role of purine monophosphate formation during DNA synthesis [83]. The compound inhibits the proliferation of B- and T-lymphocytes, whose DNA synthesis is mainly dependent on this de novo synthesis pathway [83]. Other cells use both de novo synthesis and a salvage pathway that recycles the purines; therefore, the compound explicitly affects the B- and T-lymphocytes, a highly beneficial feature for an immunosuppressant [72][83].
A long-term toxicity study in rabbits given 80 and 320 mg/kg of mycophenolic acid orally for one year showed no apparent toxicity sign [84]. In rats and mice, relatively high concentrations were needed for toxicity. For example, LD50 for rats and mice are 2500 and 700 mg/kg (oral), and 500 and 450 mg/kg (intravenous), respectively [85]. However, using the compound in immunosuppressive therapy revealed some gastrointestinal side effects, such as diarrhea, abdominal pain, mucosal changes, and submucosal inflammation, which indicate gastrointestinal toxicity [86]. The IC50 value of mycophenolic acid on Caco-2 cells representing cytotoxicity was 780 µM, the solubility limit of the compound, indicating that even at this concentration, more than 50% of the cells were viable [38]. In blue cheeses, the average mycophenolic acid concentration was reported to be 841 ± 1271 µg/kg [44]. Chronic exposure (21-day experiment) on Caco-2 cells based on the value attained by ingesting the highest contaminated cheese led to decreased intestinal barrier function [38]. However, this decrease did not result in bacterial passage. Regarding mutagenicity, the Ames test conducted on Salmonella Typhimurium strains using mycophenolic acid up to 400 µg/plate did not result in mutagenic activity [87]. Nevertheless, a study on mouse mammary carcinoma cell line FM3A indicated mycophenolic acid-induced mutations at 0.032 and 0.1 µg/mL and chromosome aberrations at 0.1 µg/mL [88].
Mycophenolic acid is a meroterpenoid, a term first used in 1968 [89], with the prefix mero meaning part, partial, or fragment [90], to describe compounds that are of mixed biosynthetic origin and derived in part from terpenoids [91]. Mycophenolic acid is a polyketide–terpenoid hybrid compound (Figure 3A) [92]. Mycophenolic acid biosynthesis in P. roqueforti is governed by a seven-gene cluster spanning 24.4 kb (Figure 3B) [93], similar in structure to the corresponding cluster in P. brevicompactum [94][95]. The silencing of each of the seven genes in the mycophenolic acid biosynthetic gene cluster of P. roqueforti resulted in significant reductions in mycophenolic acid [93]. The mycophenolic acid biosynthetic pathway has been elucidated with studies involving isotope labeling, gene silencing, and heterologous expression, mainly conducted in P. brevicompactum [92][94][95][96][97][98]. The biosynthesis begins with the formation of the tetraketide 5-methylorsellinic acid (5-MOA) from one acetyl-CoA, three units of malonyl-CoA, and one S-adenosyl-L-methionine (SAM) with the action of a polyketide synthase, coded by mpaC (Figure 3A) [93][94]. 5-MOA is then hydroxylated to produce 4,6-dihydroxy-2-(hydroxymethyl)-3-methylbenzoic acid (DHMB), and the ring is closed to form 5,7-dihydroxy-4-methylphthalide (DHMP). These two reactions are catalyzed by a bifunctional enzyme coded by the mpaDE gene having a P450 monooxygenase and a hydrolase domain [95]. After that, a mpaA-encoded prenyltransferase adds a farnesyl, a 15-carbon isoprenoid, group to DHMP to yield 6-farnesyl-5,7-dihydroxy-4-methylphthalide (FDHMP) [92][96]. Oxidative cleavage of the farnesyl side chain by an oxygenase, coded by mpaB, yields a three-fewer carbon atom containing FDHMP-3C, which is later methylated by an O-methyltranferase, encoded by mpaG, to produce MFDHMP-3C [96]. A recent fascinating discovery demonstrated the compartmentalized nature of mycophenolic acid biosynthesis [94]. While the steps until the production of MFDHMP-3C take place in the cytoplasm, MFDHMP-3C enters into the peroxisome and CoA-ligated, followed by β-oxidation-mediated chain shortening to yield mycophenolic acid-CoA [96]. An acyl-CoA hydrolase, coded by mpaH, hydrolyzes the mycophenolic acid-CoA to prevent further oxidation and yields mycophenolic acid [96][99]. There is also a gene, mpaF, coding for a mycophenolic acid-insensitive IMPDH, which has been suggested to confer self-resistance [93][100].

