Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jasmine Carter | -- | 2489 | 2023-11-16 19:50:27 | | | |
| 2 | Sirius Huang | Meta information modification | 2489 | 2023-11-17 09:27:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bettag, J.; Goldenberg, D.; Carter, J.; Morfin, S.; Borsotti, A.; Fox, J.; Reveal, M.; Natrop, D.; Gosser, D.; Kolli, S.; et al. Microbiome Influences Neurodevelopment in the Central Nervous System. Encyclopedia. Available online: https://encyclopedia.pub/entry/51706 (accessed on 08 February 2026).
Bettag J, Goldenberg D, Carter J, Morfin S, Borsotti A, Fox J, et al. Microbiome Influences Neurodevelopment in the Central Nervous System. Encyclopedia. Available at: https://encyclopedia.pub/entry/51706. Accessed February 08, 2026.
Bettag, Jeffery, Daniel Goldenberg, Jasmine Carter, Sylvia Morfin, Alison Borsotti, James Fox, Matthew Reveal, Dylan Natrop, David Gosser, Sree Kolli, et al. "Microbiome Influences Neurodevelopment in the Central Nervous System" Encyclopedia, https://encyclopedia.pub/entry/51706 (accessed February 08, 2026).
Bettag, J., Goldenberg, D., Carter, J., Morfin, S., Borsotti, A., Fox, J., Reveal, M., Natrop, D., Gosser, D., Kolli, S., & Jain, A.K. (2023, November 16). Microbiome Influences Neurodevelopment in the Central Nervous System. In Encyclopedia. https://encyclopedia.pub/entry/51706
Bettag, Jeffery, et al. "Microbiome Influences Neurodevelopment in the Central Nervous System." Encyclopedia. Web. 16 November, 2023.
Copy Citation
The brain is traditionally viewed as an immunologically privileged site; however, there are known to be multiple resident immune cells that influence the central nervous system (CNS) environment and are reactive to extra-CNS signaling. Microglia are an important component of this system, which influences early neurodevelopment in addition to modulating inflammation and regenerative responses to injury and infection. Microglia are influenced by gut microbiome-derived metabolites, both as part of their normal function and potentially in pathological patterns that may induce neurodevelopmental disabilities or behavioral changes.
microglia
gut microbiome
Gut–Brain axis
metabolite
neurodevelopment
1. Introduction
The gastrointestinal tract houses a diverse community of trillions of bacteria that exist in a symbiotic relationship with their host organism. These commensal bacteria influence the host through facilitation of digestion, immune system modulation, and influencing neurologic function and behavior [1][2][3]. Additionally, the gut microbiome has been demonstrated to be vital for the establishment of the blood–brain barrier (BBB), as germ-free (GF) mice were found to have brain vascular endothelium that were highly permeable to macromolecules and recovered selective permeability upon colonization with non-pathogenic gut microbiome or through exposure to short chain fatty acid metabolites of gut bacteria [4].
Microglia are immune cells native to the brain, which have an important role in modulating inflammation in the brain, neurogenesis, and synaptic architecture. These cells are the most abundant immune-related cells in the brain [5]. The brain is considered a relatively immunologically privileged site due to lack of lymphatic drainage and its reduced ability to present antigens to induce immune responses. However, the central nervous system (CNS) [6] does possess an array of unique cells, associated with the innate immune system, that serve a central role in mounting immune responses within the CNS [6]. Microglial cells, specifically, are prevalent in brain parenchyma. Augmentation or inhibition of immune responses have known clinical impact in controlling a host of neurodevelopmental or neurodegenerative diseases. For instance, it has been shown that SGLT2 inhibitors can reduce neuroinflammation and be neuroprotective by downregulating microglia that have been activated by bacterial factors; however, these studies were on adults who are well beyond early neurodevelopment [7]. It is becoming increasingly known that gut bacteria release certain factors similar to those seen in acute inflammatory states. Therefore, the modulation of these bacteria, the control of the factors released, the progressive development of the microbiome, and its associated bacteria and factors are likely to have an important role to play in early childhood neurodevelopment. This is becoming an increasingly important field, as, in children, who have not achieved full neurodevelopment and therefore are more prone to neurological insult, the microbiome may shape cognitive outcomes, impacting psychiatric illnesses, overall morbidity, and other behavioral factors.
