Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Igor Dmitrievich Zlotnikov | -- | 7116 | 2023-11-17 17:44:14 | | | |
| 2 | Mona Zou | + 4 word(s) | 7120 | 2023-11-20 09:08:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Savchenko, I.V.; Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic Systems Involving Macrophages in Targeted Drug Delivery. Encyclopedia. Available online: https://encyclopedia.pub/entry/51762 (accessed on 07 February 2026).
Savchenko IV, Zlotnikov ID, Kudryashova EV. Biomimetic Systems Involving Macrophages in Targeted Drug Delivery. Encyclopedia. Available at: https://encyclopedia.pub/entry/51762. Accessed February 07, 2026.
Savchenko, Ivan V., Igor D. Zlotnikov, Elena V. Kudryashova. "Biomimetic Systems Involving Macrophages in Targeted Drug Delivery" Encyclopedia, https://encyclopedia.pub/entry/51762 (accessed February 07, 2026).
Savchenko, I.V., Zlotnikov, I.D., & Kudryashova, E.V. (2023, November 17). Biomimetic Systems Involving Macrophages in Targeted Drug Delivery. In Encyclopedia. https://encyclopedia.pub/entry/51762
Savchenko, Ivan V., et al. "Biomimetic Systems Involving Macrophages in Targeted Drug Delivery." Encyclopedia. Web. 17 November, 2023.
Copy Citation
The concept of targeted drug delivery can be described in terms of the drug systems’ ability to mimic the biological objects’ property to localize to target cells or tissues. For example, drug delivery systems based on red blood cells or mimicking some of their useful features, such as long circulation in stealth mode, have been known for decades. Therapeutic strategies based on macrophages gradually gain more attention.
macrophage-mediated therapy
macrophage biomimetics
macrophage-derived particles
selective ligands
1. Introduction
Due to the increased risk of infectious diseases, with the lack of ways to effectively treat oncology, the need to develop and improve treatment methods, in particular, drug therapy, increases. However, drug therapy of inflammatory diseases, including oncology and infectious diseases, has limitations, since drugs in current use often have significant drawbacks, such as toxicity to healthy tissues, immunoreactivity, a short circulation time and low stability in the biological media. In this regard, systems of targeted drug delivery to the pathology area (site of inflammation or tumor) and/or to individual pathogens (viruses, bacteria, parasites, etc.) are of the greatest interest. Due to the selectivity of the drug in this case, it is possible to avoid its effect on healthy tissues and organs of the body and reduce the effective dose required for treatment.
Thanks to the use of nanoparticles, it became possible to partially overcome such problems as the low solubility of the drug in biological fluids, the low stability of biodegradable therapeutic agents and their toxic effect on biological systems [1][2][3]. However, despite the high ability of nanoparticles to cross many biological barriers and diffuse in intercellular and cellular media, the development of a targeted drug delivery method is still required. Recently, cell-mediated drug delivery using red blood cells, neutrophils, macrophages, stem cells and lymphocytes has attracted much attention, due to its multifunctionality and inherent stability in biological media.
2. Macrophage-Mediated Therapy via Macrophage Targeting
2.1. Design of Therapeutic Agents Targeting Macrophages
Macrophages mediate the pathological processes of inflammatory diseases, including oncology and infectious diseases. Since macrophages are closely related to tumor development, as well as due to the existence of pathogens acting through macrophages, studies aimed at the design of drug-loaded micro- and nanoparticles targeting macrophages are of great interest.
Therapeutic agents in the form of drug-loaded particles can be delivered to particular organs or cells based on their physicochemical properties, such as their size, shape, charge and solubility (passive targeting), or can be delivered to macrophages via specific targeting ligands (active targeting).
2.1.1. Passive Macrophage-Targeting Therapeutic Agents
Passive delivery is based on the pharmacokinetics of NPs, the enhanced permeability and retention (EPR) effect, and immune responses of the targeted tissue, leading to the accumulation of NPs. The efficiency of capture of particles by macrophages as part of a passive delivery strategy is affected by their following parameters: size, shape, surface charge and hydrophilicity.
-
Size
The size of the particles can affect the efficiency of their capture by macrophages, although the cellular uptake depends on environmental conditions. For instance, the uptake of non-modified liposomes by rat alveolar macrophages in vitro increased with an increase in particle size and became constant at 1000 nm, while the uptake of non-modified liposomes by alveolar macrophages in vivo increased with an increase in particle size in the range 100–2000 nm, which was due to the opsonization by lung surfactant proteins [4]. In addition, it is reported that the size of the particles affects the phagocytic capacity, endocytosis speed and endocytosis mechanism of the cell [5][6].
It is also known that bio-distribution in RES organs is affected by particle size. For example, NPs up to 500 nm in size accumulate in the liver and lungs; NPs of 10–300 nm in size accumulate predominantly in the liver and spleen; and NPs of 1–20 nm in size are usually degraded by macrophages in the kidneys [7][8]. In addition, epithelial destruction and vascular leakage occur in areas of inflammation and in solid tumors [9][10]. Therefore, NPs with a proper size can preferentially extravasate from the blood into the interstitial spaces and accumulate in inflammation sites or tumor tissues via the EPR effect [11][12].
- b.
-
Shape
Particle shape has a significant impact on macrophage cellular uptake and can be exploited for controlling the efficacy of drug delivery to macrophages. Smith et al. [13] proved that particle shape independently influences binding and internalization by macrophages. Interestingly, they found that the attachment of particles to macrophages could be ranked in the following order: prolate ellipsoids > oblate ellipsoids > spheres. However, the internalization of particles followed a different rank: oblate ellipsoids >> spheres > prolate ellipsoids. The effect of the particle shape can be explained by the fact that endocytosis is an actin-dependent process, and, therefore, the internalization of particles with a larger aspect ratio requires more energy to perform the cytoskeleton remodeling [8][13][14].
- c.
-
Surface charge and hydrophilicity
Surface charge is another factor that influences macrophage uptake, and many assays both in vitro and in vivo have indicated that charged particles are more likely to be taken up by macrophages than neutral particles. Despite the fact that the absorption of positively charged particles by cells is usually easier as a result of electrostatic interactions [15], it has been shown that the same increase in cellular uptake by macrophages can be achieved with an increase in both the negative and positive charge of particles [15][16][17]. However, the role of charge on macrophage uptake is still controversial, with contradictory observations in the literature [18].
Hydrophilicity is another parameter that strongly affects the capture of particles by macrophages in vivo. Hydrophilicity, as well as surface charge, can impact the adsorption of opsonin, thus influencing the uptake of NPs by macrophages. Increased hydrophilicity results in a lower degree of protein adsorption and reduced uptake by macrophages [19][20][21]. It is often used to hide NPs from MPS by covering them with PEG [22].
2.1.2. Active Macrophage-Targeting Therapeutic Agents
Active targeting can significantly enhance the selectivity of macrophage-mediated therapy due to specific interactions between the therapeutic agent and the cell. This approach involves the direct use of the agonists or antagonists of macrophage receptors, or modification of the surface of NPs with ligands or an antigen in order to establish selective interaction with macrophage receptors. Many studies demonstrate the advantages and therapeutic potential of the active targeting of macrophages in the treatment of oncological and infectious diseases. In this regard, promising results can be attained by using therapeutic agents specifically delivering drugs to macrophages (Table 1).
Table 1. Examples of utilizing macrophage-targeting therapeutic agents.
| Receptor Targeting | Carrier Formulation | Ligand Modification/Coating | Cargo | Purpose | Result | Refs. |
|---|---|---|---|---|---|---|
| Mannose receptor | Liposomes | Mannose | DNA | Stimulation of immune response | Mannosylated cationic liposomes exhibited significantly improved DNA delivery compared to unmodified liposomes | [23] |
| Polymeric micelles | siRNA | TAM repolarization | Modified micelles could selectively deliver efficacious amounts of functional siRNA into TAMs | [24] | ||
| Liposomes | 64Cu | PET imaging of TAMs | Highly selective accumulation of the liposomes in TAMs was observed | [25] | ||
| Selenium NPs | Isoniazid | Treatment of tuberculosis | The NPs preferentially entered macrophages and accumulated in lysosomes, releasing isoniazid | [26] | ||
| Galactose receptor | Dextran NPs | Galactose | CpG, anti-IL-10 and anti-IL-10 receptor oligonucleotides | TAM repolarization | NPs accumulated in the tumor and was taken up predominantly by TAMs | [27] |
| Chitosan-cysteine NPs | siRNA | Treatment of ulcerative colitis | Galactose modification significantly facilitated the uptake by macrophages and targeting ability of the NPs | [28] | ||
| Poly(lactic-co-glycolic acid) NPs | Dexamethasone | Development of the strategy to catch macrophages during intestinal inflammation | NPs were effectively captured by macrophages | [29] | ||
| Dectin-1 | Polymer–lipid hybrid NPs | Yeast cell wall microparticles, containing β-1,3-D-glucan | Cabazitaxel | Development of oral targeted drug delivery | The microparticles were rapidly and efficiently taken up by macrophages | [30] |
| Mesoporous silica NPs | Doxorubicin | Development of anti-tumor therapy | Drug delivery to macrophages was enhanced compared to uncoated silica NPs | [31] | ||
| Fc receptor | Alginate NPs | Tuftsin | DNA | Development of anti-inflammatory agents | Tuftsin-modified NPs were rapidly internalized in murine macrophages | [32] |
| Folate receptor-β (FRβ) | - | Anti-mouse FRβ monoclonal antibody | Pseudomonas exotoxin A | TAM depletion | Direct eliminating of TAMs was attained | [33] |
| Poly(amidoamine) dendrimers | Folic acid | Methotrexate | Alleviating of the inflammatory disease of arthritis | High degree of specific binding and internalization of the dendrimers into macrophages was observed | [34] | |
| Human serum albumin nanocapsules | - | Evaluating targeting ability of folic acid-modified agents | The internalization of nanocapsules was enhanced via FR specificity | [35] | ||
| CD44 | Hyaluronic acid–tocopherol succinate micelles | Hyaluronic acid | Rifampicin | Development of tuberculosis treatment | Micelles exhibited significant phagocytosis and a CD44-dependent uptake in comparison to free drug | [36] |
| Liposomes | Prednisolone | Development of rheumatoid arthritis therapy | Enhanced cellular uptake, mainly mediated by caveolae- and clathrin-dependent endocytosis, was achieved | [37] | ||
| Poly(lactic-co-glycolic acid) NPs | Curcumin | Alleviation of ulcerative colitis | Enhanced drug delivery to intestinal macrophages and selective accumulation in inflamed colitis tissue with minimal accumulation in healthy colon tissue was observed | [38] | ||
| Siglec-1 | Liposomes | Sialic acid | Epirubicin | Tumor therapy | The tumor-targeting efficiency and the accumulation of epirubicin in monocytes was improved compared to unmodified liposomes | [39] |
| Zoledronic acid | TAM depletion and repolarization | High targeting ability was observed | [40] |
Below are the main approaches to active macrophage targeting based on the interaction of therapeutic agents with different macrophage receptors (Figure 1).
