Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Calosci | -- | 2191 | 2023-11-17 09:42:56 | | | |
| 2 | Jason Zhu | Meta information modification | 2191 | 2023-11-20 06:35:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Calosci, D.; Passaglia, L.; Gabbiato, I.; Cartisano, F.; Affuso, R.; Sorrentino, U.; Zuccarello, D. Preimplantation Genetic Testing for Cancer Predisposition Syndromes. Encyclopedia. Available online: https://encyclopedia.pub/entry/51736 (accessed on 07 February 2026).
Calosci D, Passaglia L, Gabbiato I, Cartisano F, Affuso R, Sorrentino U, et al. Preimplantation Genetic Testing for Cancer Predisposition Syndromes. Encyclopedia. Available at: https://encyclopedia.pub/entry/51736. Accessed February 07, 2026.
Calosci, Davide, Lisa Passaglia, Ilaria Gabbiato, Francesca Cartisano, Rebecca Affuso, Ugo Sorrentino, Daniela Zuccarello. "Preimplantation Genetic Testing for Cancer Predisposition Syndromes" Encyclopedia, https://encyclopedia.pub/entry/51736 (accessed February 07, 2026).
Calosci, D., Passaglia, L., Gabbiato, I., Cartisano, F., Affuso, R., Sorrentino, U., & Zuccarello, D. (2023, November 17). Preimplantation Genetic Testing for Cancer Predisposition Syndromes. In Encyclopedia. https://encyclopedia.pub/entry/51736
Calosci, Davide, et al. "Preimplantation Genetic Testing for Cancer Predisposition Syndromes." Encyclopedia. Web. 17 November, 2023.
Copy Citation
Cancer Predisposition Syndromes (CPSs), also known as Hereditary Cancer Syndromes (HCSs), represent a group of genetic disorders associated with an increased lifetime risk of developing cancer.
Cancer Predisposition Syndromes
prenatal diagnosis
fertility preservation
PGT-M
1. Background
Hereditary transmission or de novo occurrence of pathogenic variants in a specific subset of predisposing genes is associated with an increased lifetime risk of developing tumors. These hereditary disorders, which are collectively referred to as Cancer Predisposition Syndromes (CPSs), impose a high burden on patients in terms of reduced quality of life and life expectancy but also troubled reproductive prospects. Assisted reproduction technologies, such as Preimplantation Genetic Testing for Monogenic disorders (PGT-M), offer a viable alternative to willing patients by preventing the transmission of causative variants through generations. However, according to the literature, the knowledge of these technologies among patients, and even professionals, remains limited, and so does patients’ willingness to make use of them. This appears to be influenced by a complex interplay of clinical, reproductive, demographic, socio-cultural, ethical, and psychological factors.
2. Knowledge of PGT
Articles about the use of PGT for CPSs consistently report the stance that patients should be systematically informed about this diagnostic technique, regardless of the specific syndrome [1][2]. This opinion seems to be shared by both healthcare providers and patients.
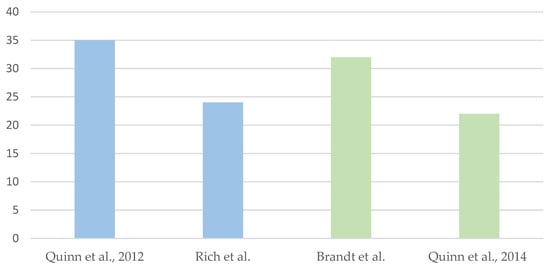
However, various studies have also highlighted that patients’ awareness of this reproductive option is still limited. In 2012, Quinn et al. published a meta-analysis of studies conducted between 1992 and 2009 on the use of PGT for CPSs. 7 articles concerned the application of PGT for Hereditary Breast and Ovarian Cancer syndrome, 2 for Familial Adenomatous Polyposis, 1 for Von Hippel–Lindau syndrome, 1 for Li–Fraumeni syndrome, and 3 for genetic predisposition to cancer in general. In this meta-analysis, only 35% of patients were aware of the applicability of PGT to CPSs [2]. In Rich et al.’s case series involving patients with MEN1, MEN2, FAP, Lynch syndrome and HBOC syndrome the percentage was 24% of 370 adults diagnosed with a CPS [3].