Figure 3. Mycophenolic acid biosynthetic pathway (A) Biosynthesis of mycophenolic acid (B) Mycophenolic acid biosynthetic gene cluster of P. roqueforti CECT 2905 and FM164. The gene IDs in the FM164 genome are as follows: mpaA (PROQFM164_S05g000561), mpaB (PROQFM164_S05g000560), mpaC (PROQFM164_S05g000559), mpaDE (PROQFM164_S05g000557), mpaF (PROQFM164_S05g000556), mpaG (PROQFM164_S05g000555), mpaH (PROQFM164_S05g000554). SAM—S-adenosyl-L-methionine, 5-MOA—5-methylorsellinic acid, DHMB—4,6-dihydroxy-2-(hydroxymethyl)-3-methylbenzoic acid, DHMP—5,7-dihydroxy-4-methylphthalide, FDHMP—6-farnesyl-5,7-dihydroxy-4-methylphthalide, FDHMP-3C—three-fewer carbon atom containing FDHMP, MFDHMP-3C—methylated FDHMP-3C, MPA—mycophenolic acid.
Mycophenolic acid production in P. roqueforti was reported to be strain-dependent [43][101]. In addition, some P. roqueforti strains were demonstrated to have a 174 bp deletion in the 3′-end of the mpaC gene, leading to a decrease in the protein length by 14 amino acids [102]. The strains with shorter mpaC produced low or no mycophenolic acid, suggesting a correlation between the deletion and the reduction or the absence of mycophenolic acid production [102]. However, even among the strains with no deletion on mpaC, significant differences in mycophenolic acid production were observed [102]; therefore, more factors, such as sequence variations in cluster genes or their regulatory regions or on a regulatory gene outside the cluster, are likely to be involved in the regulation of strain-dependent production. Although the mycophenolic acid cluster does not harbor a regulatory gene, several regulator genes for secondary metabolites were identified in P. roqueforti [103][104][105].
In addition to its variability, the mycophenolic acid production of P. roqueforti is lower than that of P. brevicompactum [106], known to be a good producer, as high as 6700 mg/L [107]. However, using a P. roqueforti mutant strain exposed to Gamma radiation and optimizing the fermentation media, El-Sayed and Zaki were able to increase the yield, which is of relevance in industrial production, up to 2933 mg/L [108].
References
- Brakhage, A.A. Regulation of Fungal Secondary Metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32.
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180.
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal Secondary Metabolism—From Biochemistry to Genomics. Nat. Rev. Microbiol. 2005, 3, 937–947.
- Conrado, R.; Gomes, T.C.; Roque, G.S.; De Souza, A.O. Overview of Bioactive Fungal Secondary Metabolites: Cytotoxic and Antimicrobial Compounds. Antibiotics 2022, 11, 1604.
- Wang, W.; Liang, X.; Li, Y.; Wang, P.; Keller, N.P. Genetic Regulation of Mycotoxin Biosynthesis. J. Fungi 2023, 9, 21.
- Lancaster, M.C.; Jenkins, F.P.; Philp, J.M. Toxicity Associated with Certain Samples of Groundnuts. Nature 1961, 192, 1095–1096.
- Nesbitt, B.F.; O’Kelly, J.; Sargeant, K.; Sheridan, A. Aspergillus flavus and Turkey X Disease: Toxic Metabolites of Aspergillus flavus. Nature 1962, 195, 1062–1063.
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56.
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392.
- Demain, A.L.; Fang, A. The Natural Functions of Secondary Metabolites. In History of Modern Biotechnology I; Fiechter, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–39. ISBN 978-3-540-44964-5.
- Skellam, E. Subcellular Localization of Fungal Specialized Metabolites. Fungal Biol. Biotechnol. 2022, 9, 11.
- Pfannenstiel, B.T.; Keller, N.P. On Top of Biosynthetic Gene Clusters: How Epigenetic Machinery Influences Secondary Metabolism in Fungi. Microb. Eng. Biotechnol. 2019, 37, 107345.
