Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sol Maria Carriazo | -- | 1585 | 2023-11-19 16:33:56 | | | |
| 2 | Sirius Huang | Meta information modification | 1585 | 2023-11-21 02:08:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carriazo, S.; Abasheva, D.; Duarte, D.; Ortiz, A.; Sanchez-Niño, M.D. COVID-19 and Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/51784 (accessed on 07 February 2026).
Carriazo S, Abasheva D, Duarte D, Ortiz A, Sanchez-Niño MD. COVID-19 and Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/51784. Accessed February 07, 2026.
Carriazo, Sol, Daria Abasheva, Deborah Duarte, Alberto Ortiz, Maria Dolores Sanchez-Niño. "COVID-19 and Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/51784 (accessed February 07, 2026).
Carriazo, S., Abasheva, D., Duarte, D., Ortiz, A., & Sanchez-Niño, M.D. (2023, November 19). COVID-19 and Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/51784
Carriazo, Sol, et al. "COVID-19 and Kidney Disease." Encyclopedia. Web. 19 November, 2023.
Copy Citation
Chronic kidney disease (CKD) is the most common risk factor for severe COVID-19 and one that most increases the risk of COVID-19-related death. Moreover, CKD increases the risk of acute kidney injury (AKI), and COVID-19 patients with AKI are at an increased risk of death.
acute kidney injury
chronic kidney disease
SCARF
COVID-19
genetic predisposition
mortality
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 769 million confirmed cases of coronavirus disease 2019 (COVID-19) and over 6.95 million deaths worldwide, as reported by the World Health Organization (WHO), but the real numbers are believed to be much higher [1]. The WHO declared the COVID-19 outbreak a pandemic on 11 March 2020, and on 5 May 2023, declared that it is no longer a public health emergency of international concern [2]. The disease is now considered endemic. In this regard, it is still topical to advance our understanding of the pathogenesis of COVID-19 and the molecular mechanisms that determine the susceptibility to severe disease. Thus, the peak number of globally diagnosed cases was observed as recently as December 2022, and an uptick of cases driven by novel varieties was observed in July–August 2023 [1]. Additionally, any acquired knowledge will be helpful in managing the next viral pandemic outbreak. Moreover, reinfection contributed to additional risks of death (HR: 2.17, 95% CI 1.93–2.45), hospitalization (HR = 3.32, 95% CI 3.13–3.51), and sequelae among different organ systems, compared with no reinfection, despite vaccination status [3]. Kidney disorders showed the highest risk after reinfection (HR = 3.55, 95% CI = 3.18–3.97; burden = 38.31, 95% CI = 32.86–44.37), and this risk remained elevated independently of the status of vaccination [3] (Figure 1).
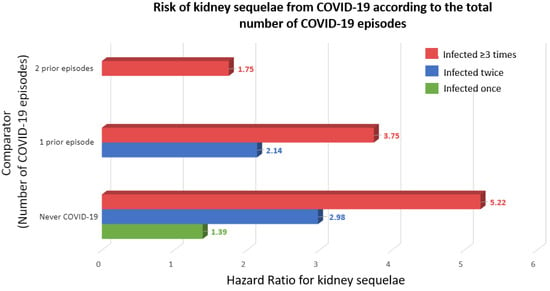
Figure 1. Risk of kidney sequelae from COVID-19 according to the total number of COVID-19 episodes [3]. Kidney sequelae were defined as acute kidney injury or chronic kidney disease. Outcomes were defined at time of first incidence of their component individual sequela.
