Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Imen Zalila-Kolsi | -- | 2525 | 2023-11-16 02:58:09 | | | |
| 2 | Rita Xu | Meta information modification | 2525 | 2023-11-16 03:02:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens. Encyclopedia. Available online: https://encyclopedia.pub/entry/51639 (accessed on 07 February 2026).
Zalila-Kolsi I, Ben-Mahmoud A, Al-Barazie R. Bacillus amyloliquefaciens. Encyclopedia. Available at: https://encyclopedia.pub/entry/51639. Accessed February 07, 2026.
Zalila-Kolsi, Imen, Afif Ben-Mahmoud, Ray Al-Barazie. "Bacillus amyloliquefaciens" Encyclopedia, https://encyclopedia.pub/entry/51639 (accessed February 07, 2026).
Zalila-Kolsi, I., Ben-Mahmoud, A., & Al-Barazie, R. (2023, November 16). Bacillus amyloliquefaciens. In Encyclopedia. https://encyclopedia.pub/entry/51639
Zalila-Kolsi, Imen, et al. "Bacillus amyloliquefaciens." Encyclopedia. Web. 16 November, 2023.
Copy Citation
Bacillus amyloliquefaciens, a Gram-positive bacterium, has emerged as a versatile microorganism with significant applications in various fields, including industry, medicine, and agriculture.
Bacillus amyloliquefaciens
industrial applications
medical applications
1. Introduction
Bacillus amyloliquefaciens, a ubiquitous Gram-positive, aerobic bacterium, is commonly found in soil environments. This versatile organism has been utilized to produce a diverse array of heterologous proteins, including β-glucanases, acid-stable alpha-amylase, mesophilic alpha-amylase, cellulase, acid-soluble proteins, keratinase, and alkaline protease [1][2][3]. These properties make B. amyloliquefaciens a valuable host for synthesising therapeutic proteins and industrially relevant enzymes. Furthermore, B. amyloliquefaciens offers several advantages for applications in agricultural biotechnology. It produces secondary metabolites that exhibit antimicrobial activities against many phytopathogenic microorganisms, promoting plant growth and enhancing overall plant health [4]. These attributes position B. amyloliquefaciens as a promising candidate for developing sustainable and eco-friendly agricultural practices.
Bacillus amyloliquefaciens possesses remarkable physiological characteristics and a highly adaptable metabolism, enabling its cultivation of cost-effective media [5]. This bacterium exhibits rapid growth, with a fermentation cycle of approximately 72 h, compared to the 180-h cycle of Saccharomyces cerevisiae [6]. B. amyloliquefaciens also benefits from robust expression systems with excellent genetic stability and lacks strong codon preferences [7].
B. amyloliquefaciens has been established as an industrially significant bacterium in the production of biological indicators for sterilization and in biodefense research [8]. The unique properties and capabilities of this microorganism make it an attractive candidate for various biotechnological applications.
Over the years, extensive research has led to the development of various genetic modification tools for Bacillus amyloliquefaciens. These tools range from classical counter-selection marker strategies to the recently developed clustered regularly interspaced short palindromic repeats (CRISPR)—based genetic toolkits. The diverse protein secretion systems, along with the novel artificial promoter and ribosome binding site (RBS) libraries, further facilitate the production of extracellular enzymes [9][10][11][12][13].
B. amyloliquefaciens is an ideal multifunctional probiotic, exhibiting significant potential in inhibiting pathogenic bacterial growth and enhancing nutrient assimilation. This microorganism is known to produce a wide array of enzymes, including α-amylase, protease, lipase, cellulase, xylanase, pectinase, aminotransferase, barnase, peroxidase, glucanase, and chitinase [12][14]. BamHI is a type II restriction endonuclease, capable of recognizing short sequences (6 bp) of DNA and specifically cleaving them at a target site [15]. The β-1,3-1,4-glucanase derived from B. amyloliquefaciens showed an important role in protecting against phytopathogenic fungi [16].
The inherent genetic background of B. amyloliquefaciens, combined with well-developed gene manipulation tools, enables the reconstruction of its cellular metabolism. The availability of public knockout collections further enhances its attractiveness as a host for metabolic engineering applications.
