Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qingyun Wan | -- | 2102 | 2023-11-21 10:40:55 | | | |
| 2 | Rita Xu | Meta information modification | 2102 | 2023-11-21 11:07:27 | | | | |
| 3 | Rita Xu | -3 word(s) | 2099 | 2023-11-24 10:05:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yan, T.; Wan, Q. Vibrational Coherence in MMLCT and MC Excited State. Encyclopedia. Available online: https://encyclopedia.pub/entry/51837 (accessed on 08 February 2026).
Yan T, Wan Q. Vibrational Coherence in MMLCT and MC Excited State. Encyclopedia. Available at: https://encyclopedia.pub/entry/51837. Accessed February 08, 2026.
Yan, Tengfei, Qingyun Wan. "Vibrational Coherence in MMLCT and MC Excited State" Encyclopedia, https://encyclopedia.pub/entry/51837 (accessed February 08, 2026).
Yan, T., & Wan, Q. (2023, November 21). Vibrational Coherence in MMLCT and MC Excited State. In Encyclopedia. https://encyclopedia.pub/entry/51837
Yan, Tengfei and Qingyun Wan. "Vibrational Coherence in MMLCT and MC Excited State." Encyclopedia. Web. 21 November, 2023.
Copy Citation
There have been significant advancements in the investigation of coherence-related phenomena in organic systems such as biological photosynthetic reaction centers. The d8 Pt(II) dinuclear complex or molecular aggregate with a metal–metal-to-ligand charge transfer (MMLCT) or metal-centered (MC) excited state was reported to show the vibrational coherence phenomenon in the intersystem crossing (ISC) process, due to the Metal–metal (M-M) interaction at excited state.
vibrational coherence
MMLCT and MC excited state
Pt complex
1. Introduction
Plants and algae exhibit remarkable efficiency in converting solar energy into stored fuel, even in challenging environments that involve oxygen and water. They are also able to withstand extreme physiological temperatures and extremely low levels of light [1]. This stands in stark contrast to conventional materials used for solar energy conversion, which typically rely on long-range periodicity [2]. Despite their strong disorder, the photosynthetic machinery in biological systems demonstrates remarkable efficiency in converting solar energy. The coherence effect is believed to play a crucial role in facilitating energy transfer during photosynthesis in plants. Coherence refers to the phase relationships among the components of a superposition of waves. When the dynamics are coherent, these phase relationships are maintained for a sufficient duration to have a significant mechanistic and functional impact [3].
The coherence effect has significant implications for the design of light-harvesting materials, particularly in organic systems. There is speculation that coherence effects could be utilized to create more efficient light-harvesting materials, both in the context of organic photovoltaics and in the development of artificial photosynthetic systems [4][5]. By leveraging coherence effects, it may be possible to overcome the challenges presented by disorder and electron-vibration couplings, and to create materials that are more resilient to energy loss and decay [6].