2. Acute Kidney Injury and Chronic Kidney Disease
According to Kidney Disease: Improving Global Outcomes (KDIGO), kidney disease may be acute or chronic, and the diagnostic criteria are used to identify patients with adverse health outcomes [4][5]. Since 2002, CKD has been defined as the presence of structural and/or functional alterations in the kidneys persisting for longer than 3 months with an impact on health [6]. However, the current, more precise definition dates from 2012 [4][7]. This timing should be considered, as when the SARS-CoV-2 pandemic hit in 2020, most healthcare professionals worldwide had not studied the current concept of CKD in medical schools and neither had the health authorities. Consequently, the striking negative impact of COVID-19 in persons with CKD flew initially under the radar, unlike other comorbidities that were soon identified as risk factors for severe COVID-19, such as diabetes, hypertension, and cardiovascular disease [8]. The diagnostic criteria that by themselves allow for the detection of CKD include an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or urinary albumin–creatinine ratio ≥ 30 mg/g, or pathological changes in the urinary sediment, kidney histology or imaging, or kidney transplantation [7]. eGFR and albuminuria allow for the categorization of CKD into mild, moderate, or severe categories, which are associated with increasing risks of CKD progression, premature all-cause death, or AKI, among others [9] (Figure 2).
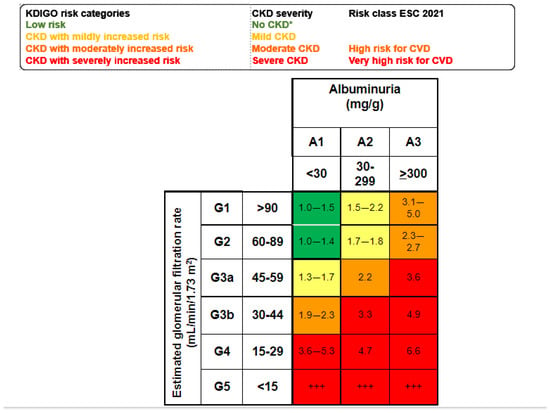
Figure 2. Mild, moderate, and severe CKD according to KDIGO risk categories based on simultaneous assessment of eGFR and albuminuria. Albuminuria is usually assessed as the urinary albumin–creatinine ratio (UACR in a spot urine sample). Figure shows the risk of all-cause death for each cell [4][9]. * No CKD if there is no other evidence of CKD as imaging, hematuria or others. +++ Kidney failure: patients needing kidney replacement therapy and cardiovascular risk is extremely high.
Globally, there are 850 million people with CKD, and the number is expected to increase as the world population ages [10][11][12]. Prior to the SARS-CoV-2 pandemic, it was estimated that, by 2040, CKD would be the fifth global cause of death, as it is growing faster than most other common causes of death [10][11]. Indeed, the loss of key kidney functions, such as the production of the anti-aging protein Klotho, or the decrease in glomerular filtration and tubular secretion of waste molecules leading to the accumulation of uremic toxins, is believed to accelerate biological aging, leading to premature death, mainly from cardiovascular causes, infection, malignancy, and the lack of access to kidney replacement therapy (KRT) [10].
The progression of CKD is frequently non-linear, as CKD predisposes to AKI, and AKI may accelerate the progression of CKD [13][14]. As for CKD, the cut-off points selected to define AKI (an increase in serum creatinine by ≥0.3 mg/dL within 48 h) are associated with an increased risk of death that increases to 45–50% in those requiring KRT and may persist for up to a year [15][16][17].