In the agricultural sector, research has demonstrated that adding an appropriate quantity of Bacillus amyloliquefaciens can significantly enhance the carbon content in compost, thereby improving soil quality and promoting crop growth [17]. Moreover, B. amyloliquefaciens is among the most prevalent bacteria known to colonize plants endophytically, playing a crucial role in the biocontrol of vascular plant pathogens [18].
B. amyloliquefaciens is also capable of forming complex biofilms, which can serve as living biological materials to produce various functional biomaterials. These include surface growth factors, antibiotics, lysozyme, and antimicrobial peptides for medical applications. The development of biofilms by Bacillus amyloliquefaciens enhances barley’s resistance to salt stress [19]. However, certain bottlenecks currently limit the yield of target heterologous proteins in B. amyloliquefaciens, such as the absence of efficient genetic editing systems, unclear transcriptional regulation of heterologous proteins, and restricted secretion of heterologous proteins [20].
Most research has focused on optimizing factors such as signal peptides, transport channel levels, chaperone protein levels, and promoters in the expression and transport systems to enhance heterologous protein production in B. amyloliquefaciens [20]. Nevertheless, there is a pressing need to direct research efforts toward designing and developing host chassis to construct sustainable, robust, and efficient microbial cell factories for heterologous protein production.
2. Genetic Manipulation of Bacillus amyloliquefaciens
Bacillus amyloliquefaciens is a bacterium of considerable industrial importance, and various genetic manipulation techniques have been employed to enhance its strains for diverse applications [1][8][10][21]. A critical target for genetic modification in B. amyloliquefaciens is the alpha-amylase gene (amyE), which has been manipulated using innovative plasmid designs for extrachromosomal and intrachromosomal purposes [22].
The amyE gene is important because it includes homologous sequences necessary for integration into the B. amyloliquefaciens chromosome. This integration can serve as a model for evaluating the success of different genetic engineering strategies: the amyE gene’s ability to integrate into the B. amyloliquefaciens chromosome can be a practical tool for checking and measuring the performance of various engineering strategies. For instance, if a modification to the amyE gene successfully integrates and improves the bacterium’s performance, the applied strategy was effective [22]. Previous research has confirmed the successful application of genetic engineering techniques in B. amyloliquefaciens and shown the potential for further optimization and industrial utilization [9][10][13][22][23]. For instance, studies [13][20][23][24][25][26][27] have exhibited enhancements in the production of enzymes, such as protease and lipase, due to genetic engineering. Other research [28][29] has focused on the bacterium’s potential in plant disease control and its role in promoting plant growth. The wide range of applications and the potential for further improvements underline the importance of ongoing research and development.
2.1. Development and Application of CRISPR-Based Genetic Toolboxes in Bacillus amyloliquefaciens Strains
Xin et al. (2022) recently reported the development of a CRISPR-based genetic toolkit designed for efficient gene editing, knockout, and integration in Bacillus amyloliquefaciens LB1ba02. By employing a single-plasmid CRISPR/Cas9n system, the researchers achieved an impressive 93% knockout efficiency for a single gene and simultaneous editing of three loci with 53.3% efficiency using a base editing CRISPR/Cas9n-AID system [9]. This innovative toolkit was successfully applied to four genes (aprE, nprE, wprA, and bamHIR), showcasing its potential as a rapid gene knockout and integration tool for B. amyloliquefaciens LB1ba02.
In a unique study, Zhao et al. (2020) developed a novel genetic toolbox to augment the endogenous expression of the mesophilic α-amylase gene in Bacillus amyloliquefaciens 205 [13]. A key component of this toolbox was the implementation of an efficient interspecific transformation method, which is a technique that allows genetic material to be transferred across different species of Bacillus [30]. This method enhanced the ability to introduce foreign DNA into B. amyloliquefaciens 205, thereby facilitating the genetic manipulation of this bacterium. The toolbox also incorporated functional CRISPR systems, which provided precise editing capabilities to further manipulate the genetic material. This innovative strategy, particularly the implementation of interspecific transformation, holds potential applications across a broad range of Bacillus species. It allows for the transfer of beneficial traits from one species to another, broadening the possibilities for genetic enhancement in this bacterial genus [30].
