Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kalliopi Andrikou | -- | 3069 | 2023-11-21 12:06:26 | | | |
| 2 | Rita Xu | -2 word(s) | 3067 | 2023-11-22 02:49:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Andrikou, K.; Rossi, T.; Verlicchi, A.; Priano, I.; Cravero, P.; Burgio, M.A.; Crinò, L.; Bandini, S.; Ulivi, P.; Delmonte, A. Circulating Tumour Cells in Advanced NSCLC. Encyclopedia. Available online: https://encyclopedia.pub/entry/51852 (accessed on 13 January 2026).
Andrikou K, Rossi T, Verlicchi A, Priano I, Cravero P, Burgio MA, et al. Circulating Tumour Cells in Advanced NSCLC. Encyclopedia. Available at: https://encyclopedia.pub/entry/51852. Accessed January 13, 2026.
Andrikou, Kalliopi, Tania Rossi, Alberto Verlicchi, Ilaria Priano, Paola Cravero, Marco Angelo Burgio, Lucio Crinò, Sara Bandini, Paola Ulivi, Angelo Delmonte. "Circulating Tumour Cells in Advanced NSCLC" Encyclopedia, https://encyclopedia.pub/entry/51852 (accessed January 13, 2026).
Andrikou, K., Rossi, T., Verlicchi, A., Priano, I., Cravero, P., Burgio, M.A., Crinò, L., Bandini, S., Ulivi, P., & Delmonte, A. (2023, November 21). Circulating Tumour Cells in Advanced NSCLC. In Encyclopedia. https://encyclopedia.pub/entry/51852
Andrikou, Kalliopi, et al. "Circulating Tumour Cells in Advanced NSCLC." Encyclopedia. Web. 21 November, 2023.
Copy Citation
Non-small cell lung cancer (NSCLC) is one of the deadliest diseases worldwide. Tissue biopsy is the current gold standard for the diagnosis and molecular profiling of NSCLC.
CTCs
epithelial-to-mesenchymal transition (EMT)
lung cancer
1. Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide [1]. Non-small cell lung cancer (NSCLC) is a heterogeneous disease that represents about 85% of all lung malignancies [2]. More than 60% of NSCLC patients are diagnosed with advanced disease, and the 5-year survival rate is lower than 15% [1][3]. In the past twenty years, there has been a great improvement in understanding the molecular biology of oncogene-driven tumours, leading to significant advances in the treatment of these patients [4]. At the same time, the treatment of advanced non-oncogene addicted NSCLC has been revolutionized with the introduction of immune checkpoint inhibitors (ICIs) in clinical practice [5]. Unfortunately, not all patients respond, and only a subgroup of them benefit from immunotherapy. Hence, in the era of personalized medicine, there is an urgent need for new predictive biomarkers that may allow early evaluation of treatment response, selection of patients, and identification of drug-resistance.
Based on the current evidence, histopathological analysis and molecular profiling of all NSCLC tissue specimens is necessary before any therapeutic decision-making [6]. However, up to 30% of NSCLC patients do not undergo molecular profiling because of limited quantity or quality of tissue available [7], representing these two features as a strong limitation. Moreover, tissue biopsy is invasive and has low repeatability, thus limiting the possibility to define the intra- and intertumour heterogenicity in both spatial and temporal terms.
Liquid biopsy is a non-invasive method that may identify cancer-related biomarkers in peripheral blood, and can be repeated at multiple timepoints with the potential to define temporal and spatial tumour heterogeneity [8]. Circulating tumour cells (CTCs), due to recent advances in molecular knowledge, represent one of the most studied components of liquid biopsy as prognostic and predictive biomarkers in the management of NSCLC patients.
2. Circulating Tumour Cells
Circulating tumour cells (CTCs) are rare; one CTC per 106–107 white blood cells, found in the bloodstream of patients with solid tumours [9]. CTCs serve as a “liquid biopsy”, together with circulating metabolites, extracellular vesicles (EVs), and circulating nucleic acids such as cell-free DNA (cfDNA), microRNAs, and messenger RNA (mRNA) [9]. Liquid biopsy refers to the cytological and molecular analysis of cancer markers shed by the tumour into blood. It represents a minimally invasive and easily repeatable test, allowing for longitudinal studies. Collection and isolation of CTCs is more complex compared to ctDNA or cfDNA, but it allows protein, transcriptomic, or methylation analyses. In Figure 1, an overview of liquid biopsy approaches, including advantages and disadvantages (Figure 1).