3. CKD as a Risk Factor for COVID-19
Identifying the factors associated with severe COVID-19 is important to protect those in high-risk groups and, eventually, develop specific therapeutic approaches if the molecular mechanisms underlying the increased susceptibility are identified. CKD is the most common and the most influential comorbidity associated with COVID-19 severity. According to the prevalence data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) together with the UN population estimates for 2020, CKD is deemed the most prevalent risk factor for severe COVID-19 in adults [18]. In addition, CKD alone explains the increased risk of severe COVID-19 in approximately 5% of the global population. Another study conducted in the UK using primary care electronic records of 17,278,392 persons examined the factors associated with COVID-19-related death [19]. While older age, expectedly, showed the strongest association with poor outcomes, CKD was among the top five comorbidities conferring the highest risk of COVID-19-related death. The adjusted HR was 2.52 (2.33–2.72) for subjects with G4–5 CKD (eGFR < 30 mL/min/1.73 m2), 3.53 (2.77–4.49) for transplant recipients, and 3.69 (3.09–4.39) for patients with kidney failure, including those receiving dialysis. The Madrid REMER Registry study also showed that, in 2020, COVID-19 was the most common cause of death among patients on kidney replacement therapy (KRT), with overall mortality increasing by 34% compared with the average in the previous decade [20]. In this regard, patients on dialysis are at high risk of death from infection as a category encompassing different microbes. While the rate of mortality from infection decreased from 224 to 163 per 10,000 person-years for those commencing dialysis in 1980–2005 and 2006–2018, respectively, it remains over 20-fold higher than in the general population and disproportionally affects women and minorities [21]. Thus, the increased severity of COVID-19 should not come as a surprise, and the key question is to what extent the predisposition of CKD patients to severe COVID-19 is a further manifestation of the non-specific immune suppression in this population, or whether it is driven by specific factors that merit specific management.
4. COVID-19, AKI, and Mortality
COVID-19 may be complicated by AKI. This may result from systemic cytokine release syndrome due to the SARS-CoV-2 infection of kidney cells, specific disease entities such as collapsing focal segmental glomerulosclerosis or thrombotic microangiopathy, or a combination of these and other factors [22][23][24][25][26]. As is the case for AKI in other contexts, AKI is associated with an increased risk of death in patients with COVID-19 [27][28][29]. In the regions and countries hit earliest and hardest by the pandemic, the lack of enough resources to provide both ventilation support and KRT to all those in need may have contributed to a further increase in the mortality of COVID-19 AKI [30]. The fact that there is evidence of the infection of kidney cells during AKI and that AKI in COVID-19 is associated with adverse outcomes means that it is also of interest to understand the susceptibility of kidney cells to SARS-CoV-2 infection. Indeed, the SARS-CoV-2 infection of kidney cells was associated with an increased risk of death in patients with COVID-19 [31].
5. Suboptimal Response to SARS-CoV-2 Vaccines in Some Patient Populations with CKD
The most obvious preventive measure for those at high risk of severe COVID-19 is vaccination [32]. However, patients on dialysis and, even more, kidney transplant recipients have suboptimal responses to vaccines. As an example, patients on dialysis require specially designed regimens to optimize the response to vaccination against hepatitis B virus, and despite that, some patients are not immunized or are immunized only transiently [33]. There are multiple anti-SARS-CoV-2 vaccines, and reviewing the CKD literature on all of them is beyond the scope of this text. However, the mRNA-based vaccines (BNT162b2 and mRNA-1273) have been prospectively assessed using the same methods in patients with CKD not on dialysis, on hemodialysis/peritoneal dialysis, and those who underwent transplantation, and this may provide a flavor of the issues. After the initial vaccination schedule, the BNT162b2 (30 μcg) vaccine was associated with a six-fold higher risk for negative humoral response than mRNA-1273 (100 μcg), likely due to the different dose, as is the case for hepatitis B virus vaccines [34]. During the first year after vaccination, patients with non-dialysis CKD and those on dialysis presented good anti-spike antibody responses. However, anti-spike antibodies decreased over time. A third dose of vaccine induced seroconversion in a high percentage of antibody-negative patients after two doses, although responses were poorer in kidney transplant recipients [35]. The fourth dose seroconverted 72% of previously negative patients. Higher anti-spike antibody titers at 12 months were independently associated with repeated exposure to antigen (fourth vaccine dose and/or previous breakthrough infections). Breakthrough COVID-19 requiring admission was observed in CKD patients with lower antibody titers, and the least benefit from the fourth dose was observed in patients with the highest need for a vaccine booster (i.e., those with lower pre-booster antibody titers or kidney transplant recipients) [36]. Overall, kidney transplant recipients presented suboptimal responses after any vaccination schedule (initial, third, and fourth dose). Some patients had a persistently negative humoral response despite boosters [37], and the mortality of patients on KRT remains high despite increased access to critical care [38].