The researchers applied several genetic engineering techniques to systematically increase gene expression levels. This included sporulation suppression, a technique that limits the bacteria’s natural process of forming spores, which allows more resources to be dedicated to the expression of the target gene. Additionally, transcript activation techniques were used to increase the production of the specific protein from its corresponding gene, and plasmid-based gene overexpression was utilized to boost the quantity of the target protein being produced. These strategic modifications can be applied across many Bacillus species [13].
Furthermore, Chen et al. (2016) successfully promoted spontaneous genetic competence in isolated B. amyloliquefaciens strains by overexpressing the master regulator ComK from Bacillus subtilis (ComKBsu). Utilizing direct transformation of PCR-generated deletion cassettes, they executed tasks such as replicative plasmid distribution and gene knockout. Artificial induction of genetic competence in B. amyloliquefaciens strains carrying the plasmid pUBXC can be achieved through the overexpression of ComKBsu [23].
2.2. Enhancing Alkaline Protease Production and Antifungal Properties of Bacillus amyloliquefaciens through Genetic Engineering
In a study focused on enhancing the alkaline protease production capacity of Bacillus amyloliquefaciens, researchers employed genetic engineering techniques to improve the production of the alkaline protease BSP-1. They cloned the bsp-1 gene from a Bacillus subtilis strain and introduced it into B. amyloliquefaciens. The results demonstrated that the recombinant strain produced a higher quantity of alkaline protease than the wild-type strain, highlighting a potential approach for genetically engineering B. amyloliquefaciens to increase the production of novel alkaline proteases [10].
More recent research has directed efforts toward genetically modifying B. amyloliquefaciens to enhance its antifungal properties and boost the production of eco-friendly antifungal lipopeptides. The study identified several genetic modifications that improved the engineered strains’ antifungal activity and lipopeptide production. These modifications included the deletion of genes involved in branched-chain amino acid biosynthesis and the overexpression of genes associated with lipopeptide synthesis. These findings suggest that genetic engineering can progressively enhance B. amyloliquefaciens’ antifungal capabilities, thereby facilitating the development of sustainable and environmentally friendly antifungal agents [11].
3. Gene Expression Using Bacillus amyloliquefaciens