- Yu, W.; Pei, R.; Zhou, J.; Zeng, B.; Tu, Y.; He, B. Molecular Regulation of Fungal Secondary Metabolism. World J. Microbiol. Biotechnol. 2023, 39, 204.
- Metin, B. Filamentous Fungi in Cheese Production. In Microbial Cultures and Enzymes in Dairy Technology; Öztürkoğlu Budak, Ş., Akal, H.C., Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 257–275. ISBN 978-1-522-55363-2.
- Morales, M.B.L.; Ardö, Y.; Berthier, F.; Karatzas, K.-A.G.; Bintsis, T. Blue-Veined Cheeses. In Global Cheesemaking Technology: Cheese Quality and Characteristics; Papademas, P., Bintsis, T., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 415–435. ISBN 978-1-119-04616-5.
- Cantor, M.D.; van den Tempel, T.; Hansen, T.K.; Ardö, Y. Blue Cheese. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Academic Press: Cambridge, MA, USA, 2004; Volume 2, pp. 175–198. ISBN 978-0-122-63653-0.
- Gripon, J.C. Mould-Ripened Cheeses. In Cheese: Chemistry, Physics and Microbiology; Springer: Boston, MA, USA, 1993; pp. 111–136. ISBN 978-1-4613-6137-4.
- Martín, J.F.; Coton, M. Chapter 12—Blue Cheese: Microbiota and Fungal Metabolites. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 275–303. ISBN 978-0-12-802309-9.
- Frisvad, J.C.; Samson, R.A. Polyphasic Taxonomy of Penicillium Subgenus Penicillium. A Guide to Identification of Food and Air-Borne Terverticillate Penicillia and Their Mycotoxins. Stud. Mycol. 2004, 49, C174.
- Sumarah, M.W.; Miller, J.D.; Blackwell, B.A. Isolation and Metabolite Production by Penicillium roqueforti, P. paneum and P. crustosum Isolated in Canada. Mycopathologia 2005, 159, 571–577.
- Mioso, R.; Toledo Marante, F.J.; Herrera Bravo de Laguna, I. Penicillium roqueforti: A Multifunctional Cell Factory of High Value-added Molecules. J. Appl. Microbiol. 2015, 118, 781–791.
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, Drugs and Other Extrolites Produced by Species in Penicillium Subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241.
- O’Brien, M.; Nielsen, K.F.; O’Kiely, P.; Forristal, P.D.; Fuller, H.T.; Frisvad, J.C. Mycotoxins and Other Secondary Metabolites Produced in Vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom Isolated from Baled Grass Silage in Ireland. J. Agric. Food Chem. 2006, 54, 9268–9276.
- Nielsen, K.F.; Sumarah, M.W.; Frisvad, J.C.; Miller, J.D. Production of Metabolites from the Penicillium roqueforti Complex. J. Agric. Food Chem. 2006, 54, 3756–3763.
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.L.; Barbier, G.; Coton, E. Filamentous Fungi and Mycotoxins in Cheese: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456.
- Martín, J.F.; Liras, P. Secondary Metabolites in Cheese Fungi. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 293–315. ISBN 978-3-319-25001-4.
- García-Estrada, C.; Martín, J.-F. Biosynthetic Gene Clusters for Relevant Secondary Metabolites Produced by Penicillium roqueforti in Blue Cheeses. Appl. Microbiol. Biotechnol. 2016, 100, 8303–8313.
- Chávez, R.; Vaca, I.; García-Estrada, C. Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms. J. Fungi 2023, 9, 459.
- Ohmomo, S.; Sato, T.; Utagawa, T.; Abe, M. Isolation of Festuclavine and Three New Indole Alkaloids, Roquefortine A, B and C from the Cultures of Penicillium roqueforti. Agric. Biol. Chem. 1975, 39, 1333–1334.
- Scott, P.M.; Merrien, M.-A.; Polonsky, J. Roquefortine and Isofumigaclavine A, Metabolites From Penicillium roqueforti. Experientia 1976, 32, 140–142.
- Martín, J.F.; Liras, P. Evolutionary Formation of Gene Clusters by Reorganization: The Meleagrin/Roquefortine Paradigm in Different Fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1579–1587.
- Martín, J.-F.; Liras, P.; García-Estrada, C. Roquefortine C and Related Prenylated Indole Alkaloids. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., García-Estrada, C., Zeilinger, S., Eds.; Springer New York: New York, NY, USA, 2014; pp. 111–128. ISBN 978-1-4939-1191-2.