References
- WHO Coronavirus Dashboard. Available online: https://covid19.who.int/ (accessed on 27 July 2023).
- Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed on 22 June 2019).
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405.
- Kidney Disease: Improving Global Outcomes (KDIGO); CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150.
- KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. 1), i–ii+S1–S266.
- Perez-Gomez, M.V.; Bartsch, L.-A.; Castillo-Rodriguez, E.; Fernandez-Prado, R.; Fernandez-Fernandez, B.; Martin-Cleary, C.; Gracia-Iguacel, C.; Ortiz, A. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin. Kidney J. 2019, 12, 258–261.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Ortiz, A.; Wanner, C.; Gansevoort, R.T.; Cozzolino, M.; Fliser, D.; Gambaro, G.; Ong, A.; Rosenkranz, A.R.; Rychlık, I.; Sarafidis, P.; et al. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: A position statement by the Council of the European Renal Association. Clin. Kidney J. 2022, 16, 403–407.
- AIRG-E; EKPF; ALCER; FRIAT; REDINREN; RICORS2040; SENEFRO; SET; ONT. CKD: The burden of disease invisible to re-search funders. Nefrologia 2022, 42, 65–84.
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090.
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805.
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66.
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083.
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493.
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initi-ative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643.
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52.
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, e1003–e1017.
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Carriazo, S.; Aparicio-Madre, M.I.; Tornero-Molina, F.; Fernández-Lucas, M.; Paraiso-Cuevas, V.; González-Parra, E.; del Río-Gallegos, F.; Marques-Vidas, M.; Alcázar-Arroyo, R.; Martins-Muñoz, J.; et al. Impact of different COVID-19 waves on kidney replacement therapy epidemiology and mortality: REMER 2020. Nephrol. Dial. Transplant. 2022, 37, 2253–2263.
- Chong, C.H.; Au, E.H.; Davies, C.E.; Jaure, A.; Howell, M.; Lim, W.H.; Craig, J.C.; Teixeira-Pinto, A.; Wong, G. Long-term Trends in Infection-Related Mortality in Adults Treated With Maintenance Dialysis. Am. J. Kidney Dis. 2023, 82, 597–607.
- Carriazo, S.; Kanbay, M.; Ortiz, A. Kidney disease and electrolytes in COVID-19: More than meets the eye. Clin. Kidney J. 2020, 13, 274–280.
- Radovic, S.; Meng, W.; Chen, L.; Paniz Mondolfi, A.E.; Bryce, C.; Grimes, Z.; Sordillo, E.M.; Cordon-Cardo, C.; Guo, H.; Huang, Y.; et al. SARS-CoV-2 infection of kidney tissues from se-vere COVID-19 patients. J. Med. Virol. 2023, 95, e28566.
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8.
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; et al. Human kidney is a target for novel severe acute respiratory syn-drome coronavirus 2 infection. Nat. Commun. 2021, 12, 2506.
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592.
- Rubin, S.; Orieux, A.; Prevel, R.; Garric, A.; Bats, M.L.; Dabernat, S.; Camou, F.; Guisset, O.; Issa, N.; Mourissoux, G.; et al. Characterization of acute kidney injury in critically ill pa-tients with severe coronavirus disease 2019. Clin Kidney J. 2020, 13, 354–361.
- Roushani, J.; Thomas, D.; Oliver, M.J.; Ip, J.; Tang, Y.; Yeung, A.; Taji, L.; Cooper, R.; Magner, P.O.; Garg, A.X.; et al. Acute kidney injury requiring renal replacement therapy in people with COVID-19 disease in Ontario, Canada: A prospective analysis of risk factors and outcomes. Clin. Kidney J. 2021, 15, 507–516.