2. 1/3MMLCT and 1/3MC Excited States of d8 Metal Complexes
Metal complexes with d8 electronic configurations, such as Rh(I), Pt(II), and Pd(II) complexes, can form a planar geometry with an open axial site (Scheme 1). When these complexes form dimers or aggregates with close metal–metal (M-M) contacts, the strong M-M Pauli repulsion and orbital overlap between two metal dz2 orbitals can lead to the formation of an M-M bonding orbital of dσ and an antibonding orbital of dσ* (Figure 1) [7]. Upon photo-excitation, an electron is promoted from the M-M antibonding orbital to the LUMO; an M-M bonding interaction is formed, leading to a contraction of the M-M distance at the excited state (Figure 1). When the LUMO is a ligand-based π* orbital, this transition is known as the singlet or triplet metal–metal-to-ligand charge transfer (1/3MMLCT) excited state transition with an M-M bond order of 0.5. When the LUMO is a metal-based pσ bonding orbital, this transition is known as the singlet or triplet metal-centered (1/3MC) excited state transition with an M-M bond order of 1. In 1975, Gray and his colleagues reported that Rh(I) aggregates are capable of forming the MMLCT excited state when undergoing self-assembly in a solution [8]. In 1990 and 1991, Gliemann, Miskowski, and their colleagues were the first to identify and report the MMLCT excited state in a series of Pt(II) complexes in the solid state [9][10]. MMLCT emission of a dinuclear Pt(II) system in solution was first observed in a guanidine-bridged binuclear Pt(II) complex by Che and co-workers in 1992 [11]. A series of butterfly-like dinuclear Pt(II) complex was synthesized and reported by Ma and Thompson in 2005 [12]. By altering the bulkiness of the bridging ligand, these dinuclear Pt(II) complexes exhibit tunable metal-to-ligand charge transfer (MLCT) and MMLCT excited states. In 2002, Yam and his colleagues observed and reported the formation of the MMLCT excited state of Pt(II) assemblies through the aggregation process in solution [13]. Despite extensive research in the Pt(II) and Rh(I) systems, there have been limited studies on the emissive excited states of Pd(II) and Au(III) d8 isoelectronic complexes. This scarcity can be attributed to the prevailing belief in the academic community that Pd(II)-Pd(II) or Au(III)-Au(III) bonded excited states are unlikely to exist due to the electrophilic nature of the Pd(II) and Au(III) atom. The first phosphorescent MMLCT emission in Pd(II) molecular aggregates was reported in 2018, where the tridentate Pd(II) isocyanide complexes form aggregates with the MMLCT emission energy at 540 nm [14]. The LMMCT (ligand-to-metal–metal charge transfer) excited state was assigned to a series of Au(III) aggregates in 2019, where the M-M bonding orbital is located at the Au-6pz orbital at the excited state for Au(III) complex [15].
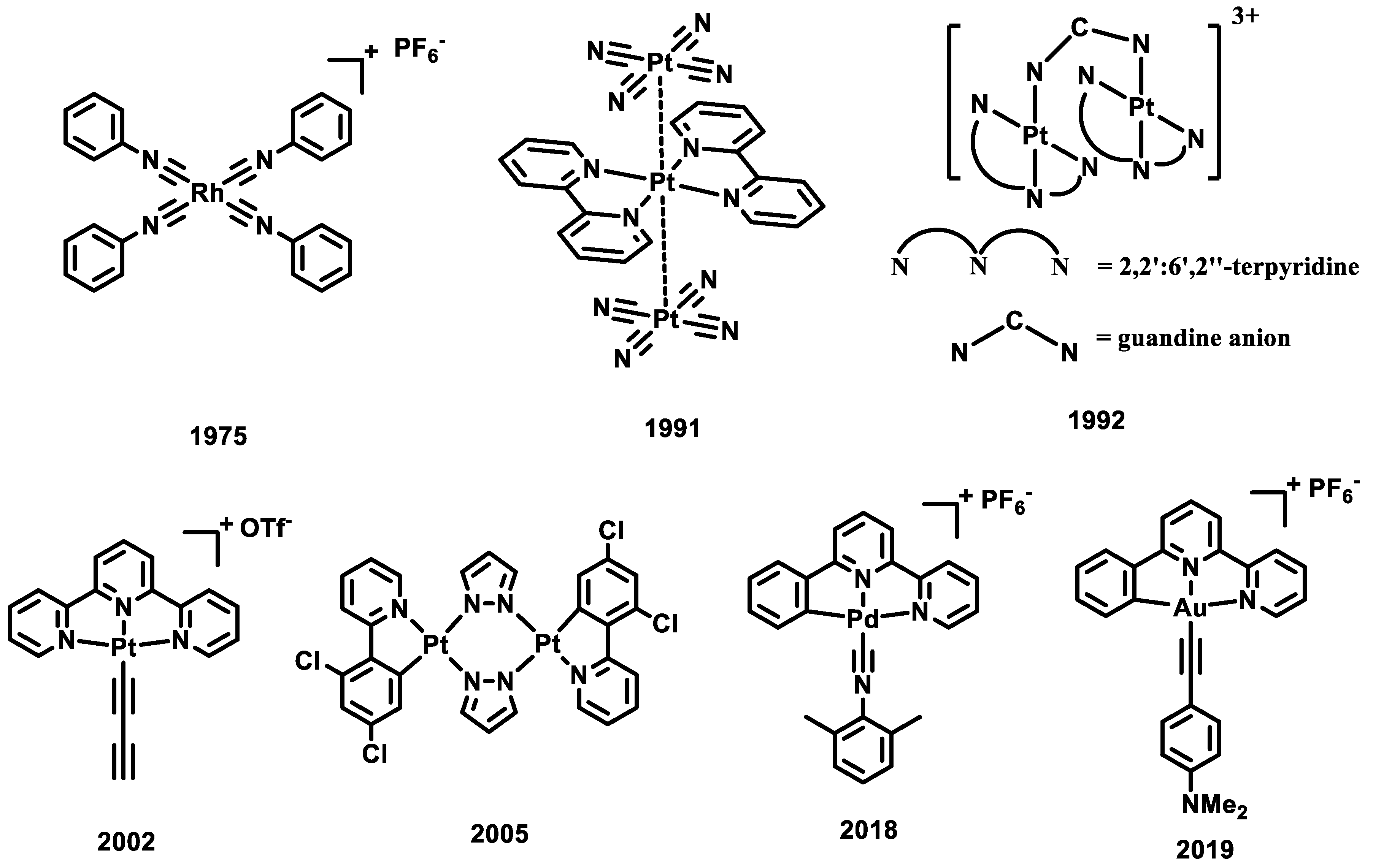
Scheme 1. Selected reported d8 metal complexes that can form the M-M bonding interaction at the excited state.

