
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Cláudia Pereira | -- | 3478 | 2023-11-16 13:45:08 | | | |
| 2 | Fanny Huang | -18 word(s) | 3460 | 2023-11-20 03:44:36 | | |
Video Upload Options
Aptamers are short, single-stranded oligonucleotides synthesized in vitro from a randomized oligonucleotide library against a specific target. These molecules are capable of binding to a wide range of biological targets with high specificity and affinity. They present great advantages over antibodies with potential applications in research, diagnosis, and therapeutics. Specifically for tumors with late-stage identification and poor prognosis, like pancreatic cancer, the study of novel aptamers holds tremendous potential for cancer diagnosis and treatment. Along with cancer treatment, aptamers have also shown high potential in regulating the immune response and modulating several critical steps of signaling cascades, such as in immune checkpoints.
1. Introduction
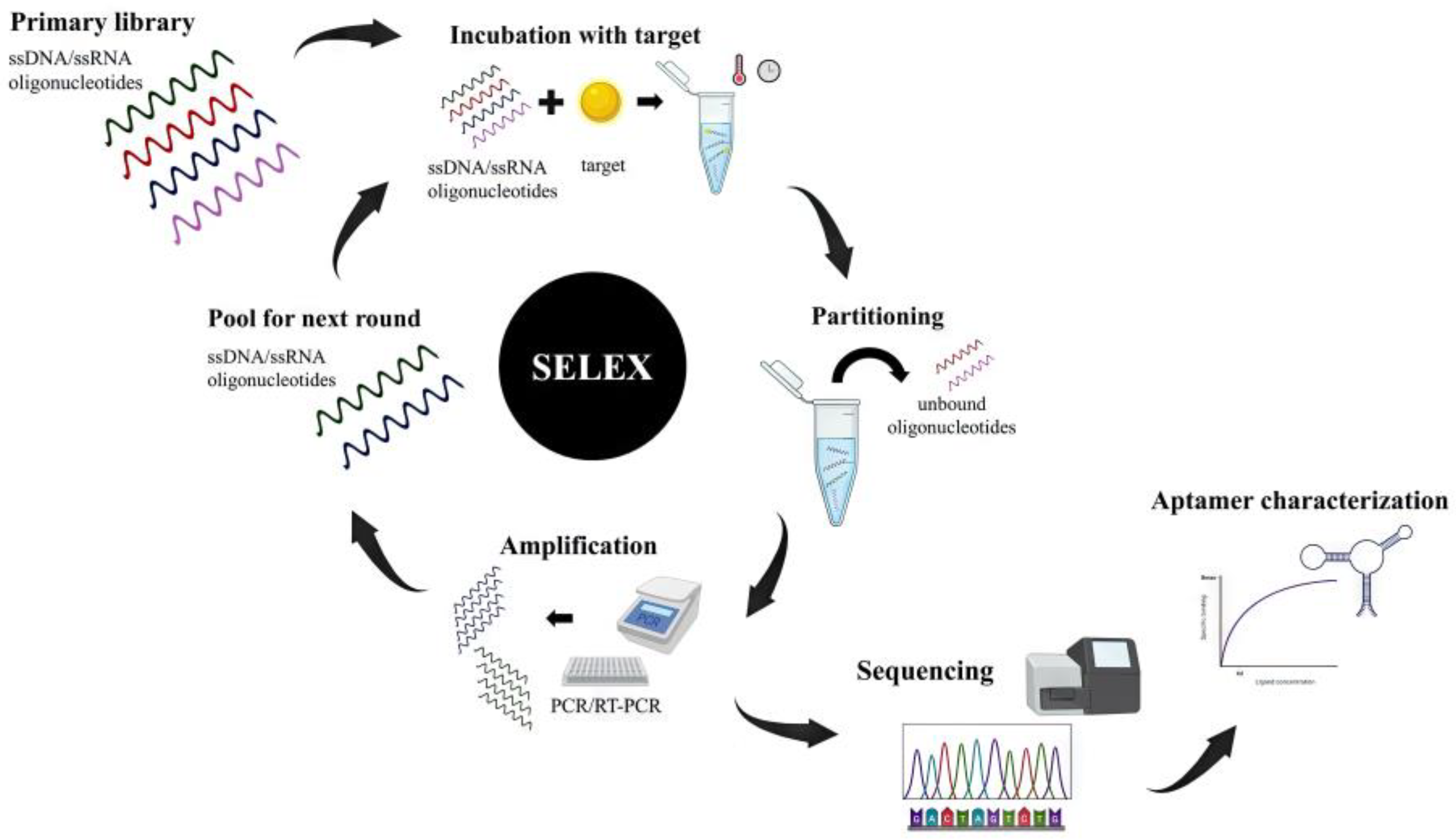
2. Cancer Therapeutics
The versatility of aptamers created an important opportunity for diverse application in the field of immunology both in diagnosis and therapy. Considering diagnostic techniques aptamers might be used for two main purposes: detection [49][78][79] and separation [80][81][82]. In therapy, aptamers have two other principal functions that include being a therapeutic drug itself [83] or being responsible for taking fewer drugs to treat the affected cell or organ and, in this way, reducing the need for higher doses of drugs that have relevant secondary effects [84][85][86].