More recently, in Villy et al.’s retrospective study, 28 patients with different CPSs were asked how they became aware of the possibility of undergoing PGT. Of them, 75% had received the information from a physician (in two-thirds of cases, from a geneticist). The cohort was mainly composed of patients with VHL syndrome (9/28), Familial Adenomatous Polyposis (8/28), and CDH1-related gastric tumor predisposition (5/28), followed by patients with variants in STK11 (2/28), AXIN2, BRCA1, MEN1, and FH (1 patient for each gene) [1].
Socioeconomic status emerged as a factor influencing patients’ awareness of the technique [1]. An income-related difference was also reported by Rich et al. (12% of patients with income below 20 K $ were aware of the technique, compared to 38% of those with 20–50 K and 22% for >100 K $) [3]. Based on these data, it could be hypothesized that information is more accessible to higher-income classes; however, according to the authors, the motivation can also be attributed to different approaches by physicians [3][4].
Regarding healthcare providers, in 2010 Brandt et al. addressed a survey of professionals involved in the management of patients affected by CPSs, such as gynecologic oncologists, obstetricians, and gynecologists, assessing their knowledge of the topic and their personal perception and experience with the application of PGT for HBOC and FAP syndromes. The questionnaire revealed that 68% of them had limited or incorrect knowledge about the use of PGT for CPSs, while over 80% expressed their willingness to refer these patients to professionals experienced in PGT [5].
The desire for further education on PGT is not limited to physicians, as shown in the 2014 study by Quinn et al. targeting nurses from the Moffitt Cancer Center. The study found that 78% declared not to be familiar with PGT, but more than half were in favor of its use for CPSs [6]. Scheme 1 provides a graphical representation of the aforementioned data.
3. Acceptability of PGT
3.1. Healthcare Providers’ Opinion
The limited available studies on CPSs show that the acceptability of PGT to professionals is often influenced by the type of syndrome and the oncological history of patients.
For example, a 2009 French study investigated the levels of acceptability of PGT among geneticists, revealing a correlation with the clinical characteristics of the different diseases. Specifically, for syndromes with childhood-onset, multifocal presentation, high penetrance and limited prevention and/or treatment options, the technique was considered acceptable by 76.3% of respondents. However, the percentage dropped to 13.2% for syndromes with adult-onset, localized presentation, high penetrance, effective prevention and/or treatment options, but with impact on quality of life. Furthermore, it reached 0% for syndromes with similar characteristics but with preserved quality of life. In order, the syndromes for which PGT was considered acceptable were: Li–Fraumeni syndrome (67.1% of respondents), retinoblastoma (47.4%), familial adenomatous polyposis (39.5%), MEN2A (11.8%), neurofibromatosis type 1 (11.8%), HBOC (7.9%), HNPCC (6.6%) [7].
More recently, a Dutch study showed high levels of approval regarding the application of PGT for individuals carrying BRCA variants (>85% of respondents, including geneticists, gynecologists, and oncologists). In particular, 92% stated that they would propose this solution to their future patients. The authors attribute this trend to the increasing development of PGT in recent years. However, most professionals still reported low to moderate knowledge of the technique [8].
3.2. Patients’ Opinion
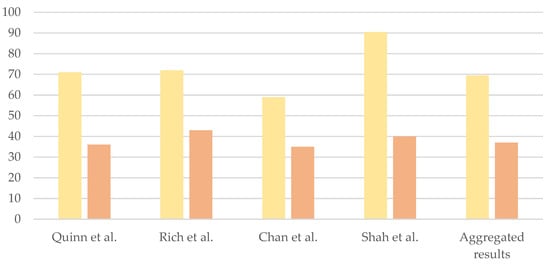
The meta-analysis by Quinn et al. revealed that the majority of interviewed patients (71%) believed that PGT should be offered as a reproductive option for couples affected by CPSs, but only 36% would use it personally [2]. This discrepancy is a recurrent finding in the scientific literature (72% vs. 43% in Rich et al.’s study; 59% vs. 35% in Chan et al.’s case series involving female patients with BRCA1/2 variants) [3][9] (Scheme 2). In Shah et al.’s study targeting patients with CDH1 variants, 40% of respondents stated they would consider PGT, 35% would not and 25% were uncertain; 90.5% of patients thought that physicians should discuss PGT with individuals carrying CDH1 variants [10].
A heterogeneous set of factors that might influence the acceptability of PGT among patients is reported to date.