2. Embryologic Characteristics and Development of Microglial Cells
Microglia are derived from primitive myeloid progenitor cells originating from the yolk sack on embryonic day 7.5 (E7.5) via stimulation by colony stimulating factor 1 receptor (CSF-1R). These primitive cells penetrate the CNS via pial surfaces and the fourth ventricle [8]. This represents a developmental path distinct from that of the hematopoietic macrophage cells present in the rest of the body such as red pulp macrophages, subcapsular sinus macrophages, alveolar macrophages of the lungs, and Kupffer cells of the liver. Microglial cells are maintained throughout the organism’s lifespan, independent of hematopoietically derived macrophages [8][9]. Evidence supporting this yolk sack origin, as distinct from other macrophage lineages, is that 30% of primitive yolk sac macrophages can be labeled early in embryonic development when exposed to tamoxifen, and when the organism is assayed for these labeled cells at different points of embryonic, fetal, and newborn development it reveals consistently a 30% proportion of labeled microglial cells in brain [10] (Figure 1).
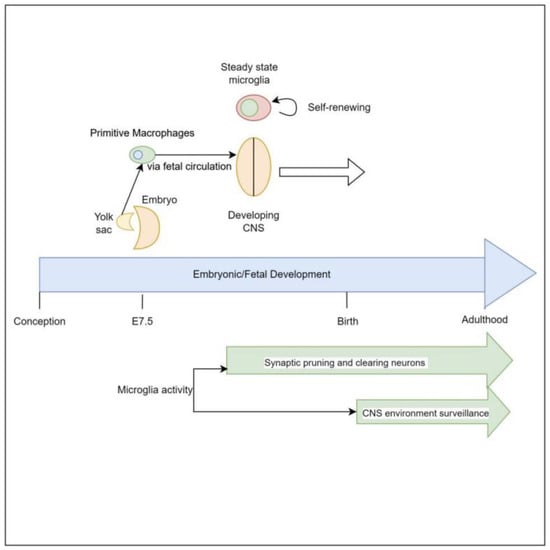
Figure 1. Both in vivo and in vitro, microglia appear to be dependent on Interleukin-34 (IL-34) for stimulation to proliferate. IL-34 is a cytokine that serves as one ligand to the receptor CSF-1R, which is central to signaling for proliferation and development of mononuclear phagocyte cells [11]. This cytokine is strongly expressed in mouse brains during embryogenesis, and strongly correlates with the proliferation of mononuclear phagocytic cells [12][13]. Additionally, IL-34 induces microglia to produce insulin degrading enzyme (IDE) and heme oxygenase-1 (HO-1), which are associated with clearing oligomeric amyloid Beta and defend against reactive oxygen species, respectively [14].
3. The Effect of Microglial Cells on Neurodevelopment
It is understood that the early brain possesses an abundance of neurons with a plethora of unneeded synaptic connections, which are progressively pruned via early sensory stimulation and motor activity to establish a network that is functional and efficient. This culling and shaping of neuronal connections takes place in designated areas of the brain in accordance with various critical periods—times in which specific stimuli are required [15][16]. This ensures both appropriate physiologic and neurologic development and aids in meeting developmental milestones. For example, in the visual system, if kittens’ eyes are sutured shut in the early months of life there is a marked drop in the number of neuronal cells that are stimulated by those eyes. This study revealed that 6 days of suturing the eyes was equivalent to a 3–4-month physiologic monocular setback [17]. This indicates that lack of sensory stimulation is a significant driving factor in neurodevelopment. Given what is known about microglia, this process is likely to be mediated by microglia.
Microglia are a vital actor during these periods, in a region-specific manner, driving maturation of neural architecture. Microglia appear to survey the extracellular medium for stimuli to induce synaptic pruning. These stimuli vary by region of the brain involved and include complement factor 3 in retinal ganglion cells, IL-33 in the reticular thalamic nucleus, and fractalkine (CX3CR1) in the hippocampus [16][18][19]. Thus, as the thalamus is commonly referred to as the ‘relay center of the brain’ and the hippocampus is known for memory formation, any interaction between gut metabolites and neurologic cell-receptor pathways may result in alterations to memory-forming ability, and thought, motor, and sensory-signaling ability as a result of over, under, or aberrant synaptic pruning.
4. Influence of Microglia on Neuropathology
The importance of microglia-driven synaptic pruning is exemplified by the neuropathology observed when it is disrupted. Autism Spectrum Disorder (ASD) refers to a grouping of communication impairments and atypical behaviors [16]. The cause of ASD is incompletely understood at this time but is believed to be multifactorial, with neuroinflammation (and thus innate immune system activation) being thought to play a strong role in the development of ASD. Autopsy of individuals with ASD, in comparison to matched controls, have revealed significantly higher densities of microglial cells in the fronto-insular cortex, visual cortex, dorsolateral prefrontal cortex, and cerebellum, indicating these cells either play a role in the progression of this syndrome or their proliferation is stimulated by a separate driver [20][21][22][23]. In mice that have overexpression of translation initiation factor eIF4E, there is noted increase in microglial density and phagocytic activity, yet reduced motility and synaptic engulfment [24]. This leads these mice to have higher synaptic density and autism-like behaviors, specifically in male mice vs. female mice.