The gold standard techniques for immune diagnosis are flow cytometry and Enzyme-linked Immunosorbent Assay (ELISA) [87] and aptamers present features useful for both strategies, offering a broad range of solutions for the detection of biomolecules like proteins, including immunoglobulins or membrane receptors [49][83][85][88], cells [80][81][89], or even Extracellular Vesicles (EVs) that have gained popularity in fighting diseases like cancer. For EVs, CD63 is being consistently used as one of the most promising targets for aptamers on the surfaces of EVs [79][82]. Aptamers might also be used for cell separation using, as an example, sorting equipment accoupled to flow cytometry, which is useful both as a diagnosis technique [80][81] and also for therapy purposes in techniques like immunotherapy or HIV therapy [80][90].
Considering therapeutical strategies, aptamers might be a drug by themselves and although there is potential for exclusive use of aptamers as a therapy [83], the most common approach is using aptamers together as co-adjuvant therapy. For example, aptamer- T cells together with anti-PD1 immune-checkpoint monoclonal antibodies have a synergetic effect in reducing cancer, in vitro and in rats, and aptamer-linked small-interfering RNA chimeras to selectively knock down genes in mouse breast cancers [91][92]. This approach allows a decrease in dose in immunotherapy applications and, in this way, reduces the secondary effects of therapies that might cause a lot of discomfort and distress in patients [83,93,94]. Aptamers are used not only in order to destroy the disease agent, like a cancer cell [89], but are often used as immune modulators, reducing immune activity [86], targeting or influencing immune checkpoint inhibitors [89][93][94], and/or promoting immune cell activity and infiltration [91][95].
Aptamers are also showing a lot of potential for drug delivery therapies, not only in immune context, but in general. This happens because aptamers are very versatile as drug-targeting tools and can be used with at least three relevant functions. Aptamers might be carriers for other drugs, allowing the encapsulation of drugs [96], be used for targeting, allowing the drug to reach in higher quantity the compromised cell/organ [84][85][86], and also might be used as a gating system that induces the release of the therapeutical drug when arriving at the target [96].
The microbiota plays a crucial role in protecting against pathogens and preventing infections, competing with pathogenic microorganisms for resources and space and preventing them from colonizing and infecting the host, producing antimicrobial peptides that can kill or inhibit the growth of pathogenic microorganisms and modulate the immune system’s response to pathogens [97][98]. However, when a disruption of this microbiota occurs, it can lead to harmful situations, particularly respiratory, gastrointestinal, and skin infections [97]. One example is antibiotics that can disrupt this balance, promoting pathogenic bacteria overgrowth and increased risk of infection, with Streptococcus and Staphylococcus being among the most common [99]. Also, the overgrowth of Clostridium difficile can lead to a severe infection, which can cause symptoms ranging from diarrhea to life-threatening complications [100].
Aptamers have been increasingly used in microbiome diagnostics, where they can identify and characterize the microorganisms present in a specific environment, such as the human gut, skin, or oral cavity, because of their unique binding properties and their ability to detect specific microbial biomarkers in complex biological samples [1] Their use in the gut microbiota field has several advantages, including high sensitivity and specificity, easy modulation and synthesis, low cost, and high stability, making them valuable tools for studying the complex interactions between the microbiota and the host and help to develop personalized microbiota-based therapies [1][2].
One application of aptamers in microbiota research is the identification and quantification of specific microbial species or strains [101]. Aptamers can be designed to specifically bind to the surface molecules or antigens of certain bacteria or other microorganisms, facilitating the identification and quantification of those organisms [101]. This method can be particularly useful in identifying and monitoring specific pathogenic or beneficial microorganisms in complex microbial communities, with studies by Hu et al. and Song et al. describing this novel approach in strains like Bifidobacterium bifidum (CCFM641-5) and Lactobacillus casei, at the same time, demonstrating the potential of aptamer-based strategies for the rapid and accurate detection and quantification of bacteria in food products [102][103].