Figure 1. Formation of the (a) MC and (b) MMLCT excited state in a d8-d8 metal complex system. (c) M-M bond contraction upon photoexcitation. 3MMLCT: triplet metal–metal-to-ligand charge transfer excited state. 3MC: triplet metal-centered excited state.
Compared to the MLCT excited state, the MMLCT or MC excited states have lower transition energy and exhibit a larger contribution from metal-ndz2 orbital for d8 metal complexes. Therefore, they can be utilized to make red or near-infrared (NIR) organic light-emitting diodes (OLEDs) with high efficiency. The solid-state thin film based on Pt(II) aggregates with the MMLCT excited state exhibits intense NIR emission with an emission peak maximum at ~740 nm and high emission quantum yield [16]. The MMLCT or MC excited state can also be used in bio-imaging [17] and inner-sphere photo-catalysis reactions [18].
3. Ultrafast Spectroscopy and CVWP
Extensive research has been dedicated to dinuclear d8-d8 metal complexes due to their remarkable photophysical and photochemical properties originating from the lowest singlet and triplet excited states. The dynamics of these excited states can be partially controlled by factors such as (1) solvent effects, (2) moiety rigidity, (3) the type of metal center, and (4) molecular structure, including ligand engineering.
Ultrafast spectroscopy stands as a potent tool for investigating the dynamics of excited states (Table 1). The advancements in laser technology have facilitated the emergence of various ultrafast spectroscopy techniques. The pump/probe wavelength range has been expanded into the extreme ultraviolet and terahertz regions, and temporal resolutions have reached sub-femtosecond scales at specific wavelengths [19][20][21]. The application of methodologies such as luminescence up-conversion, transient absorption anisotropy and multi-dimensional spectroscopies has made it possible to comprehensively track the evolution of excited states across various systems, encompassing the electronic, vibrational and spin states [22][23]. The utilization of white light (or wide range) probe is achieved with the assistance of non-collinear optical parametric amplification and super-continuum generation [24]. This enables the comprehensive monitoring of the entire energy transfer process following the initial photoexcitation, and thus provides an easily accessible but powerful tool for coherent effect study.
Table 1. Commonly used ultrafast spectroscopy techniques.