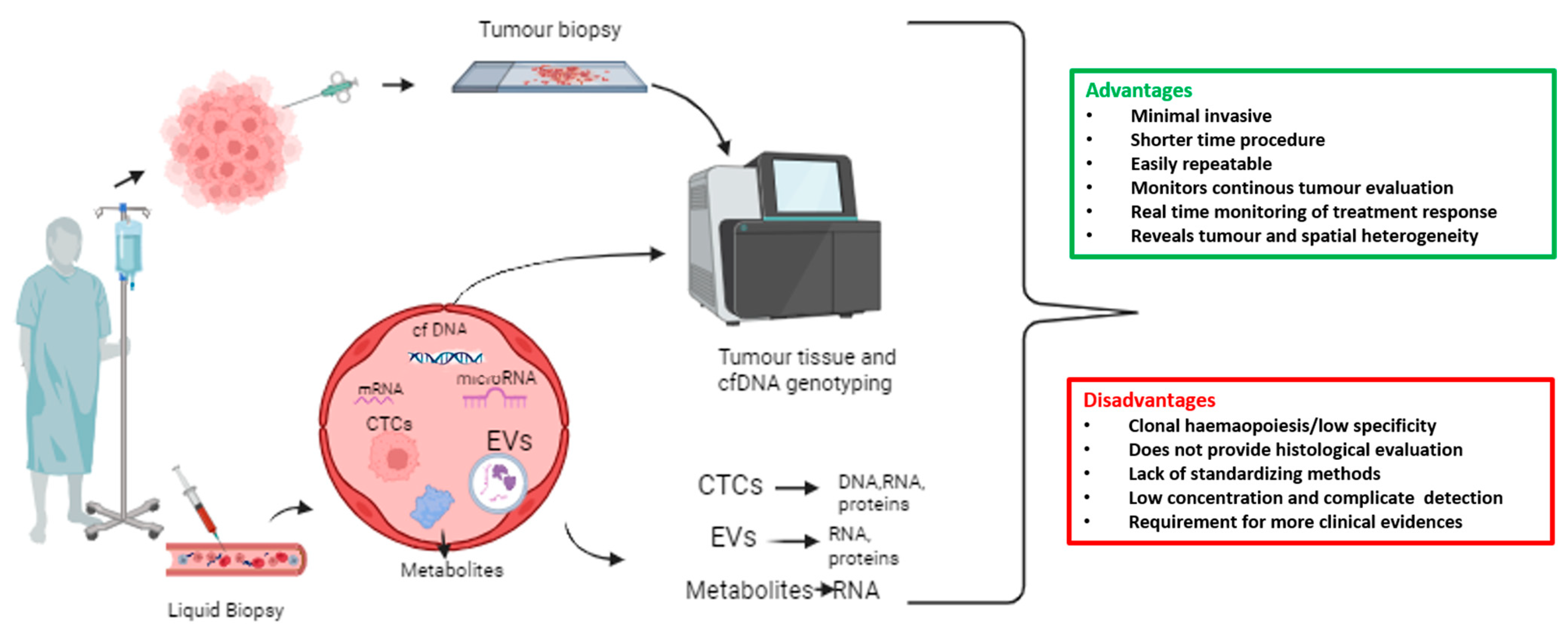
Figure 1. Overview of liquid biopsy approach with advantages and disadvantages. A wide range of biomarker sources are included in the liquid biopsy, such as circulating tumour cells (CTCs), extracellular vesicles (EVs), messenger RNAs (mRNAs), microRNAs, cell-free DNA (cfDNA), and tumour-derived metabolites that are present in bodily fluids such as blood.
CTCs slough off of the primary tumour and, through a process known as intravasation, enter the circulating blood [10], initiating the metastatic process. They can migrate either as single cells (single CTCs), or as clusters of two or more CTCs (CTC clusters or circulating microemboli), held together by intercellular junctions [11][12]. Single CTCs and CTC clusters have a short half-life in the bloodstream (6–10 min for CTC clusters and 25–30 for single CTCs) [13], as they face several insults such as attack by immune cells, cell death by anoikis, and physical stress due to fluid shear, limiting the metastatic diffusion. However, CTC clusters are associated with poor clinical outcome in cancer patients, including lung cancer, and are characterized by a significant higher metastatic potential thanks to their stem features [11][14]. CTC clusters can be further distinguished in homotypic CTC clusters, composed of cancer cells only, and heterotypic CTC clusters, formed by cancer cells and other kind of cells, including nonmalignant immune and stromal cells [12]. Clustering of CTCs with neutrophils has been widely studied, as this interaction led to cell cycle progression in circulation and metastatic seeding acceleration [15][16]. The potential of CTCs as metastatic-initiating cells was proven in xenograft models [17], while in patients, a higher number of CTCs is correlated with increased tumour aggressiveness and decreased time to relapse in different cancer types, including NSCLC [18][19][20][21].
Therefore, due to the crucial role of CTCs in the metastatic cascade, many researchers support CTC investigation, as a tool to obtain further information concerning the tumour phenotype and genotype, and to predict disease progression and survival in early and advanced stage cancer patients.
2.1. Methods for CTC Isolation and Detection in NSCLC
As the origin of most cancers is epithelial, several enrichment methods have been developed to select cells with positive expression of the epithelial cell adhesion molecule (EpCAM) antigen [22]. In this context, one of the most used platforms for CTC immune-magnetic enrichment, isolation, and enumeration relies on the EpCAM-dependent method CellSearch® technology (Veridex, Raritan, NJ). More specifically, CellSearch-based CTC enumeration is a helpful tool to stratify patients into favourable and unfavourable prognostic groups in advanced breast, colon, and prostate tumours [23][24][25][26], and represents the only FDA-approved CTC-based assay [27]. Concerning advanced NSCLC patients, the CellSearch detection of >5 CTCs/7.5 mL of blood was considered as a poor prognostic factor. However, CTC are not detectable in two-thirds of stage IV NSCLC patients, while in stage III NSCLC patients CTCs are detectable in less than 5% of patients [28][29]. Other EpCAM-dependent CTC detection methods based on immuno-affinity include the Adnatest assay (Qiagen) and the GILUPI Cell-collector [30].