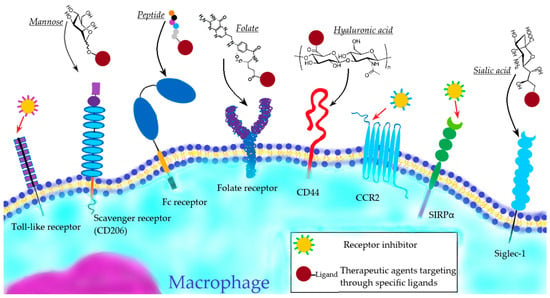
Figure 1. Active macrophage targeting via different macrophage receptors.
-
Toll-like receptor targeting
Toll-like receptors (TLRs) are well-defined pattern recognition receptors responsible for pathogen recognition and the induction of innate immune responses via signaling pathways. TLRs can detect various endogenous damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) [41]. The activation of TLRs initiate a variety of downstream signaling cascades and signaling pathways, leading to the production of inflammatory cytokines or type I IFNs [42]. The activation of TLR signaling is also crucial to the induction of antigen-specific adaptive immune responses by activating the adaptive immune cells for the clearance of invading pathogens [41].
Among the functional TLRs identified in humans, some are localized on the cell surface (TLR1, TLR2, TLR4, and TLR5) and others in intracellular compartments (TLR3, TLR7, TLR8, and TLR9) [41]. Cell-surface TLRs mainly detect membrane components of the pathogens such as proteins, lipoproteins, lipids and lipopolysaccharides (LPS), while intracellular TLRs mainly recognize nucleic acids derived from pathogens or self-nucleic acids in a pathological condition [41].
Since TLRs are involved in the production of pro-inflammatory mediators and the activation of immune responses, TLRs present an attractive target for the more precise manipulation of the function of macrophages [43]. Recent studies have demonstrated that TLR pathways play a significant role in polarizing macrophages. Therefore, TLRs can serve as a target for modeling a macrophage’s phenotype, for example, as part of tumor treatment via TAM reprogramming [44]. In addition, TLR ligands have found application in the context of infectious, inflammatory and autoimmune diseases [43].
- b.
-
Scavenger receptor targeting
Scavenger receptors (SRs) are a diverse superfamily of cell surface receptors. They are expressed by myeloid cells (macrophages and dendritic cells) and certain endothelial cells. Being a subclass of the membrane-bound pattern recognition receptors (PRRs), they play an important role in the cellular uptake and clearance of endogenous host molecules and apoptotic cells, and exogenous components marked with pathogen-associated molecular patterns (PAMPs) [45]. Removal is often carried out by simple endocytosis but might entail more complex processes, such as micropinocytosis or phagocytosis, which both require elaborate signal transduction [45][46].
Due to the fact that SRs are involved in phagocytosis and in endocytosis, these receptors are potential intermediaries in macrophage-targeting therapy, which can facilitate the selective delivery of therapeutic agents into macrophages. C-type lectin receptors (CLRs), which recognize conserved carbohydrate structures, attract a lot of attention [47]. In this regard, many assays are devoted to developing drug nanocarriers targeting mannose receptor (also known as CD206) [48][49]. Thus, mannosylated therapeutic agents are of great interest and many researchers have shown that a high targeting ability can be achieved via the modification of drug nanocarriers with mannose [50][51][52]. Other pathogen-associated components that are used for SR-mediated targeted delivery are galactose [53][54][55], dextran and its derivatives [56][57][58]. Selective delivery can also be achieved by encapsulating therapeutic agents in glucan particles derived from the yeast cell wall, which can be recognized by CLR Dectin-1 [30][31]. In addition, bio-nanocapsules (BNCs) derived from pathogens, such as virus envelope particles [59] or bacteria-like particles [60][61], can be directly used as macrophage-targeted drug carriers.
- c.
-
Fc-receptor targeting
So-called Fc-receptors (FcRs) for different immunoglobulin isotypes (IgA, IgE, IgM, and IgG) are involved in regulating and executing antibody-mediated responses [62]. FcRs are widely expressed on cells of the immune system, including macrophages [62]. These receptors recognize antibodies that are attached to infected cells or invading pathogens and stimulate phagocytosis and endocytosis [63].
Since FcR activation stimulates phagocytosis and endocytosis, FcR-mediated drug delivery strategies targeting macrophages have been developed. In this regard, tuftsin—a tetrapeptide formed by the enzymatic cleavage of the Fc portion of the immunoglobulin (IgG) molecule—has gained a lot of attention due to its ability to activate FcR [32][64][65][66]. For instance, Jain et al. [32] developed tuftsin-modified NPs and noted a much higher cellular uptake by macrophages in vitro than non-modified or scrambled peptide-modified NPs. In addition, a tuftsin derivative, tuftsin tetramer, can dramatically enhance uptake into macrophages [67].
- d.
-
Targeting of other receptors
In addition to the above-mentioned important receptors expressed by macrophages, the folate receptors [68] and CD44 [69] are considered to be potential mediators in macrophage-targeting therapy strategies.
Folate receptors are expressed on the surface of activated macrophages, known to be upregulated in the macrophages in rheumatoid arthritis and pulmonary fibrosis [70]. A high macrophage-targeting ability can be achieved by the modification of therapeutic agents with folic acid. Thus, folate-conjugated particles, such as dendrimers [34], chitosan NPs [71], liposomes [72] and human serum albumin NPs [35] exhibited enhanced macrophage uptake when compared with non-folated particles.
CD44 is a receptor for hyaluronic acid-mediated motility (RHAMM). Nanoparticles modified with hyaluronic acid (HA) can be recognized by CD44 and be taken by macrophages [69]. In recent assays, such modification of drug-loaded nanoparticles, such as micelles [36], liposomes [37] and polymeric NPs [38], enabled efficient cellular uptake by macrophages.
2.2. Macrophage Targeting in Anti-Inflammation Therapy
Since macrophages play an indispensable role in initiating and developing inflammatory processes, they are a potential target in anti-inflammatory therapy. Important factors which promote macrophage recruitment into the site of inflammation are cell adhesion molecules, such as ICAM-1 and VCAM-1, the inhibition of which can suppress inflammation. For instance, Sager et al. [73] showed that silencing endothelial cell adhesion molecules using siRNA reduced monocyte recruitment into atherosclerotic lesions.
Anti-inflammatory cytokines, such as IL-10, and anti-inflammatory drugs can also be applied to suppress inflammation. Thus, IL-10 delivered by polymeric nanocarriers was bioactive and reduced the production of pro-inflammatory cytokine IL-1β in the atherosclerotic lesion and led to significant regression in the plaque size [74]. Local inflammation treatment based on mannose-modified nanoparticles loaded with anti-inflammatory diclofenac was successfully applied to wound healing [75]; drug-loaded macrophage-targeted nanoparticles showed an enhanced anti-inflammatory effect in wound healing compared to the drug-coating-free suture.
The overactivation of TLRs leads to the production of high levels of IFN and other cytokines, which can cause chronic inflammation [76]. Moreover, the chronic activation of TLRs via interaction with DAMPs may stimulate T- and B-cells responses and contribute to the development of autoimmunity. Therefore, TLR antagonists, such as TLR2 antagonists AT1-AT8 [77], have been proposed as agents to attenuate inflammation.
2.3. Macrophage Targeting in Anti-Tumor Therapy
In the case of oncological diseases, due to the influence of macrophages on tumor development, macrophages are the preferred target for various therapeutic agents. On the one hand, M1 macrophages inhibit tumor growth and metastasis; on the other hand, TAMs (M2 macrophages) provoke tumor growth and angiogenesis. Therefore, a promising strategy is to increase the ratio of M1 macrophages/TAMs at the tumor site, which can be achieved by inhibiting macrophage recruitment, the direct depletion of TAMs, blocking “don’t eat me” signals, and reprogramming TAMs. In this regard, many strategies have been proposed, including blocking the CCL2/CCR2 axis [78] and the CD47-SIRPα pathway [79].
CCR2 is predominantly expressed by monocytes/macrophages with strong proinflammatory functions. CCR2 is a CC chemokine receptor for monocyte chemoattractant protein-1 (CCL2), which is involved in macrophage recruitment. In this regard, in order to inhibit TAM recruitment, CCR2 antagonists, such as RS102895 [80], BMS CCR2 22 (Tocris) [81] and CCX872 [82], can be used. For instance, CCX872 has exhibited good inhibition of macrophage recruitment due to its high affinity to CCR2.
Signal regulatory protein α (SIRPα) is a regulatory membrane glycoprotein from the SIRP family expressed mainly by myeloid cells (macrophages, monocytes, granulocytes, and myeloid dendritic cells) [83]. SIRPα acts as an inhibitory receptor and interacts with a broadly expressed transmembrane protein, CD47, also called the “don’t eat me” signal, which inhibits phagocytosis [84]. Therefore, SIRPα inhibitors, such as CD47 analogues or anti-SIRPα antibodies [85][86], are proposed as therapeutic agents that promote the phagocytosis of tumor cells by macrophages. For example, the monoclonal antibody KWAR23, which binds human SIRPα with high affinity and disrupts its binding to CD47, has been shown to be a promising candidate in therapy, though in combination with tumor-opsonizing monoclonal antibodies [87].
In addition, the TAM-targeted delivery of therapeutic agents is another promising strategy. In this regard, Siglec, cell surface receptors that bind sialic acid (SA), are a potential target. Among the Siglec family receptors, the SA adhesion protein Siglec-1 is one of the most abundant superficial receptors of TAMs and can mediate endocytosis after binding to SA. Recently, the modification of drug-loaded liposomes [39][40] with sialic acid has enabled a high targeting ability of drug nanocarriers. For instance, Deng et al. [88] demonstrated that the cellular uptake of liposomes modified with a sialic acid–cholesterol conjugate was increased compared with other formulations.
-
Inhibition of macrophage recruitment
Biomolecules recruiting monocytes, such as VEGF, CSF-1, CCL2 and CCL5, are involved in macrophage recruitment to the tumor area and, as a result, in increasing the number of TAMs. Inhibitors of these chemoattractants and their receptors can suppress macrophage recruitment and monocyte proliferation in TAMs, thereby reducing tumor growth and dissemination [89].
In this regard, the CCL2/CCR2 axis attracts a lot of attention; it is known that its blocking is an effective approach to inhibit macrophage recruitment in tumor sites [90]. This can be implemented via anti-CCL2 therapy or anti-CCR2 therapy.