| Technique | Advantages | Disadvantages |
|---|---|---|
| Visible transient absorption (TA) | Information about excited state dynamics such as internal conversion, ISC and so on. Simple setup. |
Blurred signals due to broadening. Mandatory requirement of optically allowed electronic transition. |
| Time-resolved vibrational spectroscopy (IR) | Information about molecular vibrations at excited state. | Mandatory requirement of symmetry allowed vibrational modes. Challenging to set up. Costly lasers and detectors. |
| Time-resolved X-ray diffraction |
Information about molecular structural changes at excited state. High temporal and spatial resolution. |
Requirement of specially prepared samples. Potentially sample damage by radiation. Complexity and synchrotron radiation facilities are usually required. |
| 2D spectroscopy | Enhanced resolution for signals mixed due to inhomogeneous broadening, compared to TA technique. Direct inspection of correlated state. |
Long data acquisition time, stable samples required. Challenging to set up. |
| Transient Raman | Information about molecular vibrations and conformational changes. | Weak signal. Interfered by fluorescence signal. |
| Time-resolved photoluminescence |
Wide range of detection time delay. Simple setup. |
Mandatory requirement of detectable photoluminescence of the sample. Inferior time resolution. |
The exceptional temporal resolution of ultrafast spectroscopy enables the unraveling of relaxation pathways during photochemical reactions, including the identification of intermediate states and their respective lifetimes. An in-depth understanding and tunability of the excited state dynamics can help optimize the energy transfer properties within or across molecules. Various efforts have been made to study the excited state dynamics of Pt complexes and other inorganic compounds. Here, several examples are listed to show the ability of ultrafast spectroscopies, to name a few. The time-resolved infrared spectroscopy is used to monitor the electron transfer in a Pt(II)-trans-acetylide motif. The electron transfer rate is altered by mode-specific infrared excitation of vibrations that are coupled to the transfer pathway [25]. Two-dimensional infrared spectroscopy is used to track energy transfer across metal centers in platinum complexes featuring a triazole-terminated alkyne ligand of two and six carbons, a perfluorophenyl ligand, and two tri(p-tolyl)phosphine ligands, respectively. Vibrational modes and their coupling even hidden under a complicated linear spectrum of the compound are identified. The transfer is efficient if the high-frequency modes involved are delocalized over both ligands’ high-frequency modes, showing an oscillation of the cross peak along the waiting time [26]. Femtosecond transient absorption is also used to clarify the excited state decay pathway. With the aid of global fitting, the ISC time is measured in a N^N Pt(II) complex with two heteroleptic ligands sample [27].
The vibrational wave packets are commonly detected during the ISC events in transition metal complex dimers and trimers. For complex [FeII(bpy)3]2+ (bpy=2,2′-bipyridine), vibrational wave packets appear in the high spin excited quintet 5T2 state shown as a beating signal over the excited-state absorption region in the first picosecond in transient absorption spectra [28][29]. In the case of [Au(CN)2−] dimer or trimer oligomer, oscillation of the transient absorption in the first picosecond region accompanied with a drastic shortening of the Au-Au bonds is observed [30][31]. Similar metal–metal bond contraction was also observed in Pt(II) dimers [32][33]. The vibrational wavepacket is able to coherently transfer from an initially excited state to another state of different spin after ISC, which is observed in Pt(pop), Pt(ppy) and Pt(bzq) [34][35].
The temporal evolution of vibrational wavepacket provides a maker to inspect, if not able to act as a handle to control the energy relaxation trajectory of chemical reaction [36][37][38][39][40]. As depicted in Figure 2, a molecule is excited by a laser pulse with a time duration so short that the excitation process is absent of a nuclear motion. Born–Oppenheimer approximation is used here to write product state wavefunctions to designate vibronic levels though it is usually not applicable near conical intersections, where energy transfer between states happens. The resulting excited state encompasses multiple vibrational states as a short-duration laser pulse typically covers a wide frequency domain. The vibrational states move in phase, forming a localized vibrational wavepacket. This vibrational wavepacket resides in a non-equilibrium position, which can be described by the Huang–Rhys factor and evolves over time. It essentially represents the molecule oscillating about its equilibrium position on the potential energy surface (PES), moving back and forth along the PES in relation to the potential coordinate. The coherence is gradually destroyed due to the coupling between the states with thermal bath.
The CVWP could be detected by various laser techniques, which usually behaves as one or several beating signals superimposed on the detected signal depending on the spectroscopy used [38][41][42][43][44]. Here, researchers limit the scope within the visible range white light pump–probe technique and diplatinum complex, in which an instantaneous light excitation ensures the vibration wavepacket by vertical transition. The evolution of the wavepacket is probed by a second laser pulse with its frequencies satisfying the Franck–Condon condition for vertical transitions, either on the S1 excited state or on other excited states. In contrary to the vibrational spectroscopy based on infrared lasers and X-ray spectroscopies, traditional pump–probe with supercontinuum white light gives an easy access for researchers to the structure and excited state dynamics of molecules. The readers could also refer to reviews for two-dimensional vibrational spectroscopy and X-ray spectroscopies [20][22][45].

Figure 2. (a) Scheme of vibrational wavepacket induced by laser pulse in the excited state. The wavepacket is a linear combination of the oscillator eigenfunctions with coefficients cn. The vertical arrow indicates an optical excitation of laser pulses. (b) Top: experimental stimulated emission spectra at different delay times for Pt-pop in ethylene glycol, excited at 370 nm. Bottom: simulated emission spectra for different delay times expressed as a function of the vibrational period T = 220 fs for the average vibrational quantum number of 6. The vertical grid lines facilitate the observation of the quantum interference fringes.