- Wagener, R.E.; Davis, N.D.; Diener, U.L. Penitrem A and Roquefortine Production by Penicillium commune. Appl. Environ. Microbiol. 1980, 39, 882–887.
- Lowes, N.; Smith, R.; Beck, B. Roquefortine in the Stomach Contents of Dogs Suspected of Strychnine Poisoning in Alberta. Can. Vet. J. Rev. Vét. Can. 1992, 33, 535–538.
- Häggblom, P. Isolation of Roquefortine C from Feed Grain. Appl. Environ. Microbiol. 1990, 56, 2924–2926.
- Arnold, D.L.; Scott, P.M.; McGuire, P.F.; Harwig, J.; Nera, E.A. Acute Toxicity Studies on Roquefortine and PR Toxin, Metabolites of Penicillium roqueforti, in the Mouse. Food Cosmet. Toxicol. 1978, 16, 369–371.
- Rasmussen, R.R.; Rasmussen, P.H.; Larsen, T.O.; Bladt, T.T.; Binderup, M.L. In Vitro Cytotoxicity of Fungi Spoiling Maize Silage. Food Chem. Toxicol. 2011, 49, 31–44.
- Hymery, N.; Mounier, J.; Coton, E. Effect of Penicillium roqueforti Mycotoxins on Caco-2 Cells: Acute and Chronic Exposure. Toxicol. In Vitro 2018, 48, 188–194.
- Aninat, C.; Hayashi, Y.; André, F.; Delaforge, M. Molecular Requirements for Inhibition of Cytochrome P450 Activities by Roquefortine. Chem. Res. Toxicol. 2001, 14, 1259–1265.
- Kopp, B.; Rehm, H.J. Antimicrobial Action of Roquefortine. Eur. J. Appl. Microbiol. Biotechnol. 1979, 6, 397–401.
- Kopp, B.; Rehm, H.J. Studies on the Inhibition of Bacterial Macromolecule Synthesis by Roquefortine, a Mycotoxin from Penicillium roqueforti. Eur. J. Appl. Microbiol. Biotechnol. 1981, 13, 232–235.
- Scott, P.M.; Kennedy, B.P.; Harwig, J.; Blanchfield, B.J. Study of Conditions of Production of Roquefortine and Other Metabolites of Penicillium roqueforti. Appl. Environ. Microbiol. 1977, 33, 249–253.
- Frisvad, J.C.; Filtenborg, O. Terverticillate Penicillia: Chemotaxonomy and Mycotoxin Production. Mycologia 1989, 81, 837–861.
- Fontaine, K.; Passeró, E.; Vallone, L.; Hymery, N.; Coton, M.; Jany, J.-L.; Mounier, J.; Coton, E. Occurrence of Roquefortine C, Mycophenolic Acid and Aflatoxin M1 Mycotoxins in Blue-Veined Cheeses. Food Control 2015, 47, 634–640.
- García-Estrada, C.; Ullán, R.V.; Albillos, S.M.; Fernández-Bodega, M.Á.; Durek, P.; von Döhren, H.; Martín, J.F. A Single Cluster of Coregulated Genes Encodes the Biosynthesis of the Mycotoxins Roquefortine C and Meleagrin in Penicillium chrysogenum. Chem. Biol. 2011, 18, 1499–1512.
- Ali, H.; Ries, M.I.; Nijland, J.G.; Lankhorst, P.P.; Hankemeier, T.; Bovenberg, R.A.L.; Vreeken, R.J.; Driessen, A.J.M. A Branched Biosynthetic Pathway Is Involved in Production of Roquefortine and Related Compounds in Penicillium chrysogenum. PLoS ONE 2013, 8, e65328.
- Kosalková, K.; Domínguez-Santos, R.; Coton, M.; Coton, E.; García-Estrada, C.; Liras, P.; Martín, J.F. A Natural Short Pathway Synthesizes Roquefortine C but Not Meleagrin in Three Different Penicillium roqueforti Strains. Appl. Microbiol. Biotechnol. 2015, 99, 7601–7612.
- Scott, P.M.; Kennedy, B.P.C. Analysis of Blue Cheese for Roquefortine and Other Alkaloids from Penicillium roqueforti. J. Agric. Food Chem. 1976, 24, 865–868.