3.2.1. Clinical Factors
The study by Rich et al. aimed at comparing the opinions of cohorts of patients affected by different syndromes (MEN1, MEN2, FAP, Lynch syndrome, and HBOC). The purpose was to highlight potential syndrome-specific factors that might influence the acceptability of PGT. The percentage of patients considering PGT was higher among those affected by MEN1 and FAP, while it was lower for patients diagnosed with MEN2 [3].
According to Hansen et al., the low interest in PGT among patients with MEN2 (especially MEN2A) might be attributed to the existence of prophylactic thyroidectomy as a primary preventive intervention for medullary thyroid carcinoma. However, it is essential to point out that this intervention carries surgical risks and does not protect against other clinical manifestations of the condition (e.g., the development of pheochromocytoma) [11].
Higher levels of acceptability were observed in subjects affected by early-onset diseases and in subjects with conditions for which prophylactic surgery is not available. Higher levels of acceptability were also reported for syndromes with a perceived higher disease burden [3].
3.2.2. Demographic Factors
Regarding demographic factors, Rich et al. found that the only factor influencing the acceptability of PGT was gender, with the highest levels among male subjects. One reason hypothesized by the authors was the concern of female patients regarding the exposure of cancerous or precancerous cells to high estrogen levels. In fact, while IVF techniques for males involve gamete donation, females undergo ovarian stimulation treatments to induce ovulation, as well as procedures for egg retrieval and subsequent embryo transfer [3]. Differently, age, ethnicity, income, and education were not found to be significant factors conditioning respondents’ opinions.
The survey conducted by Krones et al. in Germany yielded the following findings: favorable attitude towards PGT for CPSs was reported by 59.4% of male respondents and 55.8% of female respondents; regarding the percentage of respondents open to considering PGT, 40.1% of males and 32.2% of females responded positively [12].
The limitation of this survey is that it polled the general population and not patients (as emphasized by Rich et al., the personal experiences of these patients play a key role in modifying their perception of the procedure). The article by Krones et al. also compared the percentage of individuals in favor of PGT for CPSs in different countries, showing 50% of respondents in favor in Germany and 60% in the USA [12]. In the study by Marteau et al. on the British population, the percentage dropped to 34% [13].
Regarding HBOC syndrome specifically, a review by Lombardi et al. in 2022 confirmed higher levels of acceptability among male subjects [4]. Moreover, carrier status for HBOC syndrome correlated with a reduced desire for parenthood among female subjects, but not among male subjects. This same difference was also observed with regard to the desire to have a new pregnancy in BRCA1/2 patients with previous children.
3.2.3. Reproductive Factors
In the study by Rich et al. CPS couples with children, as well as couples accepting VTP as an option, appeared to have a lower degree of acceptability of PGT. In this regard, the authors suggest that parents may have concerns about the psychological consequences on a firstborn affected by a CPS if they decide to have another child who will not be affected because of PGT [3].
In the case series of BRCA carriers by Chan et al., a comparison of attitudes towards PGT in women whose families were regarded as complete vs. not complete showed similar results [9].
In Shah et al.’s study, of the two CHD1 carriers who wanted to have a biological child, one was “very likely” to use PGT, whereas the other one was “very unlikely”. Among respondents who already had children, a small majority indicated that they would have considered using PGT if it had been available [10].
3.2.4. Socio-Cultural, Ethical and Psychological Factors
In the 2021 review by Hughes et al. regarding elements influencing patients’ decisions about Preimplantation Genetic Testing, a complex set of factors emerged, including ethical and religious ones [14].
Some patients reported that they feel a sense of responsibility when considering PGT as an option, while others showed reluctance due to their religious beliefs. Similar considerations emerged from a cross-sectional study by Shah et al. targeting patients with a genetic predisposition to develop gastric tumors. Out of 38 investigated patients, 13 identified their “philosophy of life” as an influencing factor, followed by “God, religion, and morality” (10/38), the “desire to eradicate the variant for future generations” (9/38), and the desire to “minimize anxiety or suffering,” both on a personal level and for their offspring (5/38) [10].
In the investigation by Rich et al., religious patients were less favorable to the question of whether PGT should be offered compared to those who defined themselves as non-religious (69% versus 89%) [3]. According to the review by Lombardi et al., two studies stated that religious beliefs did not influence the choice of resorting to assisted reproductive techniques [4]. Conversely, Menon et al. found that women who had opted for PGT tended to be less religious [15].