The vibrational coherence revealed by ultrafast spectroscopy offers insights into the states involved in energy transfer and the molecular structural evolution. Consequently, it serves as a metric to differentiate between active and spectator relaxation channels. Nevertheless, some experiments yield conflicting results, highlighting the intricate interplay between electronic and vibrational states [32][46][47]. As a result, the full comprehension of vibrational wavepacket behavior is yet to be achieved to elucidate the dynamics of excited states in chemical reactions.
References
- Brédas, J.-L.; Sargent, E.H.; Scholes, G.D. Photovoltaic concepts inspired by coherence effects in photosynthetic systems. Nat. Mater. 2017, 16, 35–44.
- Collini, E.; Wong, C.Y.; Wilk, K.E.; Curmi, P.M.; Brumer, P.; Scholes, G.D. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 2010, 463, 644–647.
- Nelson, T.R.; Ondarse-Alvarez, D.; Oldani, N.; Rodriguez-Hernandez, B.; Alfonso-Hernandez, L.; Galindo, J.F.; Kleiman, V.D.; Fernandez-Alberti, S.; Roitberg, A.E.; Tretiak, S. Coherent exciton-vibrational dynamics and energy transfer in conjugated organics. Nat. Commun. 2018, 9, 2316.
- Haedler, A.T.; Kreger, K.; Issac, A.; Wittmann, B.; Kivala, M.; Hammer, N.; Köhler, J.; Schmidt, H.-W.; Hildner, R. Long-range energy transport in single supramolecular nanofibres at room temperature. Nature 2015, 523, 196–199.
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; Van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774.
- Sung, J.; Kim, P.; Fimmel, B.; Würthner, F.; Kim, D. Direct observation of ultrafast coherent exciton dynamics in helical π-stacks of self-assembled perylene bisimides. Nat. Commun. 2015, 6, 8646.
- Wan, Q.; Yang, J.; To, W.-P.; Che, C.-M. Strong metal-metal Pauli repulsion leads to repulsive metallophilicity in closed-shell d8 and d10 organometallic complexes. Proc. Natl. Acad. Sci. USA 2021, 118, e2019265118.
- Mann, K.R.; Gordon, J.; Gray, H.B. Characterization of oligomers of tetrakis (phenyl isocyanide) rhodium (I) in acetonitrile solution. J. Am. Chem. Soc. 1975, 97, 3553–3555.
- Biedermann, J.; Gliemann, G.; Klement, U.; Range, K.-J.; Zabel, M. Spectroscopic studies of cyclo-metallated Pt (lI) complexes: Optical absorption and emission and the structure of single crystal ·H2O (bpm = 2, 2′-bipyrimidine). Inorganica Chim. Acta 1990, 169, 63–70.
- Miskowski, V.M.; Houlding, V.H. Electronic spectra and photophysics of platinum(II) complexes with a-diimine ligands solid-state effects. 2. Metal-metal interaction in double salts and linear chains. Inorg. Chem. 1991, 30, 4446–4452.
- Yip, H.-K.; Che, C.-M.; Zhou, Z.-Y.; Mak, T. Photophysical Properties and X-ray Crystal Structure of a Luminescent Platinum (II) Dimer (CIO4)3.H2O (Gua = guanidine anion). Chem. Commun. 1992, 1369, 1369–1371.
- Ma, B.; Li, J.; Djurovich, P.I.; Yousufuddin, M.; Bau, R.; Thompson, M.E. Synthetic control of pt…pt separation and photophysics of binuclear platinum complexes. J. Am. Chem. Soc. 2005, 127, 28–29.
- Yam, V.W.-W.; Wong, K.M.-C.; Zhu, N. Solvent-Induced Aggregation through Metal…Metal/π…π Interactions: Large Solvatochromism of Luminescent Organoplatinum (II) Terpyridyl Complexes. J. Am. Chem. Soc. 2002, 124, 6506–6507.
- Wan, Q.; To, W.-P.; Yang, C.; Che, C.-M. The metal-metal-to-ligand charge transfer excited state and supramolecular polymerization of luminescent pincer PdII-isocyanide complexes. Angew. Chem. Int. Ed. 2018, 57, 3089–3093.