Loberg et al. [91] used monoclonal antibodies C1142, specifically binding to CCL2, and with their help successfully inhibited the growth and metastasis of a tumor by blocking the infiltration of TAMs. Similarly, CANTO888 antibodies were used for anti-CCL2 inhibition [92]; despite the fact that in vivo docetaxel cancer treatment was more effective than CANTO888 antibody treatment alone, the inhibition of CCL2 in combination with docetaxel significantly reduced the tumor load and induced tumor regression.
Inhibiting CCR2 is another potent therapeutic strategy, which can be implemented by anti-CCR2 antibodies, such as CCX872 [75]. An approach to blocking the CCL2/CCR2 axis through the inhibition of mRNA translation is shown in the research [93]. Wang et al. used cationic nanoparticles for targeted delivery of CCR2siRNA. Due to the charge modification, the particles were effectively engulfed by monocytes, due to which an efficient inhibition of macrophage recruitment and TAM infiltration was achieved.
- b.
-
Targeting Anti-Phagocytic Checkpoints
Being important immune cells, macrophages are able to engulf tumor cells and present tumor-specific antigens to induce adaptive immunity. However, tumor cells can evade phagocytosis by macrophages due to the high expression of “don’t eat me” signals [94].
Among “don’t eat me” signals, CD47 is the most studied antiphagocytic signal, and it is known that it prevents phagocytosis through interaction with the SIRPα integrated into the macrophage membrane [95]. Blocking the CD47-SIRPα pathway via anti-CD47 therapy or anti-SIRPα therapy is a way to restore the antitumor activity of TAMs [96].
Chao et al. [97] demonstrated that a blocking monoclonal antibody against CD47 enabled the phagocytosis of acute lymphoblastic leukemia (ALL) cells by macrophages in vitro and inhibited tumor engraftment in vivo. Moreover, anti-CD47 antibody eliminated ALL in the peripheral blood, bone marrow, spleen, and liver of mice engrafted with primary human ALL. It has been shown that in addition to antibodies, SIRPα analogues can be used to neutralize CD47. For instance, Koh et al. [98] utilized exosomes containing SIRPα variants, which induced significantly enhanced tumor phagocytosis and primed cells for an effective anti-tumor T cell response. Encouraging results can be achieved with the CD47 inhibitor magrolimab, the ongoing phase 2 trial of which is evaluating its tolerability, safety and effectiveness in the treatment of myeloma, especially in combination with other anti-cancer therapies [99].
CD47 is expressed in all types of cells, while SIRPα is only expressed on the surface of myeloid cells (macrophages, monocytes, granulocytes, and myeloid dendritic cells). Therefore, in some cases anti-SIRPα therapy is preferable [100]. In this regard, monoclonal antibodies that bind SIRPα with high affinity can be used. For instance, Ring et al. [82] showed that the anti-SIRPα antibody KWAR23 in combination with tumor-opsonizing monoclonal antibodies greatly augmented the myeloid cell-dependent killing of human tumor-derived cell lines in vitro and in vivo.
- c.
-
TAM depletion
TAM depletion is another approach to macrophage-targeted therapy that can help reduce angiogenesis, reactivate immune surveillance, and ultimately suppress tumor growth. For this purpose, various anti-cancer drugs or colony stimulating factor inhibitors, such as CSF-1, can be used.
Since TAMs cause immunosuppression via inhibiting the recruitment of T cells through cytokines, superficial immune checkpoint ligands, and exosomes, the application of immunomodulatory drugs, such as anti-programmed cell death 1 (PD-1) or anti-PD-ligand 1 drugs, is limited. Therefore, in order to improve anti-PD-1/PD-L1 therapy, TAM depletion as part of the synergetic therapy is effective in inhibiting tumor growth [101][102].
Diphtheria toxin treatment during tumor initiation or the depletion of TAMs in established tumors prevented pancreatic cancer initiation [103]; in the case of pre-established tumors, TAM depletion inhibited tumor growth and, in some cases, induced tumor regression.
Drug-carrying nanoparticles modified for targeted delivery to macrophages can also be effectively used to deplete TAMs. Zhou et al. [83] utilized sialic acid–cholesterol-conjugate modified liposomes loaded with epirubicin (EPI-SAL) and showed that EPI-SAL achieved enhanced accumulation of the drug into TAMs; the antitumor studies indicated that EPI-SAL provided strong antitumor activity by modulating the tumor microenvironment with the depletion of TAMs. Another anti-cancer drug, doxorubicin, was loaded into liposomes modified with PEG-D-mannose and PEG-L-fucose conjugates as macrophage receptor ligands [104]; the dual-ligand modified PEGylated liposomes achieved an increased distribution of DOX in tumor tissues and the superior tumor inhibitory rate via modulation of the tumor microenvironment with the exhaustion of TAMs was shown.
- d.
-
Reprogramming of TAMs
Under the influence of various factors, TAMs can switch their phenotype between tumoricidal M1- and protumorigenic M2 macrophages, which is inspiring the design of therapeutic agents targeting this macrophage plasticity. Thus, one of the promising immunotherapeutic strategies for cancer therapy is the repolarization (reprogramming) of TAMs towards an anti-tumor M1 phenotype [105]. Drugs, cytokines, immunoagonists, CpG oligonucleatids, siRNA and ROS/O2-generating nanoparticles can be used to reprogram TAMs.
In many studies, liposomes are used to encapsulate a drug and its targeted delivery to macrophages. Sousa et al. [106] showed that the effect of liposome-encapsulated zoledronate on macrophages cultured in a conditioned environment of breast cancer cells increased the content of markers of the M1 phenotype of macrophages (iNOS and TNF-α). Later, Tan et al. [40] designed liposomes modified with sialic acid (SL) and loaded zoledronic acid (ZA) into them; thanks to the modification, these drug nanocarriers (ZA-SL) could effectively deliver ZA to TAMs. In vivo experiments showed that ZA-SL cancer treatment increased the M1/M2 ratio, which was partly caused by the phenotypic remodeling of M2-like TAMs. Studies have shown that ZA reverses the polarity of TAMs from M2-like to M1-like by attenuating IL-10, VEGF, and MMP-9 production and recovering iNOS expression [107].
Polymer-based nanoparticles can also be used as nanocarriers of drugs targeted at macrophages for TAM repolarization. Wang et al. [108] developed poly β-amino ester-based NPs that could adapt by the systemic administration and release of IL-12 in the tumor microenvironment, subsequently re-educating TAMs. The nanocarriers loaded with IL-12 exhibited enhanced tumor accumulation, and extended the circulation half-life and therapeutic efficacy of encapsulated IL-12 compared to free IL-12. Cheng et al. [109] proposed a multifunctional macrophage targeting system to deliver CpG oligodeoxynucleotides to macrophages; they used mannosylated carboxymethyl chitosan/protamine sulfate/CaCO3/CpG nanoparticles, which were efficiently taken by macrophages and exerted a polarizing effect on them, increasing the production of proinflammatory cytokines including IL-12, IL-6, and TNFα.
Downregulation of CSF-1R is known to reprogram the immunosuppressive M2 macrophages to the immunostimulatory phenotype, M1 macrophages. Sialic acid-targeted cyclodextrin-based nanoparticles were developed to deliver CSF-1R siRNA to TAMs [110]; in in vitro experiments, the nanoparticles achieved cell-specific delivery to TAMs, eventually polarizing M2-like TAMs to an M1 phenotype, which enhanced the level of apoptosis in the prostate cancer cells.
Since reactive oxygen species (ROS) are important modulators of macrophage activation and polarization towards a tumoricidal M1 phenotype, ROS-generating NPs can be used as therapeutic agents modulating the tumor microenvironment. Nascimento et al. [111], using breast cancer models in vitro, found that polyaniline-coated maghemite (γ-Fe2O3) nanoparticles could be easily taken by M2-like macrophages and could re-educate them towards a pro-inflammatory profile via ROS generation. Immunotherapy can be enhanced by tumor-derived antigenic microparticles loaded with nano-Fe3O4- and CpG-containing liposomes, which can repolarize TAMs to M1 macrophages and induce the infiltration of cytotoxic T lymphocytes at the tumor site [112].
Additionally, due to TAM recruitment driven by hypoxia and the accumulation of TAMs in hypoxic regions of solid tumors, oxygen-generating NPs can regulate TAM repolarization by reducing hypoxia. Thus, Youn et al. [113] developed mannose-decorated/macrophage membrane-coated upconverting nanoparticles that contained particles generating ROS and oxygen under light irradiation.
2.4. Macrophage-Targeting in the Therapy of Infectious Diseases
Macrophages, as crucial components of immune system, can engulf and digest microbes. However, some pathogenic microorganisms have the ability to survive the digestion and utilize macrophages as reservoirs for safe haven, avoiding the action of other cells of the host immune system [114][115][116]. These microorganisms can circumvent the effectiveness of antibiotics by surviving inside host macrophages. Since therapeutics in current use have varying abilities to enter macrophages, there is an interest in using special drug delivery systems via drug-loaded nanoparticles to treat intracellular infections. The targeted delivery of drugs to macrophages is considered below on the example of HIV, tuberculosis and leishmaniasis.
-
Viral infectious diseases
Human immunodeficiency virus (HIV) is a lentivirus that leads to acquired immunodeficiency syndrome (AIDS), an immunocompromised condition that increases susceptibility to macrophage resident diseases. Since the major cellular HIV reservoirs are macrophages and CD4+ T cells, with macrophages being responsible for carrying and spreading the virus, the development of methods for the direct delivery of anti-HIV drugs to macrophages is of great interest [117][118].
Recently, drug-loaded nanoparticles, such as liposomes, polymer NPs and dendrimers, have been considered as potential therapeutic agents for treating HIV. In order to achieve a high targeting ability, Jain et al. [119] conjugated efavirenz-loaded dendrimers with tuftsin; the obtained therapeutic agents not only exhibited excellent cellular uptake but also possessed relatively low cytotoxicity with simultaneous high antiviral activity. Similarly, Jain et al. [120] developed stavudine-loaded mannosylated liposomes, which also exhibited a high targeting ability and increased biocompatibility in comparison with the pure drug. The study of the kinetics of release, the effectiveness of loading antiviral drugs in polymeric nanoparticles and their targeting ability revealed their potential as anti-HIV drug carriers [121][122][123]. Thus, Krishnan et al. [124], using chitosan carriers loaded with saquinavir, demonstrated a drug encapsulation efficiency of 75% and cell targeting efficiency greater than 92%; the saquinavir-loaded chitosan carriers exhibited superior control of the viral proliferation compared to the control drug.
- b.
-
Tuberculosis
Tuberculosis (TB) is a lung infection caused by Mycobacterium tuberculosis (Mtb). Mtb primarily infects host macrophages, developing special survival and reproduction strategies in these highly specialized cells [125]. Most of the known anti-tubercular agents are less effective in vivo due to the low macrophage permeability and rapid degradation of these drugs [126]. The use of antibiotic-loaded, macrophage-targeted nanoparticles enables a prolonged and systemic dose of anti-tubercular antibiotics [126].