In many cases, PGT may be considered an acceptable option when pregnancy termination or gamete donation is not compatible with certain ethical and religious beliefs [11]. In Kastrinos et al. case series, avoiding a potential pregnancy termination was regarded as important by 64% of women and 71% of men [16]. Lammens et al. reported that avoiding a potential termination of pregnancy was the most frequently perceived advantage of PGT among patients (32% of the total) [17].
Similarly, in the study by Derks-Smeets et al., none of the 6 couples who underwent PGT considered invasive prenatal diagnosis acceptable; specifically, all couples perceived a “moral difference” between embryo selection and pregnancy termination, considering termination too drastic of a solution [18].
In the meta-analysis by Quinn et al., the most frequently reported factor influencing the acceptability of PGT was couples’ concern for the health of the newborn. However, most studies also showed that 33% of respondents reported ethical concerns regarding the use of PGT, including those who expressed a favorable view of the procedure [2]. Derks-Smeets et al. also reported that protecting the child from the variant was one of the main psychological factors that led couples to choose PGT; factors against PGT included the fear of a loss of romance in the relationship, having to resort to IVF techniques, and the percentage of procedure failures [18].
Another key element to consider is the personal experience of these families. This includes not only the oncological history of the patient but also the one of affected family members. For example, in Kastrinos et al.’s study on patients with FAP, the main factors reported to influence a tendency toward prenatal diagnosis (invasive/preimplantation) were: already having a child affected with cancer and experiencing the loss of a family member secondary to FAP-associated cancer. In this case series, 19 out of 20 FAP patients would consider prenatal diagnosis (specifically, 90% would consider PGT and 75% would consider invasive prenatal techniques), and all of them deemed it ethical to offer PGT to affected families [16].
Regarding personal oncological history, Fortuny et al. [19] and Menon et al. [15] reported a greater tendency to consider PGT among patients with a previous diagnosis of tumor. Derks-Smeets et al. [18], Gietel-Habets et al. [8], and Ormondoryd et al. [20] found that the most important factor influencing couples’ reproductive decisions was their personal and family history of cancer.
References
- Villy, M.-C.; Frydman, N.; Moutou, C.; Thierry, G.; Raad, J.; Colas, C.; Steffann, J.; Metras, J.; Chabbert-Buffet, N.; Parc, Y.; et al. Preimplantation genetic testing in patients with genetic susceptibility to cancer. Fam. Cancer 2023, 22, 119–125.
- Quinn, G.P.; Pal, T.; Murphy, D.; Vadaparampil, S.T.; Kumar, A. High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: A systematic review and meta-analysis. Genet. Med. 2012, 14, 191–200.
- Rich, T.A.; Liu, M.; Etzel, C.J.; Bannon, S.A.; Mork, M.E.; Ready, K.; Saraiya, D.S.; Grubbs, E.G.; Perrier, N.D.; Lu, K.H.; et al. Comparison of attitudes regarding preimplantation genetic diagnosis among patients with Cancer Predisposition Syndromes. Fam. Cancer 2014, 13, 291–299.
- Lombardi, L.; Trumello, C.; Stuppia, L.; Antonucci, I.; Brandão, T.; Babore, A. BRCA1/2 pathogenetic variant carriers and reproductive decisions: Gender differences and factors associated with the choice of preimplantation genetic diagnosis (PGD) and prenatal diagnosis (PND). J. Assist. Reprod. Genet. 2022, 39, 1433–1443.
- Brandt, A.C.; Tschirgi, M.L. Knowledge, attitudes and clinical experience of physicians regarding preimplantation genetic diagnosis for hereditary cancer predisposition syndromes. Fam. Cancer 2010, 9, 479–487.
- Quinn, G.P.; Knapp, C. Knowledge and Educational Needs about Pre-Implantation Genetic Diagnosis (PGD) among Oncology Nurses. J. Clin. Med. 2014, 3, 632–645.
- Julian-Reynier, C.; Chabal, F.; Frebourg, T.; Lemery, D.; Noguès, C.; Puech, F.; Stoppa-Lyonnet, D. Professionals assess the acceptability of preimplantation genetic diagnosis and prenatal diagnosis for managing inherited predisposition to cancer. J. Clin. Oncol. 2009, 27, 4475–4480.