- Boysen, M.; Skouboe, P.; Frisvad, J.; Rossen, L. Reclassification of the Penicillium roqueforti Group into Three Species on the Basis of Molecular Genetic and Biochemical Profiles. Microbiology 1996, 142, 541–549.
- Hong, S.L.; Robbers, J.E. Genetics of Ergoline Alkaloid Formation in Penicillium roqueforti. Appl. Environ. Microbiol. 1985, 50, 558–561.
- Vinokurova, N.; Boichenko, D.; Baskunov, B.; Zelenkova, N.; Vepritskaya, I.; Arinbasarov, M.; Reshetilova, T. Minor Alkaloids of the Fungus Penicillium roqueforti Thom 1906. Appl. Biochem. Microbiol. 2001, 37, 184–187.
- McCabe, S.R.; Wipf, P. Total Synthesis, Biosynthesis and Biological Profiles of Clavine Alkaloids. Org. Biomol. Chem. 2016, 14, 5894–5913.
- Starec, M.; Fiserova, A.; Rosina, J.; Malek, J.; Krsiak, M. Effect of Agroclavine on NK Activity In Vivo under Normal and Stress Conditions in Rats. Physiol. Res. Acad. Sci. Bohemoslov. 2001, 50, 513–519.
- Martín, J.F.; Álvarez-Álvarez, R.; Liras, P. Clavine Alkaloids Gene Clusters of Penicillium and Related Fungi: Evolutionary Combination of Prenyltransferases, Monooxygenases and Dioxygenases. Genes 2017, 8, 342.
- Unsöld, I.A.; Li, S.-M. Overproduction, Purification and Characterization of FgaPT2, a Dimethylallyltryptophan Synthase from Aspergillus fumigatus. Microbiology 2005, 151, 1499–1505.
- Rigbers, O.; Li, S.-M. Ergot Alkaloid Biosynthesis in Aspergillus fumigatus: Overproduction and Biochemical Characterization of a 4-Dimethylallyltryptophan N-Methyltransferase. J. Biol. Chem. 2008, 283, 26859–26868.
- Ryan, K.L.; Moore, C.T.; Panaccione, D.G. Partial Reconstruction of the Ergot Alkaloid Pathway by Heterologous Gene Expression in Aspergillus nidulans. Toxins 2013, 5, 445–455.
- Goetz, K.E.; Coyle, C.M.; Cheng, J.Z.; O’Connor, S.E.; Panaccione, D.G. Ergot Cluster-Encoded Catalase Is Required for Synthesis of Chanoclavine-I in Aspergillus fumigatus. Curr. Genet. 2011, 57, 201–211.
- Wallwey, C.; Matuschek, M.; Li, S.-M. Ergot Alkaloid Biosynthesis in Aspergillus fumigatus: Conversion of Chanoclavine-I to Chanoclavine-I Aldehyde Catalyzed by a Short-Chain Alcohol Dehydrogenase FgaDH. Arch. Microbiol. 2010, 192, 127–134.
- Gerhards, N.; Li, S.-M. A Bifunctional Old Yellow Enzyme from Penicillium roqueforti Is Involved in Ergot Alkaloid Biosynthesis. Org. Biomol. Chem. 2017, 15, 8059–8071.
- Wallwey, C.; Matuschek, M.; Xie, X.-L.; Li, S.-M. Ergot Alkaloid Biosynthesis in Aspergillus fumigatus: Conversion of Chanoclavine-I Aldehyde to Festuclavine by the Festuclavine Synthase FgaFS in the Presence of the Old Yellow Enzyme FgaOx3. Org. Biomol. Chem. 2010, 8, 3500–3508.
- Coyle, C.M.; Cheng, J.Z.; O’Connor, S.E.; Panaccione, D.G. An Old Yellow Enzyme Gene Controls the Branch Point between Aspergillus fumigatus and Claviceps purpurea Ergot Alkaloid Pathways. Appl. Environ. Microbiol. 2010, 76, 3898–3903.
- Bilovol, Y.; Panaccione, D.G. Functional Analysis of the Gene Controlling Hydroxylation of Festuclavine in the Ergot Alkaloid Pathway of Neosartorya fumigata. Curr. Genet. 2016, 62, 853–860.