However, the use of EpCAM as the main CTC marker may limit their detection, due to the variable expression of different markers. For instance, in the case of NSCLC using label-independent procedures, a higher number of CTCs were detected than using EpCAM-dependent methods, consistent with the low CTC-positivity rates at CellSearch [28][31]. The variable marker expression is due to epithelial–mesenchymal transition (EMT), which plays a significant role in the spread of systemic cancer. During EMT, cancer cells lose their epithelial features by a downregulation of epithelial genes, such as E-cadherin, EpCAM, occludins, claudins, and α- and β-catenin [32][33]. On the other hand, they acquire mesenchymal properties by an upregulation of mesenchymal genes such as N-cadherin, matrix metalloproteinases, integrins αv and β1, vimentin, and fibronectin [32][33]. In addition, some transcriptional factors such as Snail and Twist have a crucial role in survival of CTCs during EMT process [33]. By combining epithelial (EpCAM and CK8/18/19) and mesenchymal (vimentin and TWIST1) markers, three categories of CTCs were identified: epithelial CTCs (E-CTCs), mesenchymal CTCs (M-CTCs), and hybrid epithelial/mesenchymal phenotypes (E/M-CTCs) [33][34]. While E/M-CTCs were shown to be predictive in distinguishing patients with malignant NSCLC from those with benign disease, M-CTCs were associated with the presence of metastasis [33][34][35]. Interestingly, CTCs most frequently maintain a hybrid E/M phenotype, allowing them to retain their epithelial properties, but with an increase of the aggressive potential due to mesenchymal features [36][37].
Several studies demonstrated that all of these changes confer a higher metastatic potential and chemoresistance to cancer cells. Zhang et al. demonstrated the association between the EMT phenotype of CTCs from the peripheral blood and distant metastasis in patients with NSCLC [35]. A total of 110 patients were evaluated: 85 patients had NSCLC, of which 41 were with metastatic and 44 were with nonmetastatic disease, and 25 were with benign diseases. Overall, 80% of patients were characterized as CTC-positive with EMT markers (≥ 1 cell/5 mL blood) using a Canpatrol™ CTC assay; the CTC-positive rate was not related to the nonmetastatic and metastatic status. The total number and the mean numbers of CTCs of each subpopulation were statistically higher in NSCLC than in patients with benign pulmonary diseases. In particular, E/M- and M-CTCs rates were significantly superior in patients with NSCLC (75.3 and 44.7%, respectively) than in those with benign pulmonary diseases (0% and 0%, respectively; p < 0.05). Finally, M-CTCs were significantly higher in metastatic than in nonmetastatic patients (p < 0.001) [35]. Given these data, the EpCAM-negative CTC population may be not recognized, and detection approaches based on CTC epithelial markers may fail to detect those with mesenchymal characteristics [38].
Among the marker-independent approaches, isolation by size of epithelial tumour cells (ISET) is another method which can isolate CTCs [39]. In a study by Krebs et al., conducted on chemo-naive patients with stage IIIA to IV NSCLC, the CellSearch platform detected CTCs in 9 patients (40%), and while using ISET, 32 patients (80%) emerged as CTC-positive in a subpopulation of CTCs negative for the expression of epithelial markers [28]. Moreover, the CellSearch platform did not detect circulating tumour microemboli, while the ISET system detected them in 43% of patients [28]. However, this approach could underestimate very small CTCs [9]. In addition to their size, deformability is another property that can be exploited for CTC detection through the Parsortix microfluidic platform, which allows the recovery of CTCs and CTC clusters with preserved viability [40]. In NSCLC, the Parsortix platform provided the highest CTC-positivity compared to other technologies such as ISET and Ficoll. In addition, CTC detection using Parsortix was associated with disease progression, reduced PFS, and a high risk of relapse in NSCLC patients treated with anti-PD-1 agents [41]. In addition, microfluidic devices allow the continued collection of CTCs for further downstream analysis, and offer the possibility to integrate both isolation and detection of CTCs in a single device [42].
Other emerging approaches have been described to be suitable for CTC detection, such as spectroscopy-based techniques. Surface-enhanced Raman spectroscopy (SERS) allows for the highly sensitive and accurate detection of single cells by generating molecular fingerprint signals [43]. This method was found to be rapid and cost-effective, with high recognition rates (90%), and bio-probes with specific aptamers on parylene micropore membranes could distinguish cancer cells from white blood cells [44]. Recent findings have demonstrated that SERS can be functionalised and complemented with machine learning to detect metastasis-initiating cells directly in the blood of lung cancer patients, with a 100% diagnostic sensitivity [45].
2.2. Molecular Evaluation of CTCs
2.2.1. Single Cell Analysis
Thanks to the efforts made in technology development, molecular evaluation of CTCs is now possible, even at the single-cell level, allowing for the genomic and transcriptomic characterization of individual CTCs [46].