- Wan, Q.; Xia, J.; Lu, W.; Yang, J.; Che, C.-M. Kinetically controlled self-assembly of phosphorescent AuIII aggregates and ligand-to-metal–metal charge transfer excited state: A combined spectroscopic and DFT/TDDFT study. J. Am. Chem. Soc. 2019, 141, 11572–11582.
- Ly, K.T.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68.
- Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N.S.; De Cola, L. When self-assembly meets biology: Luminescent platinum complexes for imaging applications. Chem. Soc. Rev. 2014, 43, 4144–4166.
- Roundhill, D.M.; Gray, H.B.; Che, C.-M. Pyrophosphito-bridged diplatinum chemistry. Acc. Chem. Res. 1989, 22, 55–61.
- Maiuri, M.; Garavelli, M.; Cerullo, G. Ultrafast Spectroscopy: State of the Art and Open Challenges. J. Am. Chem. Soc. 2020, 142, 3–15.
- Chergui, M.; Collet, E. Photoinduced Structural Dynamics of Molecular Systems Mapped by Time-Resolved X-ray Methods. Chem. Rev. 2017, 117, 11025–11065.
- Fayer, M.D. Dynamics of Liquids, Molecules, and Proteins Measured with Ultrafast 2D IR Vibrational Echo Chemical Exchange Spectroscopy. Annu. Rev. Phys. Chem. 2009, 60, 21–38.
- Kraack, J.P.; Hamm, P. Surface-Sensitive and Surface-Specific Ultrafast Two-Dimensional Vibrational Spectroscopy. Chem. Rev. 2017, 117, 10623–10664.
- Brixner, T.; Mančal, T.; Stiopkin, I.V.; Fleming, G.R. Phase-stabilized two-dimensional electronic spectroscopy. J. Chem. Phys. 2004, 121, 4221–4236.
- Dubietis, A.; Couairon, A. Governing Physical Effects. In Ultrafast Supercontinuum Generation in Transparent Solid-State Media; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–26.
- Delor, M.; Scattergood, P.A.; Sazanovich, I.V.; Parker, A.W.; Greetham, G.M.; Meijer, A.J.H.M.; Towrie, M.; Weinstein, J.A. Toward control of electron transfer in donor-acceptor molecules by bond-specific infrared excitation. Science 2014, 346, 1492–1495.
- Leong, T.X.; Collins, B.K.; Dey Baksi, S.; Mackin, R.T.; Sribnyi, A.; Burin, A.L.; Gladysz, J.A.; Rubtsov, I.V. Tracking Energy Transfer across a Platinum Center. J. Phys. Chem. A 2022, 126, 4915–4930.
- Wang, P.; Koo, Y.H.; Kim, W.; Yang, W.; Cui, X.; Ji, W.; Zhao, J.; Kim, D. Broadband Visible Light Harvesting N^N Pt(II) Bisacetylide Complex with Bodipy and Naphthalene Diimide Ligands: Förster Resonance Energy Transfer and Intersystem Crossing. J. Phys. Chem. C 2017, 121, 11117–11128.
- Consani, C.; Prémont-Schwarz, M.; ElNahhas, A.; Bressler, C.; van Mourik, F.; Cannizzo, A.; Chergui, M. Vibrational Coherences and Relaxation in the High-Spin State of Aqueous 2+ Angew. Chem. Int. Ed. Engl. 2009, 48, 7184–7187.
- Auböck, G.; Chergui, M. Sub-50-fs photoinduced spin crossover in 2+. Nat. Chem. 2015, 7, 629–633.
- Iwamura, M.; Nozaki, K.; Takeuchi, S.; Tahara, T. Real-Time Observation of Tight Au–Au Bond Formation and Relevant Coherent Motion upon Photoexcitation of Oligomers. J. Am. Chem. Soc. 2013, 135, 538–541.
- Iwamura, M.; Wakabayashi, R.; Maeba, J.; Nozaki, K.; Takeuchi, S.; Tahara, T. Coherent vibration and ultrafast dynamics upon bond formation in excited dimers of an Au(I) complex. Phys. Chem. Chem. Phys. 2016, 18, 5103–5107.
- van der Veen, R.M.; Cannizzo, A.; van Mourik, F.; Vlček, A., Jr.; Chergui, M. Vibrational Relaxation and Intersystem Crossing of Binuclear Metal Complexes in Solution. J. Am. Chem. Soc. 2011, 133, 305–315.