- Wan, Y.I.; Bien, Z.; Apea, V.J.; Orkin, C.M.; Dhairyawan, R.; Kirwan, C.J.; Pearse, R.M.; Puthucheary, Z.A.; Prowle, J.R. Acute kidney injury in COVID-19: Multicentre prospective analysis of registry data. Clin. Kidney J. 2021, 14, 2356–2364.
- Portolés, J.; López-Sánchez, P.; Martin-Rodríguez, L.; Serrano-Salazar, M.L.; Valdenebro-Recio, M.; Ramos, A.; Malo, R.M.; Zalamea, F.; Martin-Giner, J.M.; Marques, M.; et al. Acute and chronic kidney disease and risk of hospital mortality during COVID-19 pandemic waves in the pre-vaccination era. Clin. Kidney J. 2022, 16, 374–383.
- Isnard, P.; Vergnaud, P.; Garbay, S.; Jamme, M.; Eloudzeri, M.; Karras, A.; Anglicheau, D.; Galantine, V.; Eddine, A.J.; Gosset, C.; et al. A specific molecular signature in SARS-CoV-2-infected kidney biopsies. JCI Insight. 2023, 8, e165192.
- Barouch, D.H. COVID-19 Vaccines–Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020.
- Kong, N.C.T.; Beran, J.; Kee, S.A.; Miguel, J.L.; Sánchez, C.; Bayas, J.M.; Vilella, A.; Calbo-Torrecillas, F.; de Novales, E.L.; Srinivasa, K.; et al. A new adjuvant improves the immune response to hepati-tis B vaccine in hemodialysis patients. Kidney Int. 2008, 73, 856–862.
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Martínez Vaquera, S.; Jarava Mantecón, C.J.; Useche, G.; Sánchez Márquez, M.G.; Carnerero, M.; Jaldo Rodríguez, M.T.; Muñoz Ramos, P.; et al. Safety and immediate humoral re-sponse of COVID-19 vaccines in chronic kidney disease patients: The SENCOVAC study. Nephrol. Dial. Transplant. 2022, 37, 1868–1878.
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Orero, E.; Tejedor, S.; Mantecón, C.J.J.; Perez, V.O.G.; Franco, A.J.M.; Sánchez, C.A.; Carretero, M.P.; et al. Humoral Response to Third Dose of SARS-CoV-2 Vac-cines in the CKD Spectrum. Clin. J. Am. Soc. Nephrol. 2022, 17, 872–876.
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Jarava Mantecón, C.J.; Gomes Pérez, V.O.; Bordils, A.; Lacueva, J.; Marin Franco, A.J.; Delgado Conde, P.; Muñoz Ramos, P.; et al. Humoral response after the fourth dose of the SARS-CoV-2 vaccine in the CKD spectrum: A prespecified analysis of the SENCOVAC study. Nephrol. Dial. Transplant. 2023, 38, 969–981.
- Quiroga, B.; Soler, M.J.; Ortiz, A.; de Sequera, P.; SENCOVAC Collaborative Network. Lessons from SENCOVAC: A prospective study evaluating the re-sponse to SARS-CoV-2 vaccination in the CKD spectrum. Nefrologia 2023, in press.
- Quiroga, B.; Ortiz, A.; Cabezas-Reina, C.J.; Ruiz Fuentes, M.C.; López Jiménez, V.; Zárraga Larrondo, S.; Toapanta, N.; Molina Gómez, M.; de Sequera, P.; Sánchez-Álvarez, E.; et al. Evolving spectrum but persistent high mortality of COVID-19 among patients on kidney replacement therapy in the vaccine era: The Spanish COVID-19 KRT Registry. Clin. Kidney J. 2022, 15, 1685–1697.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
412
Revisions:
2 times
(View History)
Update Date:
21 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