Another application of aptamers in microbiome research is the study of microbial metabolites and signaling molecules, where aptamers can be designed to specifically recognize and bind to small molecules, such as short-chain fatty acids, neurotransmitters, and other signaling molecules produced by the microbiota, where aptamers can profile and characterize at the species, strain, or function level. They can be used in combination with high-throughput sequencing technologies, such as 16S rRNA sequencing to identify and quantify specific microbial populations in the gut microbiota and to identify and quantify specific microbial metabolites, which are important for gut health [104]. This approach allows researchers to better understand the interactions between the microbiome and the host and how they influence overall health and disease, providing a more accurate diagnosis and better understanding of the disease [105][106][107][108][109].
The use of aptamers in gut microbiota research is still in its early stages and there is significant potential for future applications. Aptamers have the potential to be used as a diagnostic tool for gut microbiota-related disease, such as inflammatory bowel disease and colorectal cancer, but also for gut microbiota-related diseases, such as antibiotic-resistant infections [109]. Moreover, aptamers can be integrated with other technologies, such as high-throughput sequencing and mass spectrometry, to provide a more comprehensive analysis of the gut microbiota [110]. This approach has the potential to provide a more detailed understanding of the gut microbiota and its role in human health and disease.
References
- Kumar Kulabhusan, P.A.-O.; Hussain, B.; Yuce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646.
- Byun, J. Recent Progress and Opportunities for Nucleic Acid Aptamers. Life 2021, 11, 193.
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510.
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822.
- Sousa, D.A.; Carneiro, M.; Ferreira, D.; Moreira, F.T.C.; Sales, M.G.F.; Rodrigues, L.R. Recent Advances in the Selection of Cancer-Specific Aptamers for the Development of Biosensors. Curr. Med. Chem. 2022, 29, 5850–5880.
- Agnello, L.; Camorani, S.; Fedele, M.; Cerchia, L. Aptamers and antibodies: Rivals or allies in cancer targeted therapy? Explor. Target. Antitumor Ther. 2021, 2, 107–121.
- Thevendran, R.; Citartan, M. Assays to Estimate the Binding Affinity of Aptamers. Talanta 2022, 238, 122971.
- Ozer, A.; Pagano, J.M.; Lis, J.T. New Technologies Provide Quantum Changes in the Scale, Speed, and Success of SELEX Methods and Aptamer Characterization. Mol. Ther. Nucleic Acids 2014, 3, e183.
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142.
- Radom, F.; Jurek, P.M.; Mazurek, M.P.; Otlewski, J.; Jelen, F. Aptamers: Molecules of great potential. Biotechnol. Adv. 2013, 31, 1260–1274.
- Santosh, B.; Yadava, P.K. Nucleic acid aptamers: Research tools in disease diagnostics and therapeutics. Biomed. Res. Int. 2014, 2014, 540451.
- Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20, 11959–11980.
- Wu, Y.X.; Kwon, Y.J. Aptamers: The “evolution” of SELEX. Methods 2016, 106, 21–28.
- Gijs, M.; Penner, G.; Blackler, G.B.; Impens, N.R.; Baatout, S.; Luxen, A.; Aerts, A.M. Improved Aptamers for the Diagnosis and Potential Treatment of HER2-Positive Cancer. Pharmaceuticals 2016, 9, 29.
- Lee, Y.J.; Kim, I.S.; Park, S.A.; Kim, Y.; Lee, J.E.; Noh, D.Y.; Kim, K.T.; Ryu, S.H.; Suh, P.G. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol. Ther. 2013, 21, 1004–1013.
- Pinto-Diez, C.; Ferreras-Martin, R.; Carrion-Marchante, R.; Klett-Mingo, J.I.; Garcia-Hernandez, M.; Perez-Morgado, M.I.; Sacristan, S.; Barragan, M.; Seijo-Vila, M.; Tundidor, I.; et al. An optimized MNK1b aptamer, apMNKQ2, and its potential use as a therapeutic agent in breast cancer. Mol. Ther. Nucleic Acids 2022, 30, 553–568.
- Li, Z.; Fu, X.; Huang, J.; Zeng, P.; Huang, Y.; Chen, X.; Liang, C. Advances in Screening and Development of Therapeutic Aptamers Against Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 662791.
- Esposito, C.L.; Quintavalle, C.; Ingenito, F.; Rotoli, D.; Roscigno, G.; Nuzzo, S.; Thomas, R.; Catuogno, S.; de Franciscis, V.; Condorelli, G. Identification of a novel RNA aptamer that selectively targets breast cancer exosomes. Mol. Ther. Nucleic Acids 2021, 23, 982–994.
- Aldag, J.; Persson, T.; Hartmann, R.K. 2’-Fluoro-Pyrimidine-Modified RNA Aptamers Specific for Lipopolysaccharide Binding Protein (LBP). Int. J. Mol. Sci. 2018, 19, 3883.
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365.