As previously mentioned, microglia appear to function as “gardeners” of the early brain, pruning little-utilized synapses, but they also continuously monitor the extracellular medium for signs of injury, infection, or cellular derangement to protect the CNS via modulation of inflammation, stimulation of neurogenesis, and clearing of cellular debris [25]. As the microbiome and microglia are both constantly developing from birth to death, maturing the gut, immune system, and neurodevelopment, any interaction between the two can result in such derangements or syndromes.
5. Development of Human Microbiome
The adult microbiome has been immensely studied; it has been found that the average adult microbiome is made up of over 400 species of bacteria [26][27]. An infant’s microbiome is immature and weakened, going through complex development as it progresses through new illnesses or environmental exposures into adulthood. Understanding the development of gut microbiomes is becoming increasingly important in concurrent research into cognitive development. Studies have been conducted that show that an infant’s microbiome goes through exponential development over the course of the first few years of life, eventually leading to an extensive, stable gut by the age of three [26]. Throughout these first three years of life, the infant’s microbiome experiences successive growth with several shifts between different bacterial taxonomies [26].
Major factors in the establishment and maintenance of the infant microbiome reside in both maternal and infant factors. Maternal exposures, such as infection, stress, and obesity, play a critical role in the establishment of the microbiome of the neonate after birth. Infant factors, such as mode of delivery, antibiotic exposure, and infant nutritional intake, are additional vital contributors. Mode of delivery is an integral contributor to the establishment of the gut microbiome. Infants who have been born vaginally have their initial gut flora similar to that of their mothers vaginal and anal flora, consisting heavily of Lactobacillus, while those born via cesarean section have their initial gut flora closely resembling bacteria heavily colonized on the skin surface, such as Staphylococcus [26]. Infants who were born via cesarean section have been found to have less diversity in their intestinal flora, and these changes in the gut microbiome can be seen until the age of seven [26]. Salminen et al. conducted a study in 2004 which assessed differences in intestinal flora of seven-year-old children who were born vaginally versus those who were born by cesarean section; results signified that there are significantly more Clostridium species in those who were born vaginally, signifying that changes in the microbial gut continue beyond delivery [28].
Antibiotic exposure in utero and throughout the formulation of the gut microbiome alters the trajectory and inhabitants of the gut bacteria. Studies conducted by Aloisio et al. in 2014 demonstrated that newborns whose mothers were found to be group B Streptococcus (GBS) positive and received antibiotic prophylaxis had counts of Bifidobacteria that were significantly decreased compared to the control group whose mothers did not receive prophylactic antibiotics for GBS [29]. The use of antibiotics in utero and thereafter leads to alterations in the gut microbiome and a delay in the establishment of commensal flora.
Diet composition from birth and into adulthood has a radical influence on the development of the gut microbiome [30]. Harmsen et al. conducted a study in 2000 which showed infants who were breastfed have a predominance of Bifidobacteria, while those infants who were bottle fed displayed a predominance of E. coli, staphylococcus, and clostridium [31]. There have been cited associations between the infant diet, and establishment of its microbial gut, and the development of conditions such as atopy, inflammatory bowel disease, obesity, and metabolic disorders throughout a person’s lifetime [26][32][33]. The transition from breast milk or formula to solid food leads to major alterations in the gut flora shown by a heavy increase in enterobacteria and enterococci [30]. The increase in enterobacteria and enterococci was more significant within infants who were breastfed. Following the introduction of solid food, the population size and makeup of the gut microbiome more closely resembled that of an adult [30]. After the age of three, when the microbial gut resembles that of an adult, alterations in the intestinal flora directly correlate with that of alterations in the makeup of one’s diet [34].