Owing to their remarkable capacity to express and secrete proteins, Bacillus spp. are frequently utilized in the production of commercial enzyme preparations. To accommodate the secretion requirements of diverse proteins, Bacillus spp. possess several promoters and plasmid expression systems. The three conventional protein secretion pathways in Bacillus spp. include the general protein secretion pathway (Sec), the twin-arginine translocation pathway (Tat), and the ATP-binding cassette (ABC) transporters [31][32][33] (Figure 1).
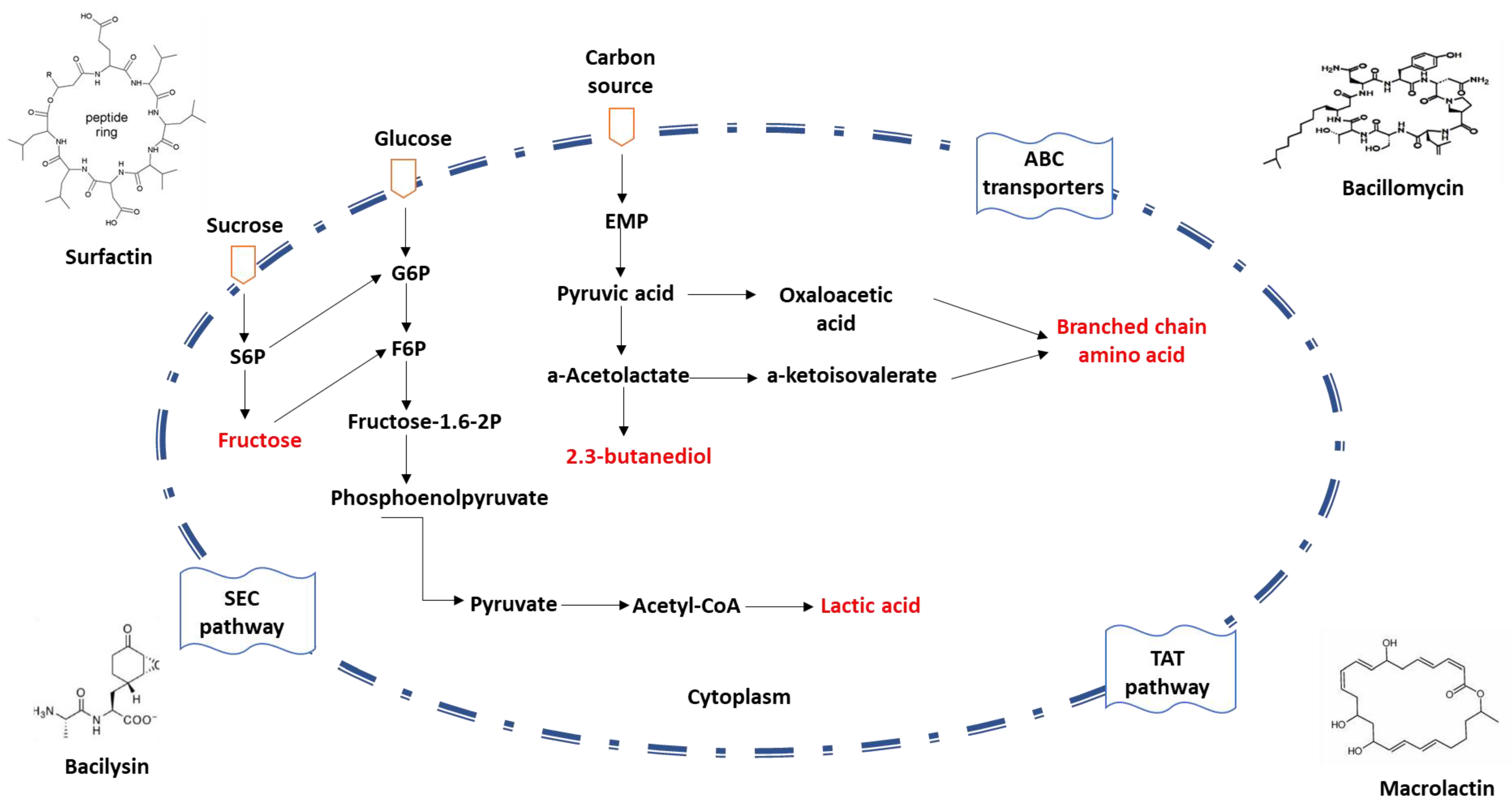
Figure 1. Schematic diagram of protein secretion pathways in Bacillus spp. The mechanism of the non-classical secretion pathway is not clear.
3.1. Investigating Plant-Bacteria Interaction: Bacillus amyloliquefaciens and Sclerotinia sclerotiorum in Soybean Plants
In a recent investigation, a technique known as digital gene expression profiling was employed to analyze the activity of genes in soybean plants interacting with Bacillus amyloliquefaciens and the plant pathogen Sclerotinia sclerotiorum [34]. Digital gene expression profiling is a next-generation sequencing method that provides an accurate snapshot of gene activity in a given cell or tissue at a particular time. It allows researchers to identify and quantify specific genes being activated or suppressed in response to various conditions or stimuli.
Using this technique, the study found distinct transcriptional responses in soybean plants interacting with these two organisms. B. amyloliquefaciens induced the expression of genes that promote growth and tolerance to stress, while S. sclerotiorum stimulated the expression of genes associated with defense and stress responses. This research provides valuable insights into the genetic dynamics of plant interactions with beneficial bacteria and pathogens. Moreover, the study identified several candidate genes differentially expressed in the soybean plants in response to both microorganisms. These ‘candidate genes’ showed significant changes in their activity levels and may play key roles in the plant’s response to B. amyloliquefaciens and S. sclerotiorum. The study also suggested potential regulatory mechanisms that control the activity of these genes during the plant-bacteria interaction [34].
These regulatory mechanisms can involve various factors and influence how the plant responds to the presence of these microorganisms. This research offers valuable insights into the intricate dynamics between beneficial bacteria, plant pathogens, and their host plants, highlighting how plants can genetically adjust their responses to different microorganisms. Such insights contribute to a deeper understanding of plant-microbe interactions and their implications for crop health and productivity, potentially informing strategies to enhance disease resistance and growth in crops [34].