- Viraka Nellore, B.P.; Kanchanapally, R.; Pramanik, A.; Sinha, S.S.; Chavva, S.R.; Hamme, A., 2nd; Ray, P.C. Aptamer-conjugated graphene oxide membranes for highly efficient capture and accurate identification of multiple types of circulating tumor cells. Bioconjug Chem. 2015, 26, 235–242.
- Sae-Lim, S.; Soontornworajit, B.; Rotkrua, P. Inhibition of Colorectal Cancer Cell Proliferation by Regulating Platelet-Derived Growth Factor B Signaling with a DNA Aptamer. Asian Pac. J. Cancer Prev. 2019, 20, 487–494.
- Ahmadyousefi, Y.; Malih, S.; Mirzaee, Y.; Saidijam, M. Nucleic acid aptamers in diagnosis of colorectal cancer. Biochimie 2019, 156, 1–11.
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2018, 8, 4016–4032.
- Lai, W.Y.; Huang, B.T.; Wang, J.W.; Lin, P.Y.; Yang, P.C. A Novel PD-L1-targeting Antagonistic DNA Aptamer With Antitumor Effects. Mol. Ther. Nucleic Acids 2016, 5, e397.
- Tsai, Y.T.; Liang, C.H.; Yu, J.H.; Huang, K.C.; Tung, C.H.; Wu, J.E.; Wu, Y.Y.; Chang, C.H.; Hong, T.M.; Chen, Y.L. A DNA Aptamer Targeting Galectin-1 as a Novel Immunotherapeutic Strategy for Lung Cancer. Mol. Ther. Nucleic Acids 2019, 18, 991–998.
- Esposito, V.; Russo, A.; Vellecco, V.; Bucci, M.; Russo, G.; Mayol, L.; Virgilio, A.; Galeone, A. Thrombin binding aptamer analogues containing inversion of polarity sites endowed with antiproliferative and anti-motility properties against Calu-6 cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2645–2650.
- Wang, H.; Qin, M.; Liu, R.; Ding, X.; Chen, I.S.Y.; Jiang, Y. Characterization of A Bifunctional Synthetic RNA Aptamer and A Truncated Form for Ability to Inhibit Growth of Non-Small Cell Lung Cancer. Sci. Rep. 2019, 9, 18836.
- Trinh, T.L.; Zhu, G.; Xiao, X.; Puszyk, W.; Sefah, K.; Wu, Q.; Tan, W.; Liu, C. A Synthetic Aptamer-Drug Adduct for Targeted Liver Cancer Therapy. PLoS ONE 2015, 10, e0136673.
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431.
- Wang, T.; Philippovich, S.; Mao, J.; Veedu, R.N. Efficient Epidermal Growth Factor Receptor Targeting Oligonucleotide as a Potential Molecule for Targeted Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4700.
- Zhang, H.; Wang, Z.; Xie, L.; Zhang, Y.; Deng, T.; Li, J.; Liu, J.; Xiong, W.; Zhang, L.; Zhang, L.; et al. Molecular Recognition and In-Vitro-Targeted Inhibition of Renal Cell Carcinoma Using a DNA Aptamer. Mol. Ther. Nucleic Acids 2018, 12, 758–768.
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N., Jr.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187.
- Speransky, S.; Serafini, P.; Caroli, J.; Bicciato, S.; Lippman, M.E.; Bishopric, N.H. A novel RNA aptamer identifies plasma membrane ATP synthase beta subunit as an early marker and therapeutic target in aggressive cancer. Breast Cancer Res. Treat. 2019, 176, 271–289.
- Dassie, J.P.; Hernandez, L.I.; Thomas, G.S.; Long, M.E.; Rockey, W.M.; Howell, C.A.; Chen, Y.; Hernandez, F.J.; Liu, X.Y.; Wilson, M.E.; et al. Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen. Mol. Ther. 2014, 22, 1910–1922.
- Jiao, Y.; Xu, P.; Luan, S.; Wang, X.; Gao, Y.; Zhao, C.; Fu, P. Molecular imaging and treatment of PSMA-positive prostate cancer with (99m)Tc radiolabeled aptamer-siRNA chimeras. Nucl. Med. Biol. 2022, 104–105, 28–37.
- Singh, S.S. Preclinical pharmacokinetics: An approach towards safer and efficacious drugs. Curr. Drug Metab. 2006, 7, 165–182.
- Marangoni, K.; Neves, A.F.; Rocha, R.M.; Faria, P.R.; Alves, P.T.; Souza, A.G.; Fujimura, P.T.; Santos, F.A.; Araujo, T.G.; Ward, L.S.; et al. Prostate-specific RNA aptamer: Promising nucleic acid antibody-like cancer detection. Sci. Rep. 2015, 5, 12090.