6. Microbiome Influence on Microglia
As noted above, microglial cells may have a myriad of interacting signals, to activate, deactivate, proliferate, or senesce, that are dependent on the specific areas of the brain affected or being affected [16][18][19]; however, microglia may also be influenced by factors outside of the CNS. This external influence may be part of normal neural development or represent pathologic disruption of physiologic microglial activity. In particular, the gut microbiome is an expansive ecosystem consisting of trillions of individual organisms comprising thousands of species of bacteria, each metabolically active and interacting with the host digestive system [35][36]. This diverse microbial community serves its’ host by maturing the intestinal mucosa, facilitating breakdown of enteral macromolecules for ease of absorption, maturation of innate immune cells, and production of a myriad of metabolites that appear to have systemic or organ-specific physiologic effects on the host [37][38][39][40]. Recent studies have demonstrated that gut microbiota does have a role in influencing normal neurodevelopment. Germ-free (GF) mice, which lack normal enteric gut commensal bacteria, have been shown to develop increased motor activity and decreased behavioral indicators of anxiety, with normal behavioral phenotypes being restored following intestinal colonization [41].
In addition to their previously mentioned roles in the development of neuroarchitecture, microglial cells modulate neuroinflammation via pro- and anti-inflammatory chemokines and cytokines [42]. In a study done by Alleva et al. in 1997, it was found that lupus-prone mice had early dysregulation of neuropsychiatric symptoms, such as anhedonia and despair, due to loss of effective TNF-α signaling. This same study linked loss of effective TNF-α production to an increase in dysfunctional cytokines, such as IL-1 and IL-6, which was hypothesized to cause the seen effects [43]. This indicates that, in mice predisposed to immune dysfunction, disruption of cytokine pathways, as is seen in gut dysbiosis, results in altered signaling of the CNS. Once the blood–brain barrier (BBB) was damaged, two separate studies found that IL-1, TNF-α, and IL-6 could diffuse across and cause increased expression of immune cell receptors, such as ICAM-1 and VCAM-1, in the brain parenchyma [44]. Subsequently, this resulted in recruitment of microglia and astrocytes, resulting in cytotoxicity [45][46].
In the study by Ballok et al., mice with uncontrolled lupus were seen to have damage to the hippocampus because of gliosis, or enlargement and proliferation of the microglial cells, while immunosuppressed mice had comparatively less degeneration than the uncontrolled mice. This same study also examined the brain of a patient with lupus and found a similar reduction in neuronal density in the hippocampal area [45]. More specifically to microglial cells, a recent review from 2022 described microglial cells as “sensors” for microbiota-derived molecules such as lipopolysaccharide (LPS), short chain fatty acids (SCFA), and tryptophan derivatives. As microglia serve in the role of patrolling brain parenchyma and surveying for signs of injury or infection, it is particularly the acute phase reactants from the microbiome that trigger inflammation [46].
The effects of microbiome metabolites on the microenvironment and behavior via microglial cell interactions are not limited to modulation of inflammation. SCFAs (acetate, propionate, and butyrate) are metabolites synthesized from glucose by fecal microbiota through well-established mechanisms [47]. In mice that have experienced thrombotic stroke, those that received supplementation with SCFAs had subsequent improved recovery of motor function of their affected limb compared to those not supplemented. Additional investigation in these mice demonstrated a modulation of CNS lymphocyte and microglial function via the supplementation of SCFAs, leading to post-stroke recovery [48].
In humans, the clinical importance is most noted primarily around children with different social structures. The term “sociobiome” refers to the environmental and social factors that influence diet. Interestingly, infants with older siblings have higher counts of facultative anaerobes and lower levels of clostridia spp. and E. Coli [49]. Other social factors, such as income and diet, may also, therefore, play a role clinically; however, given this complexity, there are currently no studies comparing the microbiomes of different social and economic spheres and associated factors, and thus on their downstream impact on microglia.
References
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84.
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666.
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, Corrected in Sci. Transl. Med. 2014, 6, 266er7.
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402.
- Nayak, D.; Zinselmeyer, B.H.; Corps, K.N.; McGavern, D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital 2012, 1, 95–106.
- Vaziri, Z.; Saleki, K.; Aram, C.; Alijanizadeh, P.; Pourahmad, R.; Azadmehr, A.; Ziaei, N. Empagliflozin treatment of cardiotoxicity: A comprehensive review of clinical, immunobiological, neuroimmune, and therapeutic implications. Biomed. Pharmacother. 2023, 168, 115686.
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845.
- Lichanska, A.M.; Hume, D.A. Origins and functions of phagocytes in the embryo. Exp. Hematol. 2000, 28, 601–611.
- Samokhvalov, I.M.; Samokhvalova, N.I.; Nishikawa, S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 2007, 446, 1056–1061.