Many assays are devoted to the development of nanoparticles for active targeted delivery, for example, by modification with mannose [48][49]. For instance, Huang et al. [50] designed mannose-modified solid lipid nanoparticles (SLNs) containing the pH-sensitive prodrug of isoniazid (INH) for the treatment of latent tuberculosis infection. A fourfold increase in intracellular antibiotic efficacy and enhanced macrophage uptake in vitro was observed, while in vivo assays showed that the level of the colony-forming unit was decreased in the SLN group compared to the free INH group. Later, in order to design macrophage-targeted delivery in TB, Ambrus et al. [127] developed nanomediated isoniazid-loaded dry powder for inhalation, based on mannosylated chitosan and hyaluronic acid hybrid nanoparticles, which were found to be a promising vehicle for targeting TB-infected macrophages. Pi et al. [26] first reported the bactericidal effects of selenium nanoparticles and introduced a novel nanomaterial-assisted anti-TB strategy manipulating isoniazid-incorporated mannosylated selenium (Ison@Man-Se) NPs for synergistic drug killing and phagolysosomal destructions of Mtb. They found that Ison@Man-Se NPs selectively entered macrophages and accumulated in lysosomes, releasing isoniazid. Furthermore, Ison@Man-Se/Man-Se NPs could trigger the fusion of Mtb into lysosomes, so that the synergistic lysosomal and isoniazid destruction of Mtb was achieved.
- c.
-
Protozoan infectious diseases
Leishmaniasis is a wide array of clinical manifestations caused by leishmania, a parasitic protozoan [128]. The intracellular localization of leishmania inside the phagolysosome of host macrophages limits chemotherapy treatment. In addition, the use of antileishmanial drugs is often compromised because of their toxicity and limited bioavailability [129]. Macrophage-targeted therapeutic agents can solve these problems [129].
Compounds of pentavalent antimony, such as sodium stibogluconate (SSG), used in the treatment of leishmaniasis have high toxicity; encapsulation of the drug in nanocarriers can help to overcome this disadvantage. For instance, Khan et al. [130] developed nano-deformable liposomes (NDLs) for the dermal delivery of SSG against cutaneous leishmaniasis; compared with the pure drug solution, NDLs displayed an increase in the selectivity index, a decrease in the cytotoxicity and a higher anti-leishmanial activity, due to effective healing of the lesion and a successful reduction in the parasitic load in vivo. Recently, targeting nanoparticles loaded with other antileishmanial drugs, such as amphotericin B (AmB) and paromomycin (PM), have also been utilized for the treatment of leishmaniasis. Heli et al. [131] investigated the effect of ligand modification of PM-loaded chitosan NPs on their anti-leishmanial activity and found the mannosylated formulation to be a suitable targeted drug delivery system for uptake into Leishmania-infected macrophages without any cytotoxic activity. Similarly, Das et al. [132] prepared a mannose containing composite hydrogel loaded with AmB and showed it to be a suitable candidate for the treatment of leishmaniasis due to its injectability, biodegradability, non-cytotoxicity and efficient drug delivery properties.
2.5. Potency of Macrophage Targeting via CD206 Receptor
Targeted delivery to macrophages (including targeting to CD206 or Siglec-1 receptors) opens up numerous opportunities to influence a wide range of diseases and pathological conditions, of which they are the driver or direct participant. These are infectious diseases that have the property of forming a reservoir of latent forms inside macrophages (tuberculosis, HIV, Ebola, etc.); a number of oncological diseases where macrophages make the main contribution to the creation of an immunosuppressive microenvironment of tumors, making them “cold”, i.e., practically invisible to the immune system; and autoimmune diseases (rheumatoid arthritis, osteoarthritis, multiple sclerosis), the pathogenesis of which is associated, on the contrary, with the excessive pro-inflammatory activity of macrophages.
The use of mannosylated drug delivery systems using the idea of biomimetics for oligosaccharidic patterns of microorganisms to target macrophage mannose receptors has been recently studied in detail by the researchers' scientific group in a series of papers [48][133][134][135][136][137] (Figure 2), in which the researchers aimed to create a targeted drug delivery system for the treatment of a number of infectious, inflammation and oncological diseases. On the basis of ligands specific to the macrophage receptor CD206, a system of targeted delivery of therapeutic “cargo”, enhanced with adjuvants (showing a synergistic effect with the main drug), into macrophages was developed [48][135][137][138][139][140][141][142][143][144] (Figure 2 shows a macrophage with its receptors recognizing mannosylated polymers). The use of a macrophage CD206 receptor as a target provides a high selectivity for drug delivery and does not require the use of immune-active compounds (interleukins, proteins, microRNAs, etc.). Systematic studies of the ligands of the CD206 receptor allowed us to develop a series of specific molecular containers of different molecular architectures, carrying an oligomannoside ligand of a complex structure with optimal affinity to the mannose receptors of macrophages. The researchers modeled the interaction of CD206 with more than a hundred relevant carbohydrate structures [133][145]; about two dozen of them were studied experimentally. As a result, optimized polymeric ligands were developed based on polyethyleneimine, mannan, and chitosan grafted with cyclodextrins (Figure 2—center) and provided the effect of accumulation of a therapeutic “cargo” in macrophages, which significantly increased organ bioavailability (and the accumulation of drugs in the lungs), and the permeability of bacterial cells to drugs was developed.
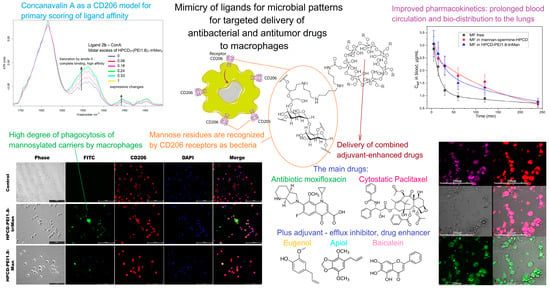
Figure 2. A brief presentation of the idea of macrophage targeting through their CD206 receptors by creating drug delivery systems that contain mannose residues mimicking pathogen patterns. The IR spectra of concanavalin A complexes (a model mannose receptor) with mannosylated polymer are shown at the top left—an example of primary screening for CD206 affinity. Confocal images of alone macrophages with FITC-labeled (green channel) mannosylated polymers are shown at the bottom left: the reseachers observed the greatest absorption by macrophages of particles with a trimannoside backbone, mimicking the oligosaccharides of bacteria. The bottom center shows the main drugs (antibacterial and anticancer drugs) that can be delivered to macrophages using the researchers' strategy, as well as their adjuvants (enhancers). Confocal images of macrophages with absorbed E. coli (as a model of intractable intramacrophage infection) are shown at the bottom right: pink—merged channels bacteria + doxorubicin. Due to the use of a high-affinity polymer to macrophages, the accumulation of the drug inside macrophages is increased by 4 times, and in addition, adjuvants (eugenol, apiol, etc.) inhibit efflux in bacteria and increase the penetration of the drug into bacteria. Polymer systems significantly increase the circulation time of moxifloxacin in the body of rats (top right) and increase the bio-distribution into the lungs to alveolar macrophages. Limitations of macrophage-mediated systems in terms of clinical translation barriers.
In addition to the optimized carbohydrate ligand providing binding to the surface receptor, the delivery system should provide a high degree of loading of therapeutic “cargo” and adjuvant, and their stimulus-sensitive release inside cells. Stimulus-sensitive release is achieved through the use of a polymer forming a compact nanoparticle at neutral pH, and unfolding at a low pH inside the bacterial endosome. A high degree of loading is achieved by grafting this polymer with cyclodextrin molecules that are able to bind therapeutic cargo (antibiotic), as well as synergistically acting adjuvants (which are otherwise poorly soluble and do not have the necessary bioavailability parameters). As a result, each polymer molecule is able to carry up to 20 “cargo” molecules (80% loading rate), which are approximately equally divided between the antibiotic and the adjuvant, and deliver them inside macrophages due to the binding of the carbohydrate ligand to CD206.
A significant increase in efficiency can be achieved by using adjuvants, which enhance the effect of an antibiotic by inhibiting efflux and increasing the permeability of the bacterial membrane [137][141][142]. Currently, the direction of biocompatible medicine is actively developing, in other words, the use of safe natural extracts and essential oils [135][141][146][147][148][149][150][151][152][153][154][155][156][157][158][159], which have a number of remarkable biological effects, including analgesic, antibacterial, anti-inflammatory, antitumor, antioxidant and regenerating properties. As adjuvants, in the the researchers' scientific group, compounds of the terpenoid, flavonoid and allylbenzene series (Figure 2—in the center from the bottom) have been extensively studied. The terpenoid adjuvants used by us demonstrated their ability to block intracellular efflux pumps that provide drug resistance to some bacteria due to the effective removal of antibiotics from the cell. Adjuvants also increase the permeability of bacterial cell membranes. The simultaneous delivery of an antibiotic and an adjuvant can increase the synergy of their action. For the combination of fluoroquinolone—a terpenoid—the reseachers observed a 2–3-fold increase in the effectiveness of the antibiotic (a 2–3-fold decrease in MIC) [137][140][142]. The reseachers showed that adjuvants (allylmethoxybenzenes, terpenoids, and flavonoids) have an enhancing effect on antibacterial drugs, including LF and MF, rifampicin, metronidazole, etc. [48][135][137][140][141][142].
However, the binding constants of both the antibiotics and adjuvants with the developed molecular containers—polymer ligands—are not high enough (about 104 M), which will cause the dissociation of the complexes upon intravenous administration and will not provide a prolonged action of the antibiotic. So, the researchers also created a moxifloxacin (fluoroquinolone) prodrug—a covalent conjugate of the antibiotic with mannosylated polymers (dendrimers) enhanced by a terpenoid adjuvant (limonene), with the function of prolonging drug action. Due to the application of such an “intelligent” prodrug system, selectivity was achieved: in microbiological experiments, an increased antibacterial effect on E. coli and B. subtilis cells was observed, while its effect on “good” Lactobacillus cells was reduced. The researchers have developed pH, thermo- and stimulus-sensitive micelles [138][142][143], which are smart molecular containers that release drugs in a slightly acidic environment and in the presence of glutathione, corresponding to the microenvironment of tumors, or in an inflammatory focus, making them potentially applicable for antibacterial and anticancer drug delivery to macrophages.
The researchers' carbohydrate ligand, as well as the whole system in the complex—(ligand–stimulus-sensitive polymer–carrier–cyclodextrin) cargo from the drug and adjuvant, demonstrated the effectiveness both in cell cultures and in experimental models of infectious diseases in vivo. A study of antibacterial activity on bacterial cell cultures showed that the combined drug moxifloxacin or levofloxacin with an adjuvant is 3–5 times more effective than the original fluoroquinolone (a decrease in MIC due to the presence of an adjuvant and inclusion in a polymer carrier) and demonstrates a prolonged effect (retains activity for up to 8 days; the original fluoroquinolone loses effectiveness for 3 days).