- Liu, X.; Wang, L.; Steffan, N.; Yin, W.-B.; Li, S.-M. Ergot Alkaloid Biosynthesis in Aspergillus fumigatus: FgaAT Catalyses the Acetylation of Fumigaclavine B. ChemBioChem 2009, 10, 2325–2328.
- Unsöld, I.A.; Li, S.-M. Reverse Prenyltransferase in the Biosynthesis of Fumigaclavine C in Aspergillus fumigatus: Gene Expression, Purification, and Characterization of Fumigaclavine C Synthase FGAPT1. ChemBioChem 2006, 7, 158–164.
- Steiner, U.; Ahimsa-Müller, M.A.; Markert, A.; Kucht, S.; Groß, J.; Kauf, N.; Kuzma, M.; Zych, M.; Lamshöft, M.; Furmanowa, M.; et al. Molecular Characterization of a Seed Transmitted Clavicipitaceous Fungus Occurring on Dicotyledoneous Plants (Convolvulaceae). Planta 2006, 224, 533–544.
- Fernández-Bodega, Á.; Álvarez-Álvarez, R.; Liras, P.; Martín, J.F. Silencing of a Second Dimethylallyltryptophan Synthase of Penicillium roqueforti Reveals a Novel Clavine Alkaloid Gene Cluster. Appl. Microbiol. Biotechnol. 2017, 101, 6111–6121.
- Havemann, J.; Vogel, D.; Loll, B.; Keller, U. Cyclolization of D-Lysergic Acid Alkaloid Peptides. Chem. Biol. 2014, 21, 146–155.
- Young, C.A.; Schardl, C.L.; Panaccione, D.G.; Florea, S.; Takach, J.E.; Charlton, N.D.; Moore, N.; Webb, J.S.; Jaromczyk, J. Genetics, Genomics and Evolution of Ergot Alkaloid Diversity. Toxins 2015, 7, 1273–1302.
- Gosio, B. Sperimentate Su Culture Pure Di Bacilli Del Carbonchio Demonstrarano Notevole Potere Antisettica. CR Acad. Med. Torino 1893, 61, 484.
- Bentley, R. Bartolomeo Gosio, 1863–1944: An Appreciation. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2001; Volume 48, pp. 229–250. ISBN 0065-2164.
- Bentley, R. Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant. Chem. Rev. 2000, 100, 3801–3826.
- Alsberg, C.; Black, O.F. Contributions to the Study of Maize Deterioration: Biochemical and Toxicological Investigations of Penicillium puberulum and Penicillium stoloniferum; US Government Printing Office: Washington, DC, USA, 1913.
- Clutterbuck, P.W.; Oxford, A.E.; Raistrick, H.; Smith, G. Studies in the Biochemistry of Micro-Organisms: The Metabolic Products of the Penicillium brevi-compactum Series. Biochem. J. 1932, 26, 1441.
- Florey, H.W.; Jennings, M.A.; Gilliver, K.; Sanders, A.G. Mycophenolic Acid an Antibiotic from Penicillium brevicompactum Dierckx. Lancet 1946, 247, 46–49.
- Abraham, E.P. The Effect of Mycophenolic Acid on the Growth of Staphylococcus aureus in Heart Broth. Biochem. J. 1945, 39, 398–408.
- Kinoshita, H.; Wongsuntornpoj, S.; Ihara, F.; Nihira, T. Anti-Rhodotorula Activity of Mycophenolic Acid Enhanced in the Presence of Polyene Antibiotic Nystatin. Lett. Appl. Microbiol. 2017, 64, 144–149.
- Chan, J.F.W.; Chan, K.-H.; Kao, R.Y.T.; To, K.K.W.; Zheng, B.-J.; Li, C.P.Y.; Li, P.T.W.; Dai, J.; Mok, F.K.Y.; Chen, H.; et al. Broad-Spectrum Antivirals for the Emerging Middle East Respiratory Syndrome Coronavirus. J. Infect. 2013, 67, 606–616.
- Silverman Kitchin, J.E.; Pomeranz, M.K.; Pak, G.; Washenik, K.; Shupack, J.L. Rediscovering Mycophenolic Acid: A Review of Its Mechanism, Side Effects, and Potential Uses. J. Am. Acad. Dermatol. 1997, 37, 445–449.