- Reyes-Reyes, E.M.; Salipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 2015, 9, 1392–1405.
- Kim, E.; Jung, Y.; Choi, H.; Yang, J.; Suh, J.S.; Huh, Y.M.; Kim, K.; Haam, S. Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polyplex. Biomaterials 2010, 31, 4592–4599.
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Vilardo, C.; Nuzzo, S.; Ricci-Vitiani, L.; De Luca, G.; Pallini, R.; Kichkailo, A.S.; et al. The Discovery of RNA Aptamers that Selectively Bind Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2019, 18, 99–109.
- Vayrynen, O.; Piippo, M.; Jamsa, H.; Vaisanen, T.; de Almeida, C.E.B.; Salo, T.; Missailidis, S.; Risteli, M. Effects of ionizing radiation and HPSE1 inhibition on the invasion of oral tongue carcinoma cells on human extracellular matrices in vitro. Exp. Cell Res. 2018, 371, 151–161.
- Cesur, O.; Nicol, C.; Groves, H.; Mankouri, J.; Blair, G.E.; Stonehouse, N.J. The Subcellular Localisation of the Human Papillomavirus (HPV) 16 E7 Protein in Cervical Cancer Cells and Its Perturbation by RNA Aptamers. Viruses 2015, 7, 3443–3461.
- Wang, Y.; Zhang, Y.; Li, P.C.; Guo, J.; Huo, F.; Yang, J.; Jia, R.; Wang, J.; Huang, Q.; Theodorescu, D.; et al. Development of Novel Aptamer-Based Targeted Chemotherapy for Bladder Cancer. Cancer Res. 2022, 82, 1128–1139.
- Mahlknecht, G.; Maron, R.; Mancini, M.; Schechter, B.; Sela, M.; Yarden, Y. Aptamer to ErbB-2/HER2 enhances degradation of the target and inhibits tumorigenic growth. Proc. Natl. Acad. Sci. USA 2013, 110, 8170–8175.
- Soundararajan, S.; Wang, L.; Sridharan, V.; Chen, W.; Courtenay-Luck, N.; Jones, D.; Spicer, E.K.; Fernandes, D.J. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol. 2009, 76, 984–991.
- Kotula, J.W.; Sun, J.; Li, M.; Pratico, E.D.; Fereshteh, M.P.; Ahrens, D.P.; Sullenger, B.A.; Kovacs, J.J. Targeted disruption of beta-arrestin 2-mediated signaling pathways by aptamer chimeras leads to inhibition of leukemic cell growth. PLoS ONE 2014, 9, e93441.
- Ul-Haq, A.; Jin, M.L.; Jeong, K.W.; Kim, H.M.; Chun, K.H. Isolation of MLL1 Inhibitory RNA Aptamers. Biomol. Ther. 2019, 27, 201–209.
- Nozari, A.; Berezovski, M.V. Aptamers for CD Antigens: From Cell Profiling to Activity Modulation. Mol. Ther. Nucleic Acids 2017, 6, 29–44.
- Kryza, D.; Debordeaux, F.; Azema, L.; Hassan, A.; Paurelle, O.; Schulz, J.; Savona-Baron, C.; Charignon, E.; Bonazza, P.; Taleb, J.; et al. Ex Vivo and In Vivo Imaging and Biodistribution of Aptamers Targeting the Human Matrix MetalloProtease-9 in Melanomas. PLoS ONE 2016, 11, e0149387.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.E.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80.
- Ray, P.; Cheek, M.A.; Sharaf, M.L.; Li, N.; Ellington, A.D.; Sullenger, B.A.; Shaw, B.R.; White, R.R. Aptamer-mediated delivery of chemotherapy to pancreatic cancer cells. Nucleic Acid. Ther. 2012, 22, 295–305.
- Yoon, S.; Huang, K.W.; Reebye, V.; Mintz, P.; Tien, Y.W.; Lai, H.S.; Saetrom, P.; Reccia, I.; Swiderski, P.; Armstrong, B.; et al. Targeted Delivery of C/EBPalpha -saRNA by Pancreatic Ductal Adenocarcinoma-specific RNA Aptamers Inhibits Tumor Growth In Vivo. Mol. Ther. 2016, 24, 1106–1116.
- Yoon, S.; Huang, K.W.; Andrikakou, P.; Vasconcelos, D.; Swiderski, P.; Reebye, V.; Sodergren, M.; Habib, N.; Rossi, J.J. Targeted Delivery of C/EBPalpha-saRNA by RNA Aptamers Shows Anti-tumor Effects in a Mouse Model of Advanced PDAC. Mol. Ther. Nucleic Acids 2019, 18, 142–154.