3.2. Cloning and Expression of Bacillus amyloliquefaciens Transglutaminase Gene in E. coli for Food Industry Applications
Duarte et al. (2020) successfully cloned and expressed the transglutaminase (TGase) gene from Bacillus amyloliquefaciens in Escherichia coli using a bicistronic vector-mediated approach. Transglutaminase enzymes are highly sought after in the food industry due to their ability to enhance food products’ flavor and nutritional value. However, their production has been challenging due to high costs and low yields [25].
To overcome these challenges, the authors constructed a plasmid in which the B. amyloliquefaciens TGase gene was fused to the prodomain of the Streptomyces caniferus protease. This prodomain is crucial in protein folding, ensuring the TGase enzyme is formed correctly. It also prevents the premature activation of TGase within the bacterial cell, which could disrupt normal cellular processes. To activate the enzyme once it is correctly folded and exported from the cell, the 3C protease gene was also incorporated in the plasmid, allowing for in vivo removal of the prodomain and subsequent activation of the enzyme. The bicistronic vector constructed in this way was then used to transform an E. coli strain ready for expression.
The study reported a successful expression of the B. amyloliquefaciens TGase gene in E. coli. The purified enzyme demonstrated activity across various substrates, indicating potential applications in the food industry [25].
3.3. Heterologous Expression and Periplasmic Secretion of an Antifungal Bacillus amyloliquefaciens BLB 369 Endo-β-1,3-1,4-Glucanase in Escherichia coli
Endo-β-1,3-1,4-glucanases, classified as glycoside hydrolases, play a crucial role in the enzymatic depolymerization of 1,3-1,4 β-glucans and exhibit antifungal properties. B. amyloliquefaciens BLB369 is known to produce this enzyme. Researchers have sequenced, cloned, and effectively expressed the glu369 full-coding sequence of the endo-β-1,3-1,4-glucanase gene in Escherichia coli Top10. To simplify the purification process, the glu369 coding sequence was integrated into the pKJD4 vector. The resultant OmpA-His-Glu369 fusion protein incorporated the OmpA signal sequence for E. coli periplasmic targeting, followed by a 6xHistidine tag for purification purposes. Owing to these beneficial attributes, endo-β-1,3-1,4-glucanase holds significant potential for various biotechnological applications [35] (Figure 2).
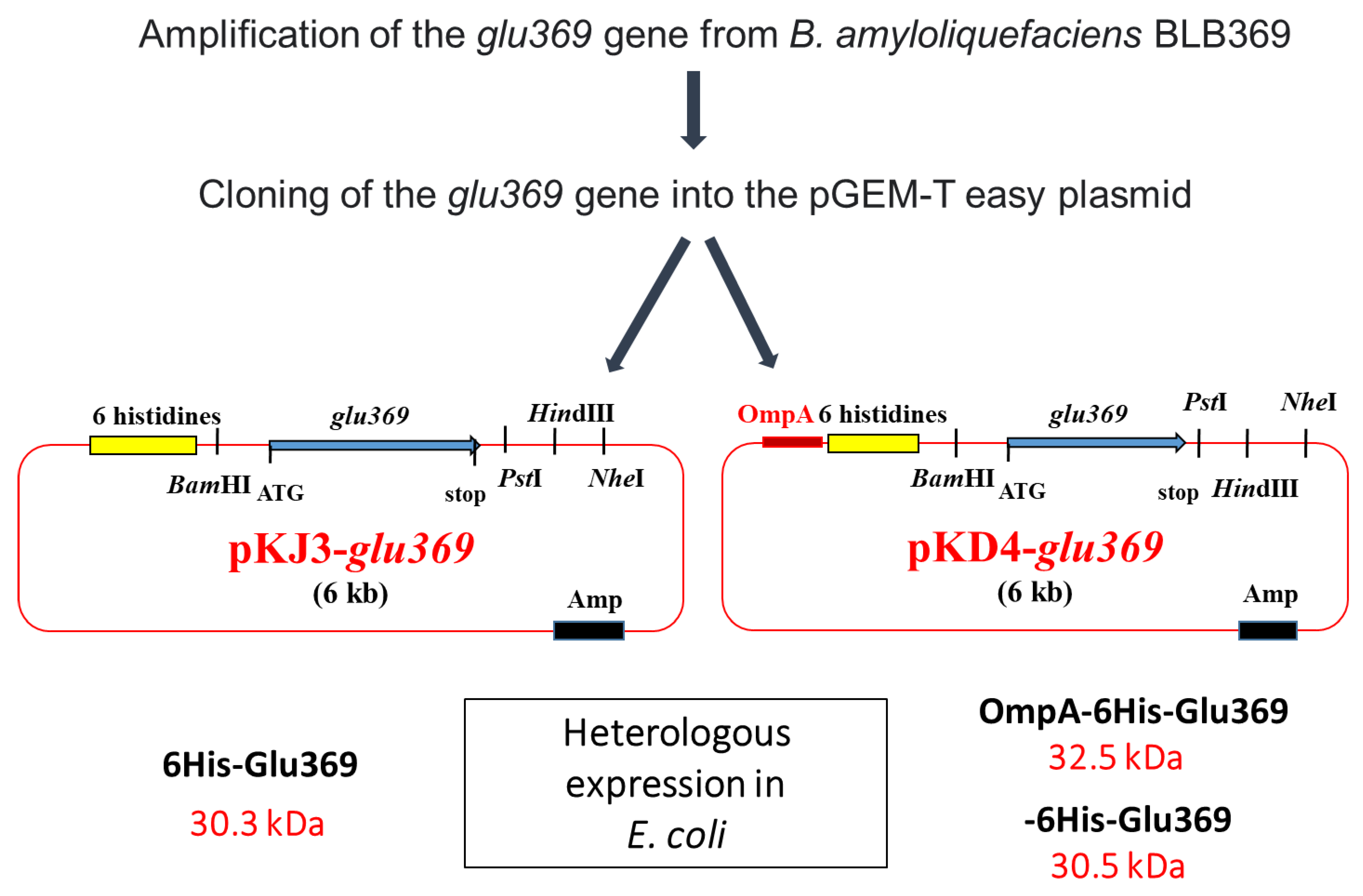