Concerning single-cell genomics of CTCs, many studies have focused on the assessment of single nucleotide variants (SNVs). The first technologies available for this application consisted of polymerase chain reaction (PCR) genotyping, especially digital PCR (dPCR). This low-input technique allows highly sensitive and specific detection of mutations; indeed, dPCR targets specific sequences due to the presence of primers, which can be combined to allow target multiplexing. dPCR-based SNV detection at the single-cell level has been demonstrated to be a useful tool for monitoring patients throughout the course of a disease, in addition to unveiling their genetic heterogeneity [47][48][49]. In NSCLC, the mutational analysis of epidermal growth factor receptor (EGFR) through dPCR on single CTCs isolated prior treatment with tyrosine kinase inhibitors (TKIs), identified a low mutation rate at the single-CTC level in patients positive for EGFR mutations. At the same time this approach can detect rare EGFR mutations in those patients considered as wild-type [50]. However, the initial limitation of these techniques was the fact that they were able to detect only specific alterations, limiting the discovery of other variants. More recently, the development of panels for next generation sequencing (NGS), including oncology-relevant genes, was implemented. Pailler et al. investigated resistance mutations in 48 cancer-related genes in single CTCs from ALK-rearranged NSCLC patients treated with crizotinib or lorlatinib at disease progression. In crizotinib-treated patients, mutational heterogeneity in multiple genes involved in ALK-independent pathways (“off-target” mutations), such as the RTK-KRAS (EGFR, KRAS, BRAF genes) and TP53 pathways, were identified. CTCs from lorlatinib-treated patients harboured mutations affecting the ALK gene, with some variants not detected in the corresponding tumour biopsy [51]. Single-cell whole exome sequencing was also demonstrated to be critical for studying drug resistance in NSCLC, as well as to discover new drug targets in NSCLC. Chang et al. exploited this approach to explore the mutational landscape of CTCs compared to matched primary and progressive tumours in platinum-based treated NSCLC patients. In addition to the frequency of mutations in cancer-driver genes (i.e., EGFR and TP53), genes associated with cell-cycle and stem cell features were frequently altered in CTCs, most of which were derived from primary tumour samples, and played crucial roles in chemo-drug resistance and metastasis for NSCLCs [52].
Transcriptomic analysis of single CTCs through RNA sequencing is a technology that may help to better understand the molecular mechanisms underlying metastasis [53], although few studies are present in the literature for NSCLC. One of the most recent technologies relies on the single-cell 3′ RNA sequencing for gene expression analysis using the 10X Chromium platform [54]. In CTCs isolated from diagnostic leukapheresis of NSCLC patients, single-cell 10X Chromium was able to identify heterogeneous CTC phenotypes. The epithelial-like phenotype was characterized by the expression of epithelial markers, as well as Ki67 (highly proliferation) and IL-1B, and interferon-response pathways (immune responsive). The mesenchymal/invasive phenotype showed expression of vimentin, hypoxia, and glycolysis pathways, whereas CTCs from the mesenchymal/stem cell-like phenotype were enriched in genes including ALDH13, and genes associated with adipogenesis [55].
2.2.2. PD-L1 Determination of CTCs
Several studies have been conducted in order to evaluate the expression of the programmed death-ligand 1 (PD-L1) in CTCs from patients treated with immune checkpoint inhibitors, especially with immunofluorescence techniques. In advanced NSCLC patients, it has been shown that CTCs were more frequently PD-L1-positive compared to tumour tissue, therefore representing a potential tool to identify patients who would benefit from immunotherapy [56][57]. In 96 NSCLC patients treated with nivolumab, baseline PD-L1 immunofluorescent expression in CTCs (83%) was higher than tissue (41%), and a higher number of PD-L1 positive CTCs was associated with nonresponders (PFS < 6 months) (p = 0.04). All of the patients had PD-L1 positive CTCs at disease progression [57]. Similar data concerning the increased PD-L1 positivity in CTCs compared to tumour tissue (53% and 43.2%, respectively) in advanced NSCLC patients were reported by Zhou et al. [56].
Several techniques have been described for the assessment of PD-L1 in CTCs. Among them, immunofluorescence methods were described, with the use of fluorescent antibodies targeting PD-L1, following CTC enrichment [56][57][58]. Other studies have proposed the CellSearch system, by including an additional antibody targeting PD-L1 in the empty fluorescence channel (AlexaFluor488 or fluorescein) [59][60]. Other techniques for PD-L1 expression assessment in CTCs include the DEPArray platform (Menarini Silicon Biosystems). This technology allows one to visually inspect single cells marked with antibodies targeting specific antigens (i.e., PD-L1), and to recover the cells of interest at a single-cell resolution by exploiting the dielectrophoretic principle [61]. One of the main advantages of the DEPArray technology relies on the possibility to analyse the expression of PD-L1 at single-cell resolution, as already reported in advanced urothelial carcinoma [62].