- Jones, E.L.; Epinette, W.W.; Hackney, V.C.; Menendez, L.; Frost, P. Treatment of Psoriasis With Oral Mycophenolic Acid. J. Investig. Dermatol. 1975, 65, 537–542.
- Domhan, S.; Muschal, S.; Schwager, C.; Morath, C.; Wirkner, U.; Ansorge, W.; Maercker, C.; Zeier, M.; Huber, P.E.; Abdollahi, A. Molecular Mechanisms of the Antiangiogenic and Antitumor Effects of Mycophenolic Acid. Mol. Cancer Ther. 2008, 7, 1656–1668.
- Siebert, A.; Cholewiński, G.; Trzonkowski, P.; Rachon, J. Immunosuppressive Properties of Amino Acid and Peptide Derivatives of Mycophenolic Acid. Eur. J. Med. Chem. 2020, 189, 112091.
- Allison, A.C.; Eugui, E.M. Mycophenolate Mofetil and Its Mechanisms of Action. Immunopharmacology 2000, 47, 85–118.
- Adams, E.; Todd, G.; Gibson, W. Long-Term Toxicity Study of Mycophenolic Acid in Rabbits. Toxicol. Appl. Pharmacol. 1975, 34, 509–512.
- Wilson, B.J. Miscellanous Penicillium Toxins. In Microbial Toxins: A Comprehensive Treatise; Ciegler, A., Kadis, S., Ajl, S.J., Eds.; Academic Press: New York, NY, USA; London, UK, 1971; pp. 460–517.
- Heischmann, S.; Dzieciatkowska, M.; Hansen, K.; Leibfritz, D.; Christians, U. The Immunosuppressant Mycophenolic Acid Alters Nucleotide and Lipid Metabolism in an Intestinal Cell Model. Sci. Rep. 2017, 7, 45088.
- Wehner, F.C.; Thiel, P.G.; van Rensburg, S.J.; Demasius, I.P.C. Mutagenicity to Salmonella Typhimurium of Some Aspergillus and Penicillium Mycotoxins. Mutat. Res. Toxicol. 1978, 58, 193–203.
- Umeda, M.; Tsutsui, T.; Saito, M. Mutagenicity and Inducibility of DNA Single-Strand Breaks and Chromosome Aberrations by Various Mycotoxins. GANN Jpn. J. Cancer Res. 1977, 68, 619–625.
- Cornforth, J. Terpenoid Biosynthesis. Chem. Br. 1968, 4, 102–106.
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997.
- Geris, R.; Simpson, T.J. Meroterpenoids Produced by Fungi. Nat. Prod. Rep. 2009, 26, 1063–1094.
- Chen, X.; Wang, L.; Zhang, J.; Jiang, T.; Hu, C.; Li, D.; Zou, Y. Immunosuppressant Mycophenolic Acid Biosynthesis Employs a New Globin-like Enzyme for Prenyl Side Chain Cleavage. Acta Pharm. Sin. B 2019, 9, 1253–1258.
- Del-Cid, A.; Gil-Durán, C.; Vaca, I.; Rojas-Aedo, J.F.; García-Rico, R.O.; Levicán, G.; Chávez, R. Identification and Functional Analysis of the Mycophenolic Acid Gene Cluster of Penicillium roqueforti. PLoS ONE 2016, 11, e0147047.
- Regueira, T.B.; Kildegaard, K.R.; Hansen, B.G.; Mortensen, U.H.; Hertweck, C.; Nielsen, J. Molecular Basis for Mycophenolic Acid Biosynthesis in Penicillium brevicompactum. Appl. Environ. Microbiol. 2011, 77, 3035–3043.
- Hansen, B.G.; Mnich, E.; Nielsen, K.F.; Nielsen, J.B.; Nielsen, M.T.; Mortensen, U.H.; Larsen, T.O.; Patil, K.R. Involvement of a Natural Fusion of a Cytochrome P450 and a Hydrolase in Mycophenolic Acid Biosynthesis. Appl. Environ. Microbiol. 2012, 78, 4908–4913.
- Zhang, W.; Du, L.; Qu, Z.; Zhang, X.; Li, F.; Li, Z.; Qi, F.; Wang, X.; Jiang, Y.; Men, P.; et al. Compartmentalized Biosynthesis of Mycophenolic Acid. Proc. Natl. Acad. Sci. USA 2019, 116, 13305–13310.