Figure 2. Strategy for cloning the glu369 gene in pKJ3 and pKJ4 and structure of recombinant plasmids pKJ3-glu369 and pKD4-glu369. Amp, ampicillin resistance; lacZ, the gene encoded by the β-galactosidase; OmpA, signal sequence.
To enhance heterologous protein synthesis in B. amyloliquefaciens, most existing research has focused on optimizing factors such as signal peptides, transport channel levels, chaperone protein levels, promoters, and other components of the expression and transport systems [20]. Nonetheless, for the establishment of sustainable, dependable, and efficient heterologous protein-producing microbial cell factories, emphasis should be placed on designing and developing host chassis.
Owing to the unique genetic backgrounds of B. amyloliquefaciens, several model strains have been extensively investigated. Advanced genome editing techniques have been employed in species like B. subtilis and B. licheniformis for heterologous protein generation through meticulous microbial chassis engineering [36]. Therefore, it is essential to augment heterologous protein production utilizing B. amyloliquefaciens, a strain distinct from B. subtilis and B. licheniformis. However, the understanding of the regulatory mechanisms governing specific enzyme secretion remains limited. This knowledge gap leads to challenges in devising accurate secretion route engineering strategies and assembling a toolbox of signal peptide sequences for various heterologous enzymes.
Cell membrane engineering is a specialized field that focuses on manipulating the structure and composition of the cell membrane to enhance its functionality. In the case of Bacillus amyloliquefaciens, this approach can increase the bacterium’s efficiency in producing and secreting proteins—essentially transforming the bacterium into a ‘cell factory’. However, one of the challenges with this approach lies in predicting the most suitable enzymes to target for a given application. Enzymes are proteins that act as catalysts for various biochemical reactions, and different enzymes are involved in different reactions. Therefore, the choice of enzymes to target can greatly influence the efficiency and effectiveness of protein production and secretion. To address this challenge, one potential strategy is to further optimize cell membrane engineering techniques to be more adaptable to different enzymes. This involves creating a modular system where the techniques can be adjusted and applied predictably depending on the specific enzymes targeted.