Study of pharmacokinetics in vivo has showed that mannosylated polymer systems based on mannan, cyclodextrins or polyethyleneimine increased the half-life of fluoroquinolones from the body of rats by more than 10 times and increased organ bioavailability, as well as the accumulation of the drug in the lungs by more than 7 times.
Safety studies have demonstrated that mannosylated polymer systems are non-toxic with respect to cells of the line HEK293 (when using concentrations up to 100 micrograms per ml, they do not cause the thrombosis and hemolysis of erythrocytes).
Therefore, the creation of the pharmaceutical composition and structure of a targeted delivery system (a biocompatible polymer modified with cyclodextrin for drug loading and carrying an oligomannoside ligand of a complex structure) for the delivery of antibiotics to macrophages, due to its high-affinity interaction with mannose receptors (CD206), significantly increases organ bioavailability (bio-distribution and accumulation of drugs in the lungs) and enhances the permeability of drugs into bacterial cells. The use of adjuvants enhances the effectiveness of antibiotics by inhibiting efflux pumps (terpenoids and flavonoids).
When the combined preparation of fluoroquinolone and its adjuvant are included in the developed delivery system, a dual mechanism of action of adjuvants is shown—an increase in the permeability of bacterial cells to the antibiotic and inhibition of the efflux proteins of bacteria (“throwing out” the drug from the cells)—which allows us to increase the accumulation of the drug in bacterial cells and reduce the load on healthy cells [107][108][109][110][111][112][113][114][115][116]. Potential perspectives of development include reducing the dosage of antibiotics, shortening the duration of treatment, and reducing the risk of developing resistance.
FTIR spectroscopy was used for the high-throughput screening of lectin–ligand interactions using concanavalin A (Figure 2—top left) as a model mannose receptor to optimize the components and molecular architecture of a delivery system [48][133][135][136][140][160]. FTIR spectroscopy can potentially help to monitor the individual status of therapy: whether drug compositions affect the bacteria, macrophages, or tumor cells or whether they are indifferent. The technique allows testing new drugs or drugs in delivery systems. The researchers have developed an original technique for detecting the selectivity of the action of drug formulations using FTIR. For example, using FTIR, the researchers demonstrated the selectivity of chitosan-based micellar systems, and observed their effect on A549 cells and, conversely, on the protection of normal HEK293 cells [143]; a similar effect was observed for bacterial E. coli cells vs Lactobacilli.
Macrophage-related biosensing system perspectives. FTIR spectroscopy provides valuable data on the interaction of cells with polymer systems, including the possibility to study the molecular mechanism of recognition, which opens prospects for the development of a biosensing system for detecting the activated pro-inflammatory macrophage (CD206+). On the other hand, with regard to biosensing using a macrophage membrane, the researchers expect that FTIR spectroscopy will become a tool for studying the affinity of the receptor–ligand interaction. For biochip development, the researchers used CD206+ macrophage membranes (membrane of macrophages dried on a polystyrene plate, then rehydrated and incubated with ligands). Macrophages are a difficult-to-grow cell culture that can be studied for several hours, so macrophage membranes as stable models are analytically significant. It turned out that CD206+ macrophage membranes demonstrate a binding ability similar to original cells.
Thus, targeting macrophages using the biomimetics of pathogen patterns is a very effective strategy for creating therapeutic systems for a range of diseases. In addition, using macrophage-derived particles, it is possible to selectively target therapeutic cargo to tumor cells, which makes it possible to bypass biological barriers, “switch” the tumor microenvironment (hot/cold) and regulate the status of inflammation. In other words, targeting macrophages and using macrophage-derived membranes as drug carriers have huge prospects for creating a golden bullet for the treatment of infectious, oncological, neurodegenerative, and autoimmune diseases.
Despite the fact that macrophage-mediated systems can turn to be more preferable than traditional methods of drug delivery, their use in clinical practice may be limited by the effectiveness and possible side effects.
Clinical trials of the macrophages reprogramming into a cytotoxic phenotype and introduced to patients with cancer resulted in only a marginal therapeutic effect [161][162] and in some cases slight fevers and chills were observed as side effects [163]. Moreover, in accordance with the nature of macrophages, their administration may lead to immune responses and increase the level of proinflammatory cytokines [164], which together with the problem of the distribution of macrophages in healthy tissues [165] may increase the risk of several side effects.
The development of macrophage-derived particles for effective targeted treatment still remains a challenge, which is mainly due to their low stability or difficulties associated with the pharmacokinetics of therapeutic agents. At the moment, most strategies are based on the release of the drug through the penetration of the cell membrane [166] or exosomal release [167] once a macrophage-derived carrier reaches its target site. These methods of therapeutic cargo transfer still need to be developed, since an important task remains to maintain a balance between the timely release of the drug and its therapeutic activity. In this regard, bypassing the need for drug to leave the macrophage-derived carrier is a potent strategy, which is implemented in photothermal therapy [168].
Since macrophages are a part of immune system, drugs targeting macrophages may trigger multiple mechanisms, which lead to immune-related side effects [169]. At the moment, only few macrophage-targeting drug delivery systems have been approved for clinical use, which is due to the lack of knowledge of their biological properties [170], as well as due to their poor stability. Biomimetic systems based on macrophages may have great potential for scientific research and subsequent medical applications [171].
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71.
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Mater. 2018, 17, 195–203.
- Tsoi, K.M.; Macparland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221.
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake Characteristics of Liposomes by Rat Alveolar Macrophages: Influence of Particle Size and Surface Mannose Modification. J. Pharm. Pharmacol. 2010, 59, 75–80.
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle Size Affects the Cellular Response in Macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657.
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934.
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat. Mater. 2014, 13, 125–138.
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458.
- Schmid-Schönbein, G.W. Analysis of Inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151.
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664.
- Gao, W.J.; Liu, J.X.; Liu, M.N.; Yao, Y.D.; Liu, Z.Q.; Liu, L.; He, H.H.; Zhou, H. Macrophage 3D Migration: A Potential Therapeutic Target for Inflammation and Deleterious Progression in Diseases. Pharmacol. Res. 2021, 167, 105563.
- Zhang, G.; Ma, L.; Bai, L.; Li, M.; Guo, T.; Tian, B.; He, Z.; Fu, Q. Inflammatory Microenvironment-Targeted Nanotherapies. J. Control. Release 2021, 334, 114–126.
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer Particle Shape Independently Influences Binding and Internalization by Macrophages. J. Control. Release 2010, 147, 408–412.
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Lehr, C.M. Nanoparticle Geometry and Surface Orientation Influence Mode of Cellular Uptake. ACS Nano 2013, 7, 1961–1973.
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666.
- Zahr, A.S.; Davis, C.A.; Pishko, M.V. Macrophage Uptake of Core-Shell Nanoparticles Surface Modified with Poly(Ethylene Glycol). Langmuir 2006, 22, 8178–8185.
- Epstein-Barash, H.; Gutman, D.; Markovsky, E.; Mishan-Eisenberg, G.; Koroukhov, N.; Szebeni, J.; Golomb, G. Physicochemical Parameters Affecting Liposomal Bisphosphonates Bioactivity for Restenosis Therapy: Internalization, Cell Inhibition, Activation of Cytokines and Complement, and Mechanism of Cell Death. J. Control. Release 2010, 146, 182–195.
- Liu, X.; Xie, X.; Jiang, J.; Lin, M.; Zheng, E.; Qiu, W.; Yeung, I.; Zhu, M.; Li, Q.; Xia, T.; et al. Use of Nanoformulation to Target Macrophages for Disease Treatment. Adv. Funct. Mater. 2021, 31, 2104487.
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between Complement Activation, Cellular Uptake and Surface Physicochemical Aspects of Novel PEG-Modified Nanocapsules. Biomaterials 2001, 22, 2967–2979.
- Motskin, M.; Müller, K.H.; Genoud, C.; Monteith, A.G.; Skepper, J.N. The Sequestration of Hydroxyapatite Nanoparticles by Human Monocyte-Macrophages in a Compartment That Allows Free Diffusion with the Extracellular Environment. Biomaterials 2011, 32, 9470–9482.
- Sarparanta, M.; Bimbo, L.M.; Rytkoänen, J.; Mäkilä, E.; Laaksonen, T.J.; Laaksonen, P.; Nyman, M.; Salonen, J.; Linder, M.B.; Hirvonen, J.; et al. Intravenous Delivery of Hydrophobin-Functionalized Porous Silicon Nanoparticles: Stability, Plasma Protein Adsorption and Biodistribution. Mol. Pharm. 2012, 9, 654–663.
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079.
- Hattori, Y.; Kawakami, S.; Suzuki, S.; Yamashita, F.; Hashida, M. Enhancement of Immune Responses by DNA Vaccination through Targeted Gene Delivery Using Mannosylated Cationic Liposome Formulations Following Intravenous Administration in Mice. Biochem. Biophys. Res. Commun. 2004, 317, 992–999.
- Ortega, R.A.; Barham, W.; Sharman, K.; Tikhomirov, O.; Giorgio, T.D.; Yull, F.E. Manipulating the NF-ΚB Pathway in Macrophages Using Mannosylated, SiRNA-Delivering Nanoparticles Can Induce Immunostimulatory and Tumor Cytotoxic Functions. Int. J. Nanomed. 2016, 11, 2163–2177.
- Locke, L.W.; Mayo, M.W.; Yoo, A.D.; Williams, M.B.; Berr, S.S. PET Imaging of Tumor Associated Macrophages Using Mannose Coated 64Cu Liposomes. Biomaterials 2012, 33, 7785–7793.
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angew. Chem. Int. Ed. 2020, 59, 3226–3234.
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted Delivery of Oligonucleotides into Tumor-Associated Macrophages for Cancer Immunotherapy. J. Control. Release 2012, 158, 286–292.
- Zhang, J.; Tang, C.; Yin, C. Galactosylated Trimethyl Chitosan-Cysteine Nanoparticles Loaded with Map4k4 SiRNA for Targeting Activated Macrophages. Biomaterials 2013, 34, 3667–3677.
- Zeeshan, M.; Ali, H.; Ain, Q.U.; Mukhtar, M.; Gul, R.; Sarwar, A.; Khan, S. A Holistic QBD Approach to Design Galactose Conjugated PLGA Polymer and Nanoparticles to Catch Macrophages during Intestinal Inflammation. Mater. Sci. Eng. C 2021, 126, 112183.
- Ren, T.; Gou, J.; Sun, W.; Tao, X.; Tan, X.; Wang, P.; Zhang, Y.; He, H.; Yin, T.; Tang, X. Entrapping of Nanoparticles in Yeast Cell Wall Microparticles for Macrophage-Targeted Oral Delivery of Cabazitaxel. Mol. Pharm. 2018, 15, 2870–2882.