- Liu, A.D.; Zhou, J.; Bi, X.Y.; Hou, G.Q.; Li, S.S.; Chen, Q.; Xu, H.; Cao, X. Aptamer-SH2 superbinder-based targeted therapy for pancreatic ductal adenocarcinoma. Clin. Transl. Med. 2021, 11, e337.
- Dua, P.; Kang, H.S.; Hong, S.M.; Tsao, M.S.; Kim, S.; Lee, D.K. Alkaline phosphatase ALPPL-2 is a novel pancreatic carcinoma-associated protein. Cancer Res. 2013, 73, 1934–1945.
- Dua, P.; Sajeesh, S.; Kim, S.; Lee, D.K. ALPPL2 Aptamer-Mediated Targeted Delivery of 5-Fluoro-2’-Deoxyuridine to Pancreatic Cancer. Nucleic Acid. Ther. 2015, 25, 180–187.
- Kim, Y.J.; Lee, H.S.; Jung, D.E.; Kim, J.M.; Song, S.Y. The DNA aptamer binds stemness-enriched cancer cells in pancreatic cancer. J. Mol. Recognit. 2017, 30, e2591.
- Wang, H.; Li, X.; Lai, L.A.; Brentnall, T.A.; Dawson, D.W.; Kelly, K.A.; Chen, R.; Pan, S. X-aptamers targeting Thy-1 membrane glycoprotein in pancreatic ductal adenocarcinoma. Biochimie 2021, 181, 25–33.
- Porciani, D.; Tedeschi, L.; Marchetti, L.; Citti, L.; Piazza, V.; Beltram, F.; Signore, G. Aptamer-Mediated Codelivery of Doxorubicin and NF-kappaB Decoy Enhances Chemosensitivity of Pancreatic Tumor Cells. Mol. Ther. Nucleic Acids 2015, 4, e235.
- Citro, A.; Pellegrini, S.; Dugnani, E.; Eulberg, D.; Klussmann, S.; Piemonti, L. CCL2/MCP-1 and CXCL12/SDF-1 blockade by L-aptamers improve pancreatic islet engraftment and survival in mouse. Am. J. Transpl. 2019, 19, 3131–3138.
- Ray, P.; Sullenger, B.A.; White, R.R. Further characterization of the target of a potential aptamer biomarker for pancreatic cancer: Cyclophilin B and its posttranslational modifications. Nucleic Acid. Ther. 2013, 23, 435–442.
- Kim, Y.H.; Sung, H.J.; Kim, S.; Kim, E.O.; Lee, J.W.; Moon, J.Y.; Choi, K.; Jung, J.E.; Lee, Y.; Koh, S.S.; et al. An RNA aptamer that specifically binds pancreatic adenocarcinoma up-regulated factor inhibits migration and growth of pancreatic cancer cells. Cancer Lett. 2011, 313, 76–83.
- Compte, M.; Harwood, S.L.; Munoz, I.G.; Navarro, R.; Zonca, M.; Perez-Chacon, G.; Erce-Llamazares, A.; Merino, N.; Tapia-Galisteo, A.; Cuesta, A.M.; et al. A tumor-targeted trimeric 4-1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat. Commun. 2018, 9, 4809.
- Dollins, C.M.; Nair, S.; Boczkowski, D.; Lee, J.; Layzer, J.M.; Gilboa, E.; Sullenger, B.A. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 2008, 15, 675–682.
- Cerchia, L.; Esposito, C.L.; Camorani, S.; Rienzo, A.; Stasio, L.; Insabato, L.; Affuso, A.; de Franciscis, V. Targeting Axl with an high-affinity inhibitory aptamer. Mol. Ther. 2012, 20, 2291–2303.
- Dinis Ano Bom, A.P.; da Costa Neves, P.C.; Bonacossa de Almeida, C.E.; Silva, D.; Missailidis, S. Aptamers as Delivery Agents of siRNA and Chimeric Formulations for the Treatment of Cancer. Pharmaceutics 2019, 11, 684.
- Bai, C.; Gao, S.; Hu, S.; Liu, X.; Li, H.; Dong, J.; Huang, A.; Zhu, L.; Zhou, P.; Li, S.; et al. Self-Assembled Multivalent Aptamer Nanoparticles with Potential CAR-like Characteristics Could Activate T Cells and Inhibit Melanoma Growth. Mol. Ther. Oncolytics 2020, 17, 9–20.
- Macdonald, J.; Henri, J.; Goodman, L.; Xiang, D.; Duan, W.; Shigdar, S. Development of a Bifunctional Aptamer Targeting the Transferrin Receptor and Epithelial Cell Adhesion Molecule (EpCAM) for the Treatment of Brain Cancer Metastases. ACS Chem. Neurosci. 2017, 8, 777–784.