In other words, this strategy aims to establish a flexible and standardized framework for cell membrane engineering, where different techniques can be plugged in or taken out as needed to optimize the bacterium’s ability to produce and secrete different target enzymes. This could potentially enhance the usefulness of B. amyloliquefaciens in various biotechnological applications.
References
- Zakataeva, N.P.; Nikitina, O.V.; Gronskiy, S.V.; Romanenkov, D.V.; Livshits, V.A. A simple method to introduce marker-free genetic modifications into the chromosome of naturally nontransformable Bacillus amyloliquefaciens strains. Appl. Microbiol. Biotechnol. 2010, 85, 1201–1209.
- Yang, L.; Wang, H.; Lv, Y.; Bai, Y.; Luo, H.; Shi, P.; Huang, H.; Yao, B. Construction of a Rapid Feather-Degrading Bacterium by Overexpression of a Highly Efficient Alkaline Keratinase in Its Parent Strain Bacillus amyloliquefaciens K11. J. Agric. Food Chem. 2016, 64, 78–84.
- Feng, J.; Gu, Y.; Quan, Y.; Cao, M.; Gao, W.; Zhang, W.; Wang, S.; Yang, C.; Song, C. Improved poly-gamma-glutamic acid production in Bacillus amyloliquefaciens by modular pathway engineering. Metab. Eng. 2015, 32, 106–115.
- Han, J.H.; Shim, H.; Shin, J.H.; Kim, K.S. Antagonistic Activities of Bacillus spp. Strains Isolated from Tidal Flat Sediment towards Anthracnose Pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol. J. 2015, 31, 165–175.
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 Culture Medium by Response Surface Methodology for Low Cost Production of Antifungal Activity. Microorganisms 2022, 10, 830.
- Ye, M.; Sun, L.; Yang, R.; Wang, Z.; Qi, K. The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed. R. Soc. Open Sci. 2017, 4, 171012.
- Zhang, J.; Zhu, B.; Li, X.; Xu, X.; Li, D.; Zeng, F.; Zhou, C.; Liu, Y.; Li, Y.; Lu, F. Multiple Modular Engineering of Bacillus amyloliquefaciens Cell Factories for Enhanced Production of Alkaline Proteases From B. Clausii. Front. Bioeng. Biotechnol. 2022, 10, 866066.
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614.
- Xin, Q.; Chen, Y.; Chen, Q.; Wang, B.; Pan, L. Development and application of a fast and efficient CRISPR-based genetic toolkit in Bacillus amyloliquefaciens LB1ba02. Microb. Cell Fact. 2022, 21, 99.
- Jiang, C.; Ye, C.; Liu, Y.; Huang, K.; Jiang, X.; Zou, D.; Li, L.; Han, W.; Wei, X. Genetic engineering for enhanced production of a novel alkaline protease BSP-1 in Bacillus amyloliquefaciens. Front. Bioeng. Biotechnol. 2022, 10, 977215.
- Wang, S.; Wang, R.; Zhao, X.; Ma, G.; Liu, N.; Zheng, Y.; Tan, J.; Qi, G. Systemically engineering Bacillus amyloliquefaciens for increasing its antifungal activity and green antifungal lipopeptides production. Front. Bioeng. Biotechnol. 2022, 10, 961535.
- Zhang, F.; Huo, K.; Song, X.; Quan, Y.; Wang, S.; Zhang, Z.; Gao, W.; Yang, C. Engineering of a genome-reduced strain Bacillus amyloliquefaciens for enhancing surfactin production. Microb. Cell Fact. 2020, 19, 223.
- Zhao, X.; Zheng, H.; Zhen, J.; Shu, W.; Yang, S.; Xu, J.; Song, H.; Ma, Y. Multiplex genetic engineering improves endogenous expression of mesophilic alpha-amylase gene in a wild strain Bacillus amyloliquefaciens 205. Int. J. Biol. Macromol. 2020, 165, 609–618.
- Su, Y.T.; Liu, C.; Long, Z.; Ren, H.; Guo, X.H. Improved Production of Spores and Bioactive Metabolites from Bacillus amyloliquefaciens in Solid-State Fermentation by a Rapid Optimization Process. Probiotics Antimicrob. Proteins 2019, 11, 921–930.