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524.
- Jain, S.; Amiji, M. Tuftsin-Modified Alginate Nanoparticles as a Noncondensing Macrophage-Targeted DNA Delivery System. Biomacromolecules 2012, 13, 1074–1085.
- Nagai, T.; Tanaka, M.; Tsuneyoshi, Y.; Xu, B.; Michie, S.A.; Hasui, K.; Hirano, H.; Arita, K.; Matsuyama, T. Targeting Tumor-Associated Macrophages in an Experimental Glioma Model with a Recombinant Immunotoxin to Folate Receptor β. Cancer Immunol. Immunother. 2009, 58, 1577–1586.
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-Targeted Nanoparticles Show Efficacy in the Treatment of Inflammatory Arthritis. Arthritis Rheum. 2011, 63, 2671–2680.
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic Acid-Functionalized Human Serum Albumin Nanocapsules for Targeted Drug Delivery to Chronically Activated Macrophages. Int. J. Pharm. 2012, 427, 460–466.
- Gao, Y.; Sarfraz, M.K.; Clas, S.D.; Roa, W.; Löbenberg, R. Hyaluronic Acid-Tocopherol Succinate-Based Self-Assembling Micelles for Targeted Delivery of Rifampicin to Alveolar Macrophages. J. Biomed. Nanotechnol. 2014, 11, 1312–1329.
- Gouveia, V.M.; Lopes-De-Araújo, J.; Costa Lima, S.A.; Nunes, C.; Reis, S. Hyaluronic Acid-Conjugated PH-Sensitive Liposomes for Targeted Delivery of Prednisolone on Rheumatoid Arthritis Therapy. Nanomedicine 2018, 13, 1037–1049.
- Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14, 2118.
- Ding, J.; Sui, D.; Liu, M.; Su, Y.; Wang, Y.; Liu, M.; Luo, X.; Liu, X.; Deng, Y.; Song, Y. Sialic Acid Conjugate-Modified Liposomes Enable Tumor Homing of Epirubicin via Neutrophil/Monocyte Infiltration for Tumor Therapy. Acta Biomater. 2021, 134, 702–715.
- Tang, X.; Sui, D.; Liu, M.; Zhang, H.; Liu, M.; Wang, S.; Zhao, D.; Sun, W.; Liu, M.; Luo, X.; et al. Targeted Delivery of Zoledronic Acid through the Sialic Acid—Siglec Axis for Killing and Reversal of M2 Phenotypic Tumor-Associated Macrophages—A Promising Cancer Immunotherapy. Int. J. Pharm. 2020, 590, 119929.
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774.
- Kaisho, T.; Akira, S. Toll-like Receptor Function and Signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987.
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J. Med. Chem. 2020, 63, 13466–13513.
- Zeng, Q.; Jewell, C.M. Directing Toll-like Receptor Signaling in Macrophages to Enhance Tumor Immunotherapy. Curr. Opin. Biotechnol. 2019, 60, 138–145.
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat. Rev. Immunol. 2013, 13, 621–634.
- Areschoug, T.; Gordon, S. Scavenger Receptors: Role in Innate Immunity and Microbial Pathogenesis. Cell. Microbiol. 2009, 11, 1160–1169.
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281.
- Zlotnikov, I.D.; Ezhov, A.A.; Petrov, R.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Belogurova, N.G.; Kudryashova, E.V. Mannosylated Polymeric Ligands for Targeted Delivery of Antibacterials and Their Adjuvants to Macrophages for the Enhancement of the Drug Efficiency. Pharmaceuticals 2022, 15, 1172.
- Serrasqueiro, F.; Barbosa, A.I.; Lima, S.A.C.; Reis, S. Targeting the Mannose Receptor with Functionalized Fucoidan/Chitosan Nanoparticles Triggers the Classical Activation of Macrophages. Int. J. Mol. Sci. 2023, 24, 9908.
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, X.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable Solid Lipid Nanoparticles for Intracellular Tuberculosis Infection Therapy: Macrophage-Targeting and PH-Sensitive Properties. Drug Deliv. Transl. Res. 2021, 11, 1218–1235.
- Peng, Y.; Yao, W.; Wang, B.; Zong, L. Mannosylated Chitosan Nanoparticles Based Macrophage-Targeting Gene Delivery System Enhanced Cellular Uptake and Improved Transfection Efficiency. J. Nanosci. Nanotechnol. 2015, 15, 2619–2627.
- Chen, P.; Zhang, X.; Jia, L.; Prud’Homme, R.K.; Szekely, Z.; Sinko, P.J. Optimal Structural Design of Mannosylated Nanocarriers for Macrophage Targeting. J. Control. Release 2014, 194, 341–349.
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Nuti, M. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945.
- Chen, X.; Wu, Y.; Li, R.; Li, C.; Xu, L.; Qiao, W.; Dong, N. Galactose-Modified Nanoparticles for Delivery of MicroRNA to Mitigate the Progress of Abdominal Aortic Aneurysms via Regulating Macrophage Polarization. Nanomedicine 2022, 44, 102564.
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. J. Control. Release 2021, 337, 179–192.
- Foerster, F.; Bamberger, D.; Schupp, J.; Weilbächer, M.; Kaps, L.; Strobl, S.; Radi, L.; Diken, M.; Strand, D.; Tuettenberg, A.; et al. Dextran-Based Therapeutic Nanoparticles for Hepatic Drug Delivery. Nanomedicine 2016, 11, 2663–2677.
- Shah, N.K.; Gupta, S.K.; Wang, Z.; Meenach, S.A. Enhancement of Macrophage Uptake via Phosphatidylserine-Coated Acetalated Dextran Nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 50, 57–65.
- Han, J.; Na, R.; Zhao, N.; Yuan, X.; Fu, L.; Jing, J.; Qian, A.; Ye, W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules 2023, 28, 591.
- Li, H.; Tatematsu, K.; Somiya, M.; Iijima, M.; Kuroda, S. Development of a Macrophage-Targeting and Phagocytosis-Inducing Bio-Nanocapsule-Based Nanocarrier for Drug Delivery. Acta Biomater. 2018, 73, 412–423.
- Tsutsui, Y.; Tomizawa, K.; Nagita, M.; Michiue, H.; Nishiki, T.; Ohmori, I.; Seno, M.; Matsui, H. Development of Bionanocapsules Targeting Brain Tumors. J. Control. Release 2007, 122, 159–164.
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial Ghosts for Targeting Delivery and Subsequent Responsive Release of Ciprofloxacin to Destruct Intracellular Bacteria. Chem. Eng. J. 2020, 399, 125700.
- Nimmerjahn, F.; Ravetch, J.V. Fc-Receptors as Regulators of Immunity. Adv. Immunol. 2007, 96, 179–204.
- Mkaddem, S.B.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811.
- Bar-Shavit, Z.; Stabinsky, Y.; Fridkin, M.; Goldman, R. Tuftsin-Macrophage Interaction: Specific Binding and Augmentation of Phagocytosis. J. Cell. Physiol. 1979, 100, 55–62.
- Khan, M.A. Targeted Drug Delivery Using Tuftsin-Bearing Liposomes: Implications in the Treatment of Infectious Diseases and Tumors. Curr. Drug Targets 2020, 22, 770–778.
- Liang, D.S.; Wen, Z.J.; Wang, J.H.; Zhu, F.F.; Guo, F.; Zhou, J.L.; Xu, J.J.; Zhong, H.J. Legumain Protease-Sheddable PEGylated, Tuftsin-Modified Nanoparticles for Selective Targeting to Tumour-Associated Macrophages. J. Drug Target. 2022, 30, 82–93.
- Horváti, K.; Bacsa, B.; Szabó, N.; Dávid, S.; Mezo, G.; Grolmusz, V.; Vértessy, B.; Hudecz, F.; Bo’sze, S. Enhanced Cellular Uptake of a New, in Silico Identified Antitubercular Candidate by Peptide Conjugation. Bioconjug Chem. 2012, 23, 900–907.
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359.
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthc. Mater. 2017, 6, 1601012.
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate Receptor-Mediated Targeting of Therapeutic and Imaging Agents to Activated Macrophages in Rheumatoid Arthritis. Adv. Drug Deliv. Rev. 2004, 56, 1205–1217.
- Yang, C.; Gao, S.; Kjems, J. Folic Acid Conjugated Chitosan for Targeted Delivery of SiRNA to Activated Macrophages in Vitro and in Vivo. J. Mater. Chem. B 2014, 2, 8608–8615.
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective Liposome Targeting of Folate Receptor Positive Immune Cells in Inflammatory Diseases. Nanomedicine 2018, 14, 1033–1043.
- Sager, H.B.; Dutta, P.; Dahlman, J.E.; Hulsmans, M.; Courties, G.; Sun, Y.; Heidt, T.; Vinegoni, C.; Borodovsky, A.; Fitzgerald, K.; et al. RNAi Targeting Multiple Cell Adhesion Molecules Reduces Immune Cell Recruitment and Vascular Inflammation after Myocardial Infarction. Sci. Transl. Med. 2016, 8, 342ra80.
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.-S.; Chan Kwon, I.; Tae, G. Targeted Delivery of Anti-Inflammatory Cytokine by Nanocarrier Reduces Atherosclerosis in Apo E−/− Mice. Biomaterials 2020, 226, 119550.
- Kim, H.; Kim, B.H.; Huh, B.K.; Yoo, Y.C.; Heo, C.Y.; Bin Choy, Y.; Park, J.H. Surgical Suture Releasing Macrophage-Targeted Drug-Loaded Nanoparticles for an Enhanced Anti-Inflammatory Effect. Biomater. Sci. 2017, 5, 1670–1677.
- Ospelt, C.; Gay, S. TLRs and Chronic Inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505.
- Murgueitio, M.S.; Henneke, P.; Glossmann, H.; Santos-Sierra, S.; Wolber, G. Prospective Virtual Screening in a Sparse Data Scenario: Design of Small-Molecule TLR2 Antagonists. ChemMedChem 2014, 9, 813–822.
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 Signalling Axis in Cancer: Mechanisms and Therapeutic Targeting. Cell Prolif. 2021, 54, e13115.
- Hu, J.; Xiao, Q.; Dong, M.; Guo, D.; Wu, X.; Wang, B. Glioblastoma Immunotherapy Targeting the Innate Immune Checkpoint CD47-SIRPα Axis. Front. Immunol. 2020, 11, 593219.
- Lindholm, P.F.; Sivapurapu, N.; Jovanovic, B.; Kajdacsy-Balla, A. Monocyte-Induced Prostate Cancer Cell Invasion Is Mediated by Chemokine Ligand 2 and Nuclear Factor-ΚB Activity. J. Clin. Cell. Immunol. 2015, 6, 308.