- Ma, Y.; Ai, G.; Zhang, C.; Zhao, M.; Dong, X.; Han, Z.; Wang, Z.; Zhang, M.; Liu, Y.; Gao, W.; et al. Novel Linear Peptides with High Affinity to alphavbeta3 Integrin for Precise Tumor Identification. Theranostics 2017, 7, 1511–1523.
- Fechter, P.; Cruz Da Silva, E.; Mercier, M.C.; Noulet, F.; Etienne-Seloum, N.; Guenot, D.; Lehmann, M.; Vauchelles, R.; Martin, S.; Lelong-Rebel, I.; et al. RNA Aptamers Targeting Integrin alpha5beta1 as Probes for Cyto- and Histofluorescence in Glioblastoma. Mol. Ther. Nucleic Acids 2019, 17, 63–77.
- Jeong, H.; Lee, S.H.; Hwang, Y.; Yoo, H.; Jung, H.; Kim, S.H.; Mok, H. Multivalent Aptamer-RNA Conjugates for Simple and Efficient Delivery of Doxorubicin/siRNA into Multidrug-Resistant Cells. Macromol. Biosci. 2017, 17, 1600343.
- Wang, C.Y.; Lin, B.L.; Chen, C.H. An aptamer targeting shared tumor-specific peptide antigen of MAGE-A3 in multiple cancers. Int. J. Cancer 2016, 138, 918–926.
- Wang, C.Y.; Lin, B.L.; Chen, C.H. Targeted drug delivery using an aptamer against shared tumor-specific peptide antigen of MAGE-A3. Cancer Biol. Ther. 2021, 22, 12–18.
- Liu, Z.; Parveen, N.; Rehman, U.; Aziz, A.; Sheikh, A.; Abourehab, M.A.S.; Guo, W.; Huang, J.; Wang, Z.; Kesharwani, P. Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer. Mol. Cancer 2023, 22, 8.
- Zhang, X.H.; Wang, W.; Chen, X. Selection and identification of an ssDNA aptamer to NB4 cell. J. Clin. Lab. Anal. 2021, 35, e23718.
- Gao, X.; Teng, X.; Dai, Y.; Li, J. Rolling Circle Amplification-Assisted Flow Cytometry Approach for Simultaneous Profiling of Exosomal Surface Proteins. ACS Sens. 2021, 6, 3611–3620.
- Zhou, Q.; Liu, Y.; Shin, D.S.; Silangcruz, J.; Tuleuova, N.; Revzin, A. Aptamer-containing surfaces for selective capture of CD4 expressing cells. Langmuir 2012, 28, 12544–12549.
- Cheng, E.L.; Kacherovsky, N.; Pun, S.H. Aptamer-Based Traceless Multiplexed Cell Isolation Systems. ACS Appl. Mater. Interfaces 2022, 14, 44136–44146.
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585.
- Zhou, J.; Satheesan, S.; Li, H.; Weinberg, M.S.; Morris, K.V.; Burnett, J.C.; Rossi, J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015, 22, 379–390.
- Lopes-Nunes, J.; Lifante, J.; Shen, Y.; Ximendes, E.C.; Jaque, D.; Iglesias-de la Cruz, M.C.; Cruz, C. Biological studies of an ICG-tagged aptamer as drug delivery system for malignant melanoma. Eur. J. Pharm. Biopharm. 2020, 154, 228–235.
- Zhong, Y.; Zhao, J.; Li, J.; Liao, X.; Chen, F. Advances of aptamers screened by Cell-SELEX in selection procedure, cancer diagnostics and therapeutics. Anal. Biochem. 2020, 598, 113620.
- Wang, C.W.; Chung, W.H.; Cheng, Y.F.; Ying, N.W.; Peck, K.; Chen, Y.T.; Hung, S.I. A new nucleic acid-based agent inhibits cytotoxic T lymphocyte-mediated immune disorders. J. Allergy Clin. Immunol. 2013, 132, 713–722.
- Ott, L.E.; Carson, S. Immunological tools: Engaging students in the use and analysis of flow cytometry and enzyme-linked immunosorbent assay (ELISA). Biochem. Mol. Biol. Educ. 2014, 42, 382–397.
- Bognár, Z.; Gyurcsányi, R.E. Aptamers against Immunoglobulins: Design, Selection and Bioanalytical Applications. Int. J. Mol. Sci. 2020, 21, 5748.
- Camorani, S.; Passariello, M.; Agnello, L.; Esposito, S.; Collina, F.; Cantile, M.; Di Bonito, M.; Ulasov, I.V.; Fedele, M.; Zannetti, A.; et al. Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2020, 39, 180.