3. Prognostic Value of CTCs in Advanced NSCLC
The prognostic role of CTC count, evaluated at the baseline and after one cycle of chemotherapy by a semiautomated EpCAM–based immunomagnetic technique, has been evaluated for the first time in 101 chemotherapy-naive patients with stage III and IV NSCLC [29]. The number of CTCs in 7.5 mL of blood was higher in 60 patients with stage IV NSCLC (range, 0 to 146) than in 27 patients with stage IIIB (range, 0 to 3); in 14 IIIA patients, CTCs were not detected. In a univariate analysis, progression-free survival (PFS) was 6.8 vs. 2.4 months (p = 0.001), and overall survival (OS) was 8.1 vs. 4.3 months (p = 0.001) for patients with <5 CTCs per 7.5 mL of blood compared with ≥5 CTCs before chemotherapy, respectively. In a multivariate analysis, CTC number at baseline was the strongest predictor of OS, with a hazard ratio (HR) of 7.92 (95% CI, 2.85 to 22.01; p = 0.001) [29]. Similar results have been seen after a cycle of chemotherapy. Similarly, Nieva et al. evaluated a method using enrichment-free fluorescent labeling of CTCs, followed by automated digital microscopy in 28 patients with NSCLC [63]. CTCs were identified in 68% of analysed samples, with a median concentration of 1.6 per ml (range 0–182.6 CTCs/mL). Increased CTC detection seems to be related to progressive disease in individual patients; patients were grouped into those with ≥ 5 CTCs/mL and those with <5: the first group had a median survival of 244 days, while the second did not reach the median survival, with a median follow-up of 304 days. The HR for death was 4.0 in the group, with ≥5 CTCs/mL relative to those patients with a lower count, with a four-fold risk of dying at any given timepoint for patients with higher CTC counts (p = 0.0084) [63]. In another series of 43 advanced NSCLC, blood samples were obtained at the baseline, before the second and fifth cycles of chemotherapy. At baseline, 18 (41.9%) patients were positive for intact CTC counts, and 10 (23.2%) of them had ≥5 CTCs. The group of patients with CTC > 5 at baseline presented worse PFS and OS than those with <5 CTC (p = 0.034 and p = 0.008, respectively); during therapy, patients with a high amount of CTCs during the treatment demonstrated lower OS and PFS rates [64]. Moreover, they demonstrated that PFS and OS were shorter in patients with a progressive increase of CTC count from the baseline to final timepoint (p = 0.003 and p = 0.019, respectively) [64]. The prognostic role of CTCs has been evaluated in a pooled analysis of data from 550 patients with NSCLC enrolled in seven European centres. CTC counts of ≥ 2 and ≥ 5 per 7.5 mL were associated with reduced PFS (≥2 CTCs: HR = 1.72, p < 0.001; ≥ 5 CTCs: HR = 2.21, p < 0.001) and OS (≥2 CTCs: HR = 2.18, p < 0.001; ≥ 5 CTCs: HR = 2.75, p < 0.001), respectively [65]. In general, clinical trials performed with CellSearch methodology highlight the prognostic value of CTC count, even if negative studies have been reported in the literature [66][67]. Several methodologies, other than CellSearch, have been evaluated, including flow cytometry, the antigen-based platform Cytell, and ISET [39][68][69].
Despite interesting insights suggesting that these alternative methods may have higher sensitivity, the CellSearch approach remains the only clinically validated, FDA-cleared system for identification, isolation, and CTC counts.
Furthermore, interesting results regarding the prognostic role of CTCs in different molecular subtypes of advanced NSCLC have been reported in some studies. Tong et al. investigated the prognostic value of CTC count in 43 patients with EGFR -mutated or ALK -rearranged NSCLC at baseline and at progression of disease. Increased CTC count at baseline (≥8 CTCs/3.2 mL) was significantly associated with both shorter PFS and OS (11.6 vs. 8.5 months, p = 0.004 for PFS and 21 vs. 17.7 months, p = 0.013 for OS) [70]. Similar results were reported regarding the prognostic role of CTCs in terms of PFS in NSCLC patients treated with osimertinib [71]. According to CTC values at the baseline, patients were divided into favourable (CTCs < 5) and unfavourable (CTCs ≥ 5) prognostic groups; PFS was 9.3 in the former group and 6.5 months in the later one (HR 5.712, 95% CI: 3.781–9.577; p = 0.0002) [71]. The negative prognostic role of CTC count was also reported in a small cohort comprising 24 metastatic EGFR -positive NSCLC patients [48]. Patients with CTCs ≥ 1 in 7.5 mL had significantly shorted OS compared to patients without CTCs (3.0 months vs. not reached, p = 0.012, HR = 2.9, 95% CI 1.6–54.1 months) [72].
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Zhang, Y.; Luo, G.; Etxeberria, J.; Hao, Y. Global Patterns and Trends in Lung Cancer Incidence: A Population-Based Study. J. Thorac. Oncol. 2021, 16, 933–944.
- Huang, C.Y.; Chen, B.H.; Chou, W.C.; Yang, C.T.; Chang, J.W. Factors associated with the prognosis and long-term survival of patients with metastatic lung adenocarcinoma: A retrospective analysis. J. Thorac. Dis. 2018, 10, 2070–2078, Erratum in: J. Thorac. Dis. 2018, 10, E604.