- Gietel-Habets, J.; de Die-Smulders, C.; Tjan-Heijnen, V.; Derks-Smeets, I.; van Golde, R.; Gomez-Garcia, E.; van Osch, L. Professionals’ knowledge, attitude and referral behaviour of preimplantation genetic diagnosis for hereditary breast and ovarian cancer. Reprod. Biomed. Online 2018, 36, 137–144.
- Chan, J.; Lnc, J.; Sammel, M.; DiGiovanni, L.; Voong, C. Reproductive Decision-Making in Women with BRCA1/2 Mutations. J. Genet. Couns. 2017, 26, 594–603.
- Shah, I.H.; Salo-Mullen, E.E.; Amoroso, K.A.; Kelsen, D.; Stadler, Z.K.; Hamilton, J.G. Attitudes toward preimplantation genetic testing and quality of life among individuals with hereditary diffuse gastric cancer syndrome. Hered. Cancer Clin. Pract. 2022, 20, 31.
- Würgler Hansen, A.; Sønderberg Roos, L.K.; Løssl, K.; Godballe, C.; Mathiesen, J.S. Preimplantation Genetic Testing of Multiple Endocrine Neoplasia Type 2A. Front. Endocrinol. 2020, 11, 10–13.
- Krones, T.; Schlüter, E.; Manolopoulos, K.; Bock, K.; Tinneberg, H.-R.; Koch, M.C.; Lindner, M.; Hoffmann, G.F.; Mayatepek, E.; Huels, G.; et al. Public, expert and patients’ opinions on preimplantation genetic diagnosis (PGD) in Germany. Reprod. Biomed. Online 2005, 10, 116–123.
- Marteau, T.; Michie, S.; Drake, H.; Bobrow, M. Public attitudes towards the selection of desirable characteristics in children. J. Med. Genet. 1996, 32, 796–798.
- Hughes, T.; Bracewell-Milnes, T.; Saso, S.; Jones, B.P.; Almeida, P.A.; Maclaren, K.; Norman-Taylor, J.; Johnson, M.; Nikolaou, D. A review on the motivations, decision-making factors, attitudes and experiences of couples using pre-implantation genetic testing for inherited conditions. Hum. Reprod. Update 2021, 27, 944–966.
- Menon, U.; Harper, J.; Sharma, A.; Fraser, L.; Burnell, M.; ElMasry, K.; Rodeck, C.; Jacobs, I. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum. Reprod. 2007, 22, 1573–1577.
- Kastrinos, F.; Stoffel, E.M.; Balmaña, J.; Syngal, S. Attitudes toward prenatal genetic testing in patients with familial adenomatous polyposis. Am. J. Gastroenterol. 2007, 102, 1284–1290.
- Lammens, C.; Bleiker, E.; Aaronson, N.; Vriends, A.; Ausems, M.; Jansweijer, M.; Wagner, A.; Sijmons, R.; Ouweland, A.V.D.; van der Luijt, R.; et al. Attitude towards pre-implantation genetic diagnosis for hereditary cancer. Fam. Cancer 2009, 8, 457–464.
- Derks-Smeets, I.A.P.; Gietel-Habets, J.J.G.; Tibben, A.; Tjan-Heijnen, V.C.G.; Meijer-Hoogeveen, M.; Geraedts, J.P.M.; van Golde, R.; Gomez-Garcia, E.; Bogaart, E.v.D.; van Hooijdonk, M.; et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: A challenge for couples with hereditary breast and ovarian cancer. Hum. Reprod. 2014, 29, 1103–1112.
- Fortuny, D.; Balmaña, J.; Graña, B.; Torres, A.; Ramón y Cajal, T.; Darder, E.; Gadea, N.; Velasco, A.; López, C.; Sanz, J.; et al. Opinion about reproductive decision making among individuals undergoing BRCA1/2 genetic testing in a multicentre Spanish cohort. Hum. Reprod. 2009, 24, 1000–1006.
- Ormondroyd, E.; Donnelly, L.; Moynihan, C.; Savona, C.; Bancroft, E.; Evans, D.G.; Eeles, R.; Lavery, S.; Watson, M. Attitudes to reproductive genetic testing in women who had a positive BRCA test before having children: A qualitative analysis. Eur. J. Hum. Genet. 2012, 20, 4–10.
More
Information
Subjects:
Genetics & Heredity; Oncology; Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
698
Revisions:
2 times
(View History)
Update Date:
20 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