- Haldrup, K.; Dohn, A.O.; Shelby, M.L.; Mara, M.W.; Stickrath, A.B.; Harpham, M.R.; Huang, J.; Zhang, X.; Møller, K.B.; Chakraborty, A.; et al. Butterfly Deformation Modes in a Photoexcited Pyrazolate-Bridged Pt Complex Measured by Time-Resolved X-ray Scattering in Solution. J. Phys. Chem. A 2016, 120, 7475–7483.
- Kim, P.; Valentine, A.J.S.; Roy, S.; Mills, A.W.; Chakraborty, A.; Castellano, F.N.; Li, X.; Chen, L.X. Ultrafast Excited-State Dynamics of Photoluminescent Pt(II) Dimers Probed by a Coherent Vibrational Wavepacket. J. Phys. Chem. C 2021, 12, 6794–6803.
- Monni, R.; Auböck, G.; Kinschel, D.; Aziz-Lange, K.M.; Gray, H.B.; Vlček, A.; Chergui, M. Conservation of vibrational coherence in ultrafast electronic relaxation: The case of diplatinum complexes in solution. Chem. Phys. Lett. 2017, 683, 112–120.
- Rose, T.S.; Rosker, M.J.; Zewail, A.H. Femtosecond real-time probing of reactions. IV. The reactions of alkali halides. J. Chem. Phys. 1989, 91, 7415–7436.
- Shen, Y.-C.; Cina, J.A. What can short-pulse pump-probe spectroscopy tell us about Franck-Condon dynamics? J. Chem. Phys. 1999, 110, 9793–9806.
- Vos, M.H.; Rappaport, F.; Lambry, J.-C.; Breton, J.; Martin, J.-L. Visualization of coherent nuclear motion in a membrane protein by femtosecond spectroscopy. Nature 1993, 363, 320–325.
- Jonas, D.M.; Bradforth, S.E.; Passino, S.A.; Fleming, G.R. Femtosecond Wavepacket Spectroscopy: Influence of Temperature, Wavelength, and Pulse Duration. J. Phys. Chem. 1995, 99, 2594–2608.
- Dantus, M.; Lozovoy, V.V. Experimental Coherent Laser Control of Physicochemical Processes. Chem. Rev. 2004, 104, 1813–1860.
- Letokhov, V.S.; Tyakht, V.V. Coherent Vibrational Wave Packet Dynamics in Femtosecond Laser Excitation of Diatomic Molecules. Isr. J. Chem. 1990, 30, 189–195.
- Song, Y.; Clafton, S.N.; Pensack, R.D.; Kee, T.W.; Scholes, G.D. Vibrational coherence probes the mechanism of ultrafast electron transfer in polymer–fullerene blends. Nat. Commun. 2014, 5, 4933.
- Cina, J.A.; Fleming, G.R. Vibrational Coherence Transfer and Trapping as Sources for Long-Lived Quantum Beats in Polarized Emission from Energy Transfer Complexes. J. Phys. Chem. A 2004, 108, 11196–11208.
- Duan, H.G.; Jha, A.; Li, X.; Tiwari, V.; Ye, H.; Nayak, P.K.; Zhu, X.L.; Li, Z.; Martinez, T.J.; Thorwart, M.; et al. Intermolecular vibrations mediate ultrafast singlet fission. Sci. Adv. 2020, 6, eabb0052.
- Turner, D.B.; Wilk, K.E.; Curmi, P.M.G.; Scholes, G.D. Comparison of Electronic and Vibrational Coherence Measured by Two-Dimensional Electronic Spectroscopy. J. Phys. Chem. Lett. 2011, 2, 1904–1911.
- Kim, P.; Kelley, M.S.; Chakraborty, A.; Wong, N.L.; Van Duyne, R.P.; Schatz, G.C.; Castellano, F.N.; Chen, L.X. Coherent vibrational wavepacket dynamics in platinum (II) dimers and their implications. J. Phys. Chem. C 2018, 122, 14195–14204.
- Cho, S.; Mara, M.W.; Wang, X.; Lockard, J.V.; Rachford, A.A.; Castellano, F.N.; Chen, L.X. Coherence in metal-metal-to-ligand-charge-transfer excited states of a dimetallic complex investigated by ultrafast transient absorption anisotropy. J. Phys. Chem. A 2011, 115, 3990–3996.
More
Information
Subjects:
Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
764
Revisions:
3 times
(View History)
Update Date:
24 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