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365.
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362.
- European Society for Medical Oncology. Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Available online: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf (accessed on 3 June 2023).
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta. Cytol. 2019, 63, 489–496.
- Malapelle, U.; Tiseo, M.; Vivancos, A.; Kapp, J.; Serrano, M.J.; Tiemann, M. Liquid Biopsy for Biomarker Testing in Non-Small Cell Lung Cancer: A European Perspective. J. Mol. Pathol. 2021, 2, 255–273.
- Andree, K.C.; van Dalum, G.; Terstappen, L.W. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 2016, 10, 395–407.
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840.
- Aceto, N.; Toner, M.; Maheswaran, S.; Haber, D.A. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-To-Mesenchymal Transition. Trends Cancer 2015, 1, 44–52.
- Aceto, N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020, 43, 18–23.
- Yu, M. Metastasis Stemming from Circulating Tumor Cell Clusters. Trends Cell Biol. 2019, 29, 275–276.
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491.
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Aceto, N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557.
- Rozenberg, J.M.; Buzdin, A.A.; Mohammad, T.; Rakitina, O.A.; Didych, D.A.; Pleshkan, V.V.; Alekseenko, I.V. Molecules promoting circulating clusters of cancer cells suggest novel therapeutic targets for treatment of metastatic cancers. Front. Immunol. 2023, 14, 1099921.
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544.
- Wang, C.; Mu, Z.; Ye, Z.; Zhang, Z.; Abu-Khalaf, M.M.; Silver, D.P.; Palazzo, J.P.; Jagannathan, G.; Fellin, F.M.; Bhattacharya, S.; et al. Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors. Breast Cancer Res. Treat. 2020, 181, 679–689.
- Paoletti, C.; Regan, M.M.; Niman, S.M.; Dolce, E.M.; Darga, E.P.; Liu, M.C.; Marcom, P.K.; Hart, L.L.; Smith, J.W., II; Tedesco, K.L.; et al. Circulating tumor cell number and endocrine therapy index in ER positive metastatic breast cancer patients. NPJ Breast Cancer 2021, 7, 77.
- de Kruijff, I.E.; Sieuwerts, A.M.; Onstenk, W.; Kraan, J.; Smid, M.; Van, M.N.; van der Vlugt-Daane, M.; Hoop, E.O.; Mathijssen, R.H.J.; Lolkema, M.P.; et al. Circulating Tumor Cell Enumeration and Characterization in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Cabazitaxel. Cancers 2019, 11, 1212.
- Lindsay, C.R.; Faugeroux, V.; Michiels, S.; Pailler, E.; Facchinetti, F.; Ou, D.; Bluthgen, M.V.; Pannet, C.; Ngo-Camus, M.; Bescher, G.; et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann. Oncol. 2017, 28, 1523–1531.
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987.
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221, Erratum in: J. Clin. Oncol. 2009, 27, 1923.
- Cristofanilli, M.; Hayes, D.F.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Reuben, J.M.; Doyle, G.V.; Matera, J.; Allard, W.J.; Miller, M.C.; et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 2005, 23, 1420–1430, Erratum in: J. Clin. Oncol. 2005, 23, 4808.
- de Bono, J.S.; Attard, G.; Adjei, A.; Pollak, M.N.; Fong, P.C.; Haluska, P.; Roberts, L.; Melvin, C.; Repollet, M.; Chianese, D.; et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 2007, 13, 3611–3616.
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006, 12, 4218–4224.
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904.
- Krebs, M.G.; Hou, J.M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315.
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 1556–1563.
- Li, X.; Li, Y.; Shao, W.; Li, Z.; Zhao, R.; Ye, Z. Strategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2020, 9, 2012–2025.
- Wang, J.; Lu, W.; Tang, C.; Liu, Y.; Sun, J.; Mu, X.; Zhang, L.; Dai, B.; Li, X.; Zhuo, H.; et al. Label-Free Isolation and mRNA Detection of Circulating Tumor Cells from Patients with Metastatic Lung Cancer for Disease Diagnosis and Monitoring Therapeutic Efficacy. Anal. Chem. 2015, 87, 11893–11900.
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196.
- Pantazaka, E.; Vardas, V.; Roumeliotou, A.; Kakavogiannis, S.; Kallergi, G. Clinical Relevance of Mesenchymal- and Stem-Associated Phenotypes in Circulating Tumor Cells Isolated from Lung Cancer Patients. Cancers 2021, 13, 2158.
- Zhang, Y.; Men, Y.; Wang, J.; Xing, P.; Zhao, J.; Li, J.; Xu, D.; Hui, Z.; Cui, W. Epithelial circulating tumor cells with a heterogeneous phenotype are associated with metastasis in NSCLC. J. Cancer Res. Clin. Oncol. 2022, 148, 1137–1146.
- Zhang, X.; Wei, L.; Li, J.; Zheng, J.; Zhang, S.; Zhou, J. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol. Med. Rep. 2019, 19, 601–608.