- Zhang, W.; Cao, S.; Qiu, L.; Qi, F.; Li, Z.; Yang, Y.; Huang, S.; Bai, F.; Liu, C.; Wan, X.; et al. Functional Characterization of MpaG′, the O-Methyltransferase Involved in the Biosynthesis of Mycophenolic Acid. ChemBioChem 2015, 16, 565–569.
- Canonica, L.; Kroszczynski, W.; Ranzi, B.M.; Rindone, B.; Santaniello, E.; Scolastico, C. Biosynthesis of Mycophenolic Acid. J. Chem. Soc. Perkin 1 1972, 116, 2639–2643.
- You, C.; Li, F.; Zhang, X.; Ma, L.; Zhang, Y.-Z.; Zhang, W.; Li, S. Structural Basis for Substrate Specificity of the Peroxisomal Acyl-CoA Hydrolase MpaH’ Involved in Mycophenolic Acid Biosynthesis. FEBS J. 2021, 288, 5768–5780.
- Hansen, B.G.; Genee, H.J.; Kaas, C.S.; Nielsen, J.B.; Regueira, T.B.; Mortensen, U.H.; Frisvad, J.C.; Patil, K.R. A New Class of IMP Dehydrogenase with a Role in Self-Resistance of Mycophenolic Acid Producing Fungi. BMC Microbiol. 2011, 11, 202.
- Fontaine, K.; Hymery, N.; Lacroix, M.Z.; Puel, S.; Puel, O.; Rigalma, K.; Gaydou, V.; Coton, E.; Mounier, J. Influence of Intraspecific Variability and Abiotic Factors on Mycotoxin Production in Penicillium roqueforti. Int. J. Food Microbiol. 2015, 215, 187–193.
- Gillot, G.; Jany, J.-L.; Dominguez-Santos, R.; Poirier, E.; Debaets, S.; Hidalgo, P.I.; Ullán, R.V.; Coton, E.; Coton, M. Genetic Basis for Mycophenolic Acid Production and Strain-Dependent Production Variability in Penicillium roqueforti. Food Microbiol. 2017, 62, 239–250.
- Rojas-Aedo, J.F.; Gil-Durán, C.; Goity, A.; Vaca, I.; Levicán, G.; Larrondo, L.F.; Chávez, R. The Developmental Regulator Pcz1 Affects the Production of Secondary Metabolites in the Filamentous Fungus Penicillium roqueforti. Microbiol. Res. 2018, 212–213, 67–74.
- Torrent, C.; Gil-Durán, C.; Rojas-Aedo, J.F.; Medina, E.; Vaca, I.; Castro, P.; García-Rico, R.O.; Cotoras, M.; Mendoza, L.; Levicán, G.; et al. Role of Sfk1 Gene in the Filamentous Fungus Penicillium roqueforti. Front. Microbiol. 2017, 8, 2424.
- García-Rico, R.; Chavez, R.; Fierro, F.; Martin, J. Effect of a Heterotrimeric G Protein α Subunit on Conidia Germination, Stress Response, and Roquefortine C Production in Penicillium roqueforti. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2009, 12, 123–129.
- Vinokurova, N.G.; Ivanushkina, N.E.; Kochkina, G.A.; Arinbasarov, M.U.; Ozerskaya, S.M. Production of Mycophenolic Acid by Fungi of the Genus Penicillium Link. Appl. Biochem. Microbiol. 2005, 41, 83–86.
- Chen, M.; Wang, J.; Lin, L.; Xu, X.; Wei, W.; Shen, Y.; Wei, D. Synergistic Regulation of Metabolism by Ca2+/Reactive Oxygen Species in Penicillium brevicompactum Improves Production of Mycophenolic Acid and Investigation of the Ca2+ Channel. ACS Synth. Biol. 2022, 11, 273–285.
- El-Sayed, E.-S.R.; Zaki, A.G. Unlocking the Biosynthetic Potential of Penicillium roqueforti for Hyperproduction of the Immunosuppressant Mycophenolic Acid: Gamma Radiation Mutagenesis and Response Surface Optimization of Fermentation Medium. Biotechnol. Appl. Biochem. 2023, 70, 306–317.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
799
Revisions:
2 times
(View History)
Update Date:
24 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