- Pastor, F.; Berraondo, P.; Etxeberria, I.; Frederick, J.; Sahin, U.; Gilboa, E.; Melero, I. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 2018, 17, 751–767.
- Liu, C.G.; Wang, Y.; Liu, P.; Yao, Q.L.; Zhou, Y.Y.; Li, C.F.; Zhao, Q.; Liu, G.H.; Zhang, X.L. Aptamer-T Cell Targeted Therapy for Tumor Treatment Using Sugar Metabolism and Click Chemistry. ACS Chem. Biol. 2020, 15, 1554–1565.
- Lai, X.; Yao, F.; An, Y.; Li, X.; Yang, X. Novel Nanotherapeutics for Cancer Immunotherapy by PD-L1-Aptamer-Functionalized and Fexofenadine-Loaded Albumin Nanoparticles. Molecules 2023, 11, 2556.
- Shigdar, S.; Schrand, B.; Giangrande, P.H.; de Franciscis, V. Aptamers: Cutting edge of cancer therapies. Mol. Ther. 2021, 29, 2396–2411.
- Camorani, S.; Granata, I.; Collina, F.; Leonetti, F.; Cantile, M.; Botti, G.; Fedele, M.; Guarracino, M.R.; Cerchia, L. Novel Aptamers Selected on Living Cells for Specific Recognition of Triple-Negative Breast Cancer. iScience 2020, 23, 100979.
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.Y.; Ling, X.; Caruso, H.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2019, 129, 137–149.
- Tan, X.; Jia, F.; Wang, P.; Zhang, K. Nucleic acid-based drug delivery strategies. J. Control Release 2020, 323, 240–252.
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89.
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell Infect. Microbiol. 2021, 11, 716299.
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39.
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221.
- Chen, J.; Zhou, J.; Peng, Y.; Xie, Y.; Xiao, Y. Aptamers: A prospective tool for infectious diseases diagnosis. J. Clin. Lab. Anal. 2022, 36, e24725.
- Hu, L.; Wang, L.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Selection, Characterization and Interaction Studies of a DNA Aptamer for the Detection of Bifidobacterium bifidum. Int. J. Mol. Sci. 2017, 18, 883.
- Song, S.; Wang, X.; Xu, K.; Ning, L.; Yang, X. Rapid identification and quantitation of the viable cells of Lactobacillus casei in fermented dairy products using an aptamer-based strategy powered by a novel cell-SELEX protocol. J. Dairy. Sci. 2019, 102, 10814–10824.
- Xing, H.; Zhang, Y.; Krämer, M.; Kissmann, A.K.; Amann, V.; Raber, H.F.; Weil, T.; Stieger, K.R.; Knippschild, U.; Henkel, M.; et al. A Polyclonal Aptamer Library for the Specific Binding of the Gut Bacterium Roseburia intestinalis in Mixtures with Other Gut Microbiome Bacteria and Human Stool Samples. Int. J. Mol. Sci. 2022, 23, 7744.
- Bhardwaj, J.; Chaudhary, N.; Kim, H.; Jang, J. Subtyping of influenza A H1N1 virus using a label-free electrochemical biosensor based on the DNA aptamer targeting the stem region of HA protein. Anal. Chim. Acta 2019, 1064, 94–103.
- Bai, C.; Lu, Z.; Jiang, H.; Yang, Z.; Liu, X.; Ding, H.; Li, H.; Dong, J.; Huang, A.; Fang, T.; et al. Aptamer selection and application in multivalent binding-based electrical impedance detection of inactivated H1N1 virus. Biosens. Bioelectron. 2018, 110, 162–167.
- Chen, H.L.; Hsiao, W.H.; Lee, H.C.; Wu, S.C.; Cheng, J.W. Selection and Characterization of DNA Aptamers Targeting All Four Serotypes of Dengue Viruses. PLoS ONE 2015, 10, e0131240.
- Morais, L.M.; Chaves, T.S.; Medeiros, M.A.; Pereira, K.A.B.; Jurgilas, P.B.; Barbosa de Lima, S.M.; Missailidis, S.; Bispo de Filippis, A.M. Selection and Characterization of Single-Stranded DNA Aptamers of Diagnostic Potential against the Whole Zika Virus. Viruses 2022, 14, 1867.
- Afrasiabi, S.; Pourhajibagher, M.; Raoofian, R.; Tabarzad, M.; Bahador, A. Therapeutic applications of nucleic acid aptamers in microbial infections. J. Biomed. Sci. 2020, 27, 6.
- Wang, K.; Wang, M.; Ma, T.; Li, W.; Zhang, H. Review on the Selection of Aptamers and Application in Paper-Based Sensors. Biosensors 2022, 13, 39.