- Kallergi, G.; Aggouraki, D.; Zacharopoulou, N.; Stournaras, C.; Georgoulias, V.; Martin, S.S. Evaluation of α-tubulin, detyrosinated α-tubulin, and vimentin in CTCs: Identification of the interaction between CTCs and blood cells through cytoskeletal elements. Breast Cancer Res. 2018, 20, 67.
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, R59.
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836.
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853.
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The Parsortix™ Cell Separation System-A versatile liquid biopsy platform. Cytometry A 2018, 93, 1234–1239.
- Papadaki, M.A.; Sotiriou, A.I.; Vasilopoulou, C.; Filika, M.; Aggouraki, D.; Tsoulfas, P.G.; Apostolopoulou, C.A.; Rounis, K.; Mavroudis, D.; Agelaki, S. Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy. Cancers 2020, 12, 1556.
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics Based Point-Of-Care Diagnostics. Biotechnol. J. 2018, 13, 1700047.
- Kamińska, A.; Szymborski, T.; Witkowska, E.; Kijeńska-Gawrońska, E.; Świeszkowski, W.; Niciński, K.; Trzcińska-Danielewicz, J.; Girstun, A. Detection of Circulating Tumor Cells Using Membrane-Based SERS Platform: A New Diagnostic Approach for ‘Liquid Biopsy’. Nanomaterials 2019, 9, 366.
- Lv, W.; Fu, B.; Liu, W.; Huang, W.; Li, M.; Liu, Y.; Kang, Y.; Wang, J.; Bai, S.; Lu, C.; et al. Efficient detection of single circulating tumor cell in blood using Raman mapping based on Aptamer-SERS bio-probe coupled with micropore membrane filtration. Talanta 2023, 267, 125220.
- Premachandran, S.; Dhinakaran, A.K.; Das, S.; Venkatakrishnan, K.; Tan, B.; Sharma, M. Detection of lung cancer metastasis from blood using L-MISC nanosensor: Targeting circulating metastatic cues for improved diagnosis. Biosens. Bioelectron. 2023, 243, 115782.
- Han, Y.; Wang, D.; Peng, L.; Huang, T.; He, X.; Wang, J.; Ou, C. Single-cell sequencing: A promising approach for uncovering the mechanisms of tumor metastasis. J. Hematol. Oncol. 2022, 15, 59.
- Owen, S.; Lo, T.W.; Fouladdel, S.; Zeinali, M.; Keller, E.; Azizi, E.; Ramnath, N.; Nagrath, S. Simultaneous Single Cell Gene Expression and EGFR Mutation Analysis of Circulating Tumor Cells Reveals Distinct Phenotypes in NSCLC. Adv. Biosyst. 2020, 4, e2000110.
- Rowlands, V.; Rutkowski, A.J.; Meuser, E.; Carr, T.H.; Harrington, E.A.; Barrett, J.C. Optimisation of robust singleplex and multiplex droplet digital PCR assays for high confidence mutation detection in circulating tumour DNA. Sci. Rep. 2019, 9, 12620.
- Denis, J.A.; Patroni, A.; Guillerm, E.; Pépin, D.; Benali-Furet, N.; Wechsler, J.; Manceau, G.; Bernard, M.; Coulet, F.; Larsen, A.K.; et al. Droplet digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery. Mol. Oncol. 2016, 10, 1221–1231.
- Zhang, Q.; Nong, J.; Wang, J.; Yan, Z.; Yi, L.; Gao, X.; Liu, Z.; Zhang, H.; Zhang, S. Isolation of circulating tumor cells and detection of EGFR mutations in patients with non-small-cell lung cancer. Oncol. Lett. 2019, 17, 3799–3807.
- Pailler, E.; Faugeroux, V.; Oulhen, M.; Mezquita, L.; Laporte, M.; Honoré, A.; Lecluse, Y.; Queffelec, P.; NgoCamus, M.; Nicotra, C.; et al. Acquired Resistance Mutations to ALK Inhibitors Identified by Single Circulating Tumor Cell Sequencing in ALK-Rearranged Non-Small-Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6671–6682.
- Chang, Y.; Wang, Y.; Li, B.; Lu, X.; Wang, R.; Li, H.; Yan, B.; Gu, A.; Wang, W.; Huang, A.; et al. Whole-Exome Sequencing on Circulating Tumor Cells Explores Platinum-Drug Resistance Mutations in Advanced Non-small Cell Lung Cancer. Front. Genet. 2021, 12, 722078.
- Negishi, R.; Yamakawa, H.; Kobayashi, T.; Horikawa, M.; Shimoyama, T.; Koizumi, F.; Sawada, T.; Oboki, K.; Omuro, Y.; Funasaka, C.; et al. Transcriptomic profiling of single circulating tumor cells provides insight into human metastatic gastric cancer. Commun. Biol. 2022, 5, 20.
- Wang, X.; He, Y.; Zhang, Q.; Ren, X.; Zhang, Z. Direct Comparative Analyses of 10X Genomics Chromium and Smart-seq2. Genom. Proteom. Bioinform. 2021, 19, 253–266.
- Rieckmann, L.M.; Spohn, M.; Selbuz, E.; Schubert, C.; Agorku, D.; Becker, L.; Borchers, A.; Krause, J.; Ruff, L.; Heinemann, S.; et al. Abstract 3374: Large-scale single-cell whole transcriptomic analyses reveal distinct malignant phenotypes of CTCs from NSCLC patients. Cancer Res. 2022, 82 (Suppl. 12), 3374.