- Baghdadi, M.; Umeyama, Y.; Hama, N.; Kobayashi, T.; Han, N.; Wada, H.; Seino, K.-I. Interleukin-34, a comprehensive review. J. Leukoc. Biol. 2018, 104, 931–951.
- Wei, S.; Nandi, S.; Chitu, V.; Yeung, Y.-G.; Yu, W.; Huang, M.; Williams, L.T.; Lin, H.; Stanley, E.R. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 2010, 88, 495–505.
- Garceau, V.; Balic, A.; Garcia-Morales, C.; Sauter, K.A.; McGrew, M.J.; Smith, J.; Vervelde, L.; Sherman, A.; Fuller, T.E.; Oliphant, T.; et al. The development and maintenance of the mononuclear phagocyte system of the chick is controlled by signals from the macrophage colony-stimulating factor receptor. BMC Biol. 2015, 13, 12.
- Mizuno, T.; Doi, Y.; Mizoguchi, H.; Jin, S.; Noda, M.; Sonobe, Y.; Takeuchi, H.; Suzumura, A. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-β neurotoxicity. Am. J. Pathol. 2011, 179, 2016–2027.
- Hua, J.Y.; Smith, S.J. Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 2004, 7, 327–332.
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458.
- Hubel, D.H.; Wiesel, T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970, 206, 419–436.
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705.
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273.
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas 2013, 25, 191–192.
- Tetreault, N.A.; Hakeem, A.Y.; Jiang, S.; Williams, B.A.; Allman, E.; Wold, B.J.; Allman, J.M. Microglia in the cerebral cortex in autism. J. Autism Dev. Disord. 2012, 42, 2569–2584.
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmermann, A.W.; Pardo, C.A. Neuroglial activtion and neuroinflammation in the brains of patients with autism. Ann. Neurol. 2005, 57, 67–81.
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 2010, 68, 368–376.
- Xu, Z.X.; Kim, G.H.; Tan, J.W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.-Y.; Xu, H.; Lee, S.-H.; Do, N.-Y.; et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 2020, 11, 1797.
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2016, 136 (Suppl. S1), 10–17.
- Dunn, G.A.; Mitchell, A.J.; Selby, M.; Fair, D.A.; Gustafsson, H.C.; Sullivan, E.L. Maternal diet and obesity shape offspring central and peripheral inflammatory outcomes in juvenile non-human primates. Brain Behav. Immun. 2022, 102, 224–236.
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs. Res. 2016, 65, 76–88.
- Salminen, S.; Gibson, G.R.; McCartney, A.L.; Isolauri, E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 2004, 53, 1388–1389.
- Aloisio, I.; Mazzola, G.; Corvaglia, L.T.; Tonti, G.; Faldella, G.; Biavati, B.; Di Gioia, D. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2014, 98, 6051–6060.
- Stark, P.L.; Lee, A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 1982, 15, 189–203.
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67.
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17.
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597.
- Kim, M.; Benayoun, B.A. The microbiome: An emerging key player in aging and longevity. Transl. Med. Aging 2020, 4, 103–116.
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51.
- Frank, D.N.; Pace, N.R. Gastrointestinal microbiology enters the metagenomics era. Curr. Opin. Gastroenterol. 2008, 24, 4–10.
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884.
- Geuking, M.B.; Cahenzli, J.; Lawson, M.A.; Ng, D.C.K.; Slack, E.; Hapfelmeier, S.; McCoy, K.D.; Macpherson, A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011, 34, 794–806.
- Donia, M.S.; Fischbach, M.A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015, 349, 1254766.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Charo, I.F.; Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006, 354, 610–621.
- Alleva, D.G.; Kaser, S.B.; Beller, D.I. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J. Immunol. 1997, 159, 5610–5619.
- McHale, J.F.; Harari, O.A.; Marshall, D.; Haskard, D.O. TNF-alpha and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. J. Immunol. 1999, 163, 3993–4000.
- Ballok, D.A.; Woulfe, J.; Sur, M.; Cyr, M.; Sakić, B. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus 2004, 14, 649–661.
- D’Alessandro, G.; Marrocco, F.; Limatola, C. Microglial cells: Sensors for neuronal activity and microbiota-derived molecules. Front. Immunol. 2022, 13, 1011129.
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592.
- Sadler, R.; Cramer, J.V.; Heindl, S.; Kostidis, S.; Betz, D.; Zuurbier, K.R.; Northoff, B.H.; Heijink, M.; Goldberg, M.P.; Plautz, E.J.; et al. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J. Neurosci. 2020, 40, 1162–1173.
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
400
Revisions:
2 times
(View History)
Update Date:
17 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