- Grossman, J.G.; Nywening, T.M.; Belt, B.A.; Panni, R.Z.; Krasnick, B.A.; DeNardo, D.G.; Hawkins, W.G.; Goedegebuure, S.P.; Linehan, D.C.; Fields, R.C. Recruitment of CCR2+ Tumor Associated Macrophage to Sites of Liver Metastasis Confers a Poor Prognosis in Human Colorectal Cancer. Oncoimmunology 2018, 7, e1470729.
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 Inhibition Reduces Tumor Myeloid Cells and Unmasks a Checkpoint Inhibitor Effect to Slow Progression of Resistant Murine Gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138.
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752.
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Bin Riaz, I.; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “Don’t Eat Me” Signal of CD47-SIRPα in Hematological Malignancies, an in-Depth Review. Blood Rev. 2018, 32, 480–489.
- Ho, C.C.M.; Guo, N.; Sockolosky, J.T.; Ring, A.M.; Weiskopf, K.; Özkan, E.; Mori, Y.; Weissman, I.L.; Garcia, K.C. “Velcro” Engineering of High Affinity CD47 Ectodomain as Signal Regulatory Protein α (SIRPα) Antagonists That Enhance Antibody-Dependent Cellular Phagocytosis. J. Biol. Chem. 2015, 290, 12650–12663.
- Murata, Y.; Tanaka, D.; Hazama, D.; Yanagita, T.; Saito, Y.; Kotani, T.; Oldenborg, P.A.; Matozaki, T. Anti-Human SIRPα Antibody Is a New Tool for Cancer Immunotherapy. Cancer Sci. 2018, 109, 1300–1308.
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585.
- Zhou, S.; Zhang, T.; Peng, B.; Luo, X.; Liu, X.; Hu, L.; Liu, Y.; Di, D.; Song, Y.; Deng, Y. Targeted Delivery of Epirubicin to Tumor-Associated Macrophages by Sialic Acid-Cholesterol Conjugate Modified Liposomes with Improved Antitumor Activity. Int. J. Pharm. 2017, 523, 203–216.
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-Associated Macrophages: A Promising Target for a Cancer Immunotherapeutic Strategy. Pharmacol. Res. 2020, 161, 105111.
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210.
- Loberg, R.D.; Ying, C.; Craig, M.; Yan, L.; Snyder, L.A.; Pienta, K.J. CCL2 as an Important Mediator of Prostate Cancer Growth in Vivo through the Regulation of Macrophage Infiltration. Neoplasia 2007, 9, 556–562.
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with Systemic Delivery of Neutralizing Antibodies Induces Prostate Cancer Tumor Regression in Vivo. Cancer Res. 2007, 67, 9417–9424.
- Shen, S.; Zhang, Y.; Chen, K.G.; Luo, Y.L.; Wang, J. Cationic Polymeric Nanoparticle Delivering CCR2 SiRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol. Pharm. 2018, 15, 3642–3653.
- Zhou, X.; Liu, X.; Huang, L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv. Funct. Mater. 2021, 31, 2006220.
- Veillette, A.; Tang, Z. Signaling Regulatory Protein (SIRP)a-CD47 Blockade Joins the Ranks of Immune Checkpoint Inhibition. J. Clin. Oncol. 2019, 37, 1012–1014.
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S.; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPα Antibodies as a Potential New Tool for Cancer Immunotherapy. JCI Insight 2017, 2, 89140.
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2011, 71, 1374–1384.
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 Blockade Increases Cancer Cell Phagocytosis. Biomaterials 2017, 121, 121–129.
- Paul, B.; Liedtke, M.; Khouri, J.; Rifkin, R.; Gandhi, M.D.; Kin, A.; Levy, M.Y.; Silbermann, R.; Cottini, F.; Sborov, D.W.; et al. A Phase II Multi-Arm Study of Magrolimab Combinations in Patients with Relapsed/Refractory Multiple Myeloma. Future Oncol. 2023, 19, 7–17.
- Voets, E.; Paradé, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; Van Den Tillaart, G.; Van Duijnhoven, S.; Driessen, L.; et al. Functional Characterization of the Selective Pan-Allele Anti-SIRPα Antibody ADU-1805 That Blocks the SIRPα-CD47 Innate Immune Checkpoint. J. Immunother. Cancer 2019, 7, 340.
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.-T.T.; et al. Dissecting the Immunosuppressive Tumor Microenvironments in Glioblastoma-on-a-Chip for Optimized PD-1 Immunotherapy. eLife 2020, 9, e52253.
- Mao, Y.; Eissler, N.; Le Blanc, K.; Johnsen, J.I.; Kogner, P.; Kiessling, R. Targeting Suppressive Myeloid Cells Potentiates Checkpoint Inhibitors to Control Spontaneous Neuroblastoma. Clin. Cancer Res. 2016, 22, 3849–3859.
- Zhang, Y.; Velez-Delgado, A.; Mathew, E.; Li, D.; Mendez, F.M.; Flannagan, K.; Rhim, A.D.; Simeone, D.M.; Beatty, G.L.; Di Magliano, M.P. Myeloid Cells Are Required for PD-1/PD-L1 Checkpoint Activation and the Establishment of an Immunosuppressive Environment in Pancreatic Cancer. Gut 2017, 66, 124–136.
- Li, C.; Lai, C.; Qiu, Q.; Luo, X.; Hu, L.; Zheng, H.; Lu, Y.; Liu, M.; Zhang, H.; Liu, X.; et al. Dual-Ligand Modification of PEGylated Liposomes Used for Targeted Doxorubicin Delivery to Enhance Anticancer Efficacy. AAPS PharmSciTech 2019, 20, 188.
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 165–174.
- Sousa, S.; Auriola, S.; Mönkkönen, J.; Määttä, J. Liposome Encapsulated Zoledronate Favours M1-like Behaviour in Murine Macrophages Cultured with Soluble Factors from Breast Cancer Cells. BMC Cancer 2015, 15, 4.
- Giraudo, E.; Inoue, M.; Hanahan, D. An Amino-Bisphosphonate Targets MMP-9–Expressing Macrophages and Angiogenesis to Impair Cervical Carcinogenesis. J. Clin. Investig. 2004, 114, 623–633.
- Wang, Y.; Lin, Y.X.; Qiao, S.L.; An, H.W.; Ma, Y.; Qiao, Z.Y.; Rajapaksha, R.P.Y.J.; Wang, H. Polymeric Nanoparticles Enable Reversing Macrophage in Tumor Microenvironment for Immunotherapy. Biomaterials 2017, 112, 153–163.
- He, X.Y.; Liu, B.Y.; Ai, S.L.; Xu, L.; Zhuo, R.X.; Cheng, S.X. Functional Polymer/Inorganic Hybrid Nanoparticles for Macrophage Targeting Delivery of Oligodeoxynucleotides in Cancer Immunotherapy. Mater. Today Chem. 2017, 4, 106–116.
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. Sialic Acid-Targeted Cyclodextrin-Based Nanoparticles Deliver CSF-1R SiRNA and Reprogram Tumour-Associated Macrophages for Immunotherapy of Prostate Cancer. Eur. J. Pharm. Sci. 2023, 185, 106427.
- Nascimento, C.; Castro, F.; Domingues, M.; Lage, A.; Alves, É.; de Oliveira, R.; de Melo, C.; Eduardo Calzavara-Silva, C.; Sarmento, B. Reprogramming of Tumor-Associated Macrophages by Polyaniline-Coated Iron Oxide Nanoparticles Applied to Treatment of Breast Cancer. Int. J. Pharm. 2023, 636, 122866.
- Zhao, H.; Zhao, B.; Wu, L.; Xiao, H.; Ding, K.; Zheng, C.; Song, Q.; Sun, L.; Wang, L.; Zhang, Z. Amplified Cancer Immunotherapy of a Surface-Engineered Antigenic Microparticle Vaccine by Synergistically Modulating Tumor Microenvironment. ACS Nano 2019, 13, 12553–12566.
- Yoon, J.; Le, X.T.; Kim, J.; Lee, H.; Nguyen, N.T.; Lee, W.T.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Macrophage-Reprogramming Upconverting Nanoparticles for Enhanced TAM-Mediated Antitumor Therapy of Hypoxic Breast Cancer. J. Control. Release 2023, 360, 482–495.
- Djaldetti, M.; Salman, H.; Bergman, M.; Djaldetti, R.; Bessler, H. Phagocytosis—The Mighty Weapon of the Silent Warriors. Microsc. Res. Tech. 2002, 57, 421–431.
- Kirsh, R.; Bugelski, P.J.; Poste, G. Drug Delivery to Macrophages for the Therapy of Cancer and Infectious Diseases. Ann. N. Y. Acad. Sci. 1987, 507, 141–154.
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-Based Nanocarriers and Their Application to Target Macrophages and Deliver Antimicrobial Agents. Adv. Drug Deliv. Rev. 2019, 151–152, 94–129.
- Kruize, Z.; Kootstra, N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front. Microbiol. 2019, 10, 2828.
- Kumar, A.; Herbein, G. The Macrophage: A Therapeutic Target in HIV-1 Infection. Mol. Cell. Ther. 2014, 2, 10.
- Dutta, T.; Garg, M.; Jain, N.K. Targeting of Efavirenz Loaded Tuftsin Conjugated Poly(Propyleneimine) Dendrimers to HIV Infected Macrophages in Vitro. Eur. J. Pharm. Sci. 2008, 34, 181–189.
- Garg, M.; Asthana, A.; Agashe, H.B.; Agrawal, G.P.; Jain, N.K. Stavudine-Loaded Mannosylated Liposomes: In-Vitro Anti-HIV-I Activity, Tissue Distribution and Pharmacokinetics. J. Pharm. Pharmacol. 2010, 58, 605–616.
- Adlin Jino Nesalin, J.; Anton Smith, A. Preparation and Evaluation of Stavudine Loaded Chitosan Nanoparticles. J. Pharm. Res. 2013, 6, 268–274.
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of Poly(Lactic Acid)/Chitosan Nanoparticles for Anti-HIV Drug Delivery Applications. Carbohydr. Polym. 2010, 80, 833–838.
- Varshosaz, J.; Taymouri, S.; Jafari, E.; Jahanian-Najafabadi, A.; Taheri, A. Formulation and Characterization of Cellulose Acetate Butyrate Nanoparticles Loaded with Nevirapine for HIV Treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 9–20.
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of Chitosan Nanoformulations as Potent Anti-HIV Therapeutic Systems. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 476–484.
- Pieters, J. Mycobacterium Tuberculosis and the Macrophage: Maintaining a Balance. Cell Host Microbe 2008, 3, 399–407.
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 2100193.
- Mukhtar, M.; Csaba, N.; Robla, S.; Varela-Calviño, R.; Nagy, A.; Burian, K.; Kókai, D.; Ambrus, R. Dry Powder Comprised of Isoniazid-Loaded Nanoparticles of Hyaluronic Acid in Conjugation with Mannose-Anchored Chitosan for Macrophage-Targeted Pulmonary Administration in Tuberculosis. Pharmaceutics 2022, 14, 1543.