- Zhou, Q.; Liu, X.; Li, J.; Tong, B.; Xu, Y.; Chen, M.; Liu, X.; Gao, X.; Shi, Y.; Zhao, J.; et al. Circulating tumor cells PD-L1 expression detection and correlation of therapeutic efficacy of immune checkpoint inhibition in advanced non-small-cell lung cancer. Thorac. Cancer 2023, 14, 470–478.
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112.
- Dall’Olio, F.G.; Gelsomino, F.; Conci, N.; Marcolin, L.; De Giglio, A.; Grilli, G.; Sperandi, F.; Fontana, F.; Terracciano, M.; Fragomeno, B.; et al. PD-L1 Expression in Circulating Tumor Cells as a Promising Prognostic Biomarker in Advanced Non-small-cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, 423–431.
- Bergmann, S.; Coym, A.; Ott, L.; Soave, A.; Rink, M.; Janning, M.; Stoupiec, M.; Coith, C.; Peine, S.; von Amsberg, G.; et al. Evaluation of PD-L1 expression on circulating tumor cells (CTCs) in patients with advanced urothelial carcinoma (UC). Oncoimmunology 2020, 9, 1738798.
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782.
- Di Trapani, M.; Manaresi, N.; Medoro, G. DEPArray™ system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytometry A 2018, 93, 1260–1266.
- Cappelletti, V.; Verzoni, E.; Ratta, R.; Vismara, M.; Silvestri, M.; Montone, R.; Miodini, P.; Reduzzi, C.; Claps, M.; Sepe, P.; et al. Analysis of Single Circulating Tumor Cells in Renal Cell Carcinoma Reveals Phenotypic Heterogeneity and Genomic Alterations Related to Progression. Int. J. Mol. Sci. 2020, 21, 1475.
- Nieva, J.; Wendel, M.; Luttgen, M.S.; Marrinucci, D.; Bazhenova, L.; Kolatkar, A.; Santala, R.; Whittenberger, B.; Burke, J.; Torrey, M.; et al. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: A longitudinal analysis. Phys. Biol. 2012, 9, 016004.
- Muinelo-Romay, L.; Vieito, M.; Abalo, A.; Nocelo, M.A.; Barón, F.; Anido, U.; Brozos, E.; Vázquez, F.; Aguín, S.; Abal, M.; et al. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers 2014, 6, 153–165.
- Lindsay, C.R.; Blackhall, F.H.; Carmel, A.; Fernandez-Gutierrez, F.; Gazzaniga, P.; Groen, H.J.M.; Hiltermann, T.J.; Krebs, M.G.; Loges, S.; López-López, R.; et al. EPAC-lung: Pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur. J. Cancer 2019, 117, 60–68.
- Hirose, T.; Murata, Y.; Oki, Y.; Sugiyama, T.; Kusumoto, S.; Ishida, H.; Shirai, T.; Nakashima, M.; Yamaoka, T.; Okuda, K.; et al. Relationship of circulating tumor cells to the effectiveness of cytotoxic chemotherapy in patients with metastatic non-small-cell lung cancer. Oncol. Res. 2012, 20, 131–137.
- Juan, O.; Vidal, J.; Gisbert, R.; Muñoz, J.; Maciá, S.; Gómez-Codina, J. Prognostic significance of circulating tumor cells in advanced non-small cell lung cancer patients treated with docetaxel and gemcitabine. Clin. Transl. Oncol. 2014, 16, 637–643.
- Zhang, Z.; Xiao, Y.; Zhao, J.; Chen, M.; Xu, Y.; Zhong, W.; Xing, J.; Wang, M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016, 21, 519–525.
- Pawlikowska, P.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Tayoun, T.; Pailler, E.; Farace, F. Circulating tumor cells (CTCs) for the noninvasive monitoring and personalization of non-small cell lung cancer (NSCLC) therapies. J. Thorac. Dis. 2019, 11, S45–S56.
- Tong, B.; Xu, Y.; Zhao, J.; Chen, M.; Zhong, W.; Xing, J.; Wang, M. Prognostic role of circulating tumor cells in patients with EGFR-mutated or ALK-rearranged non-small cell lung cancer. Thorac. Cancer 2018, 9, 640–645.
- Yang, B.; Zheng, D.; Zeng, Υ.; Qin, A.; Gao, J.; Yu, G. Circulating tumor cells predict prognosis following secondline AZD 9291 treatment in EGFR-T790M mutant non-small cell lung cancer patients. J. BUON 2018, 23, 1077–1081.
- Isobe, K.; Hata, Y.; Kobayashi, K.; Hirota, N.; Sato, K.; Sano, G.; Sugino, K.; Sakamoto, S.; Takai, Y.; Shibuya, K.; et al. Clinical significance of circulating tumor cells and free DNA in non-small cell lung cancer. Anticancer Res. 2012, 32, 3339–3344.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
314
Revisions:
2 times
(View History)
Update Date:
22 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