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615.
- Kunjachan, S.; Jose, S.; Thomas, C.A.; Joseph, E.; Kiessling, F.; Lammers, T. Physicochemical and Biological Aspects of Macrophage-Mediated Drug Targeting in Anti-Microbial Therapy. Fundam. Clin. Pharmacol. 2012, 26, 63–71.
- Dar, M.J.; Din, F.U.; Khan, G.M. Sodium Stibogluconate Loaded Nano-Deformable Liposomes for Topical Treatment of Leishmaniasis: Macrophage as a Target Cell. Drug Deliv. 2018, 25, 1595–1606.
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Erratum: Paromomycin-Loaded Mannosylated Chitosan Nanoparticles: Synthesis, Characterization and Targeted Drug Delivery against Leishmaniasis (Acta Tropica (2019) 197 (105045) PII: S0001-706X(19)30864-2). Acta Trop. 2019, 197, 105072.
- Dowari, P.; Roy, S.; Das, S.; Chowdhuri, S.; Kushwaha, R.; Das, B.K.; Ukil, A.; Das, D. Mannose-Decorated Composite Peptide Hydrogel with Thixotropic and Syneresis Properties and Its Application in Treatment of Leishmaniasis. Chem. Asian J. 2022, 17, e202200550.
- Zlotnikov, I.D.; Kudryashova, E.V. Computer Simulation of the Receptor–Ligand Interactions of Mannose Receptor CD206 in Comparison with the Lectin Concanavalin A Model. Biochemistry 2022, 87, 54–69.
- Zlotnikov, I.D.; Ezhov, A.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Dyachkova, U.D.; Belogurova, N.G.; Kudryashova, E.V. Application Prospects of FTIR Spectroscopy and CLSM to Monitor the Drugs Interaction with Bacteria Cells Localized in Macrophages for Diagnosis and Treatment Control of Respiratory Diseases. Diagnostics 2023, 13, 698.
- Zlotnikov, I.D.; Kudryashova, E.V. Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. Pharmaceuticals 2022, 15, 625.
- Zlotnikov, I.D.; Vanichkin, D.A.; Kudryashova, E.V. Methods for Determining the Parameters of Receptor-Ligand Interactions on the Model of Concanavalin A and Mannosylated Chitosans Promising Carriers for Drug Delivery to Alveolar Macrophages. Biotekhnologiya 2021, 37, 28–40.
- Zlotnikov, I.D.; Davydova, M.P.; Danilov, M.R.; Krylov, S.S.; Belogurova, N.G.; Kudryashova, E.V. Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action. Pharmaceuticals 2023, 16, 1102.
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. Pharmaceutics 2023, 15, 1613.
- Zlotnikov, I.D.; Malashkeevich, S.M.; Belogurova, N.G.; Kudryashova, E.V. Thermoreversible Gels Based on Chitosan Copolymers as “Intelligent” Drug Delivery System with Prolonged Action for Intramuscular Injection. Pharmaceutics 2023, 15, 1478.
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E.V. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int. J. Mol. Sci. 2022, 23, 16144.
- Zlotnikov, I.D.; Belogurova, N.G.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Plant Alkylbenzenes and Terpenoids in the Form of Cyclodextrin Inclusion Complexes as Antibacterial Agents and Levofloxacin Synergists. Pharmaceuticals 2022, 15, 861.
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446.
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135.
- Zlotnikov, I.D.; Dobryakova, N.V.; Ezhov, A.A.; Kudryashova, E.V. Achievement of the Selectivity of Cytotoxic Agents against Cancer Cells by Creation of Combined Formulation with Terpenoid Adjuvants as Prospects to Overcome Multidrug Resistance. Int. J. Mol. Sci. 2023, 24, 8023.
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and Molecular Insight in Guest–Host Complexes of Fluoroquinolones with β-Cyclodextrin Derivatives, as Revealed by ATR-FTIR Spectroscopy and Molecular Modeling Experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462.
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing Essential Oils (Trans-Cinnamaldehyde, Eugenol, Cinnamon Bark, and Clove Bud Extracts) for Antimicrobial Delivery Applications. LWT 2013, 51, 86–93.
- Cardoso, N.N.R.; Alviano, C.S.; Blank, A.F.; Romanos, M.T.V.; Fonseca, B.B.; Rozental, S.; Rodrigues, I.A.; Alviano, D.S. Synergism Effect of the Essential Oil from Ocimum Basilicum Var. Maria Bonita and Its Major Components with Fluconazole and Its Influence on Ergosterol Biosynthesis. Evid. Based Complement. Altern. Med. 2016, 2016, 5647182.
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786.
- Boire, N.A.; Riedel, S.; Parrish, N.M. Essential Oils and Future Antibiotics: New Weapons against Emerging’Superbugs’? J. Anc. Dis. Prev. Rem. 2013, 1, 105.
- Samet, A.V.; Shevchenko, O.G.; Rusak, V.V.; Chartov, E.M.; Myshlyavtsev, A.B.; Rusanov, D.A.; Semenova, M.N.; Semenov, V.V. Antioxidant Activity of Natural Allylpolyalkoxybenzene Plant Essential Oil Constituents. J. Nat. Prod. 2019, 82, 1451–1458.
- Bin Yoo, C.; Han, K.T.; Cho, K.S.; Ha, J.; Park, H.J.; Nam, J.H.; Kil, U.H.; Lee, K.T. Eugenol Isolated from the Essential Oil of Eugenia Caryophyllata Induces a Reactive Oxygen Species-Mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Cancer Lett. 2005, 225, 41–52.
- Tadtong, S.; Watthanachaiyingcharoen, R.; Kamkaen, N. Antimicrobial Constituents and Synergism Effect of the Essential Oils from Cymbopogon Citratus and Alpinia Galanga. Nat. Prod. Commun. 2014, 9, 277–280.
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium Aromaticum Essential Oil and Eugenol. Evid. Based Complement. Altern. Med. 2021, 2021, 6663255.
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and Antioxidant Activities of Mexican Oregano Essential Oils (Lippia Graveolens H. B. K.) with Different Composition When Microencapsulated Inβ-Cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590.
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172.
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as Specific Inhibitors of the Biosynthesis of Aflatoxin G1 in Aspergillus Parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332.
- Semenov, V.V.; Rusak, V.V.; Chartov, E.M.; Zaretskii, M.I.; Konyushkin, L.D.; Firgang, S.I.; Chizhov, A.O.; Elkin, V.V.; Latin, N.N.; Bonashek, V.M.; et al. Polyalkoxybenzenes from Plant Raw Materials 1. Isolation of Polyalkoxybenzenes from CO2 Extracts of Umbelliferae Plant Seeds. Russ. Chem. Bull. 2007, 56, 2448–2455.
- Leite, A.M.; Lima, E.D.O.; De Souza, E.L.; Diniz, M.D.F.F.M.; Trajano, V.N.; De Medeiros, I.A. Inhibitory Effect of β-Pinene, α-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126.
- Puiu, R.A.; Bîrcă, A.C.; Grumezescu, V.; Duta, L.; Oprea, O.C.; Holban, A.M.; Hudiță, A.; Gălățeanu, B.; Balaure, P.C.; Grumezescu, A.M.; et al. Multifunctional Polymeric Biodegradable and Biocompatible Coatings Based on Silver Nanoparticles: A Comparative In Vitro Study on Their Cytotoxicity towards Cancer and Normal Cell Lines of Cytostatic Drugs versus Essential-Oil-Loaded Nanoparticles and on Their Antimicrobial and Antibiofilm Activities. Pharmaceutics 2023, 15, 1882.
- Zlotnikov, I.D.; Kudryashova, E.V. Mannose Receptors of Alveolar Macrophages as a Target for the Addressed Delivery of Medicines to the Lungs. Russ. J. Bioorg. Chem. 2022, 48, 46–75.
- Faradji, A.; Bohbot, A.; Frost, H.; Schmitt-Goguel, M.; Siffert, J.C.; Dufour, P.; Eber, M.; Lallot, C.; Wiesel, M.L.; Bergerat, J.P. Phase I Study of Liposomal MTP-PE-Activated Autologous Monocytes Administered Intraperitoneally to Patients with Peritoneal Carcinomatosis. J. Clin. Oncol. 1991, 9, 1251–1260.
- Hennemann, B.; Scheibenbogen, C.; SchuUmichen, C.; Andreesen, R. Intrahepatic Adoptive Immunotherapy with Autologous Tumorcytotoxic Macrophages in Patients with Cancer. J. Immunother. 1995, 18, 19–27.
- Andreesen, R.; Hennemann, B.; Krause, S.W. Adoptive Immunotherapy of Cancer Using Monocyte-Derived Macrophages: Rationale, Current Status, and Perspectives. J. Leukoc. Biol. 1998, 64, 419–426.
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bloom, O. Pilot Study: Elevated Circulating Levels of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor in Patients with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 1498–1507.
- Fu, J.; Wang, D.; Mei, D.; Zhang, H.; Wang, Z.; He, B.; Zhang, Q. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J. Control. Release 2015, 204, 11–19.
- Bressani, R.F.; Nowacek, A.S.; Singh, S.; Balkundi, S.; Rabinow, B.; McMillan, J.; Gendelman, H.E.; Kanmogne, G.D. Pharmacotoxicology of Monocyte-Macrophage Nanoformulated Antiretroviral Drug Uptake and Carriage. Nanotoxicology 2011, 5, 592–605.
- Zhao, Y.; Haney, M.J.; Gupta, R.; Bohnsack, J.P.; He, Z.; Kabanov, A.V.; Batrakova, E.V. GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model. PLoS ONE 2014, 9, e106867.
- Yang, T.D.; Choi, W.; Yoon, T.H.; Lee, K.J.; Lee, J.-S.; Joo, J.H.; Lee, M.-G.; Yim, H.S.; Choi, K.M.; Kim, B.; et al. In Vivo Photothermal Treatment by the Peritumoral Injection of Macrophages Loaded with Gold Nanoshells. Biomed. Opt. Express 2016, 7, 185.
- Tack, C.J.; Kleijwegt, F.S.; Van Riel, P.L.C.M.; Roep, B.O. Development of Type 1 Diabetes in a Patient Treated with Anti-TNF-α Therapy for Active Rheumatoid Arthritis. Diabetologia 2009, 52, 1442–1444.
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of Physicochemical and Surface Properties on in Vivo Fate of Drug Nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21.
- Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic System Based on Reconstituted Macrophage Membranes for Analyzing and Selection of Higher-Affinity Ligands Specific to Mannose Receptor to Develop the Macrophage-Focused Medicines. Biomedicines 2023, 11, 2769.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
20 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