- Roberts, R.J.; Wilson, G.A.; Young, F.E. Recognition sequence of specific endonuclease Bam HI from Bacillus amyloliquefaciens H. Nature 1977, 265, 82–84.
- Wang, R.; Long, Z.; Liang, X.; Guo, S.; Ning, N.; Yang, L.; Wang, X.; Lu, B.; Gao, J. The role of a β-1, 3-1, 4-glucanase derived from Bacillus amyloliquefaciens FS6 in the protection of ginseng against Botrytis cinerea and Alternaria panax. Biol. Control 2021, 164, 104765.
- Sabaté, D.C.; Brandán, C.P. Bacillus amyloliquefaciens strain enhances rhizospheric microbial growth and reduces root and stem rot in a degraded agricultural system. Rhizosphere 2022, 22, 100544.
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513.
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227.
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642.
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016.
- Vehmaanpera, J.; Steinborn, G.; Hofemeister, J. Genetic manipulation of Bacillus amyloliquefaciens. J. Biotechnol. 1991, 19, 221–240.
- Chen, X.T.; Ji, J.B.; Liu, Y.C.; Ye, B.; Zhou, C.Y.; Yan, X. Artificial induction of genetic competence in Bacillus amyloliquefaciens isolates. Biotechnol. Lett. 2016, 38, 2109–2117.
- Devaraj, K.; Aathika, S.; Periyasamy, K.; Manickam Periyaraman, P.; Palaniyandi, S.; Subramanian, S. Production of thermostable multiple enzymes from Bacillus amyloliquefaciens KUB29. Nat. Prod. Res. 2019, 33, 1674–1677.
- Duarte, L.S.; Barse, L.Q.; Dalberto, P.F.; da Silva, W.T.S.; Rodrigues, R.C.; Machado, P.; Basso, L.A.; Bizarro, C.V.; Ayub, M.A.Z. Cloning and expression of the Bacillus amyloliquefaciens transglutaminase gene in E. coli using a bicistronic vector construction. Enzym. Microb. Technol. 2020, 134, 109468.
- Gao, W.; He, Y.; Zhang, F.; Zhao, F.; Huang, C.; Zhang, Y.; Zhao, Q.; Wang, S.; Yang, C. Metabolic engineering of Bacillus amyloliquefaciens LL3 for enhanced poly-gamma-glutamic acid synthesis. Microb. Biotechnol. 2019, 12, 932–945.
- Gould, A.R.; May, B.K.; Elliott, W.H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J. Bacteriol. 1975, 122, 34–40.
- Xue, M.; Wu, Y.; Hong, Y.; Meng, Y.; Xu, C.; Jiang, N.; Li, Y.; Liu, W.; Fan, Y.; Zhou, Y. Effects of dietary Bacillus amyloliquefaciens on the growth, immune responses, intestinal microbiota composition and disease resistance of yellow catfish, Pelteobagrus fulvidraco. Front. Cell Infect. Microbiol. 2022, 12, 1047351.
- Yang, W. Components of rhizospheric bacterial communities of barley and their potential for plant growth promotion and biocontrol of Fusarium wilt of watermelon. Braz. J. Microbiol. 2019, 50, 749–757.
- Zhao, X.; Xu, J.; Tan, M.; Yu, Z.; Yang, S.; Zheng, H.; Song, H. Construction of a plasmid interspecific transfer system in Bacillus species with the counter-selectable marker mazF. J. Ind. Microbiol. Biotechnol. 2018, 45, 417–428.
- Tjalsma, H.; Antelmann, H.; Jongbloed, J.D.; Braun, P.G.; Darmon, E.; Dorenbos, R.; Dubois, J.Y.; Westers, H.; Zanen, G.; Quax, W.J.; et al. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004, 68, 207–233.
- Jongbloed, J.D.; Antelmann, H.; Hecker, M.; Nijland, R.; Bron, S.; Airaksinen, U.; Pries, F.; Quax, W.J.; van Dijl, J.M.; Braun, P.G. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 2002, 277, 44068–44078.
- Freudl, R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb. Cell Fact. 2018, 17, 52.
- Liu, J.; Hu, X.; He, H.; Zhang, X.; Guo, J.; Bai, J.; Cheng, Y. Digital gene expression profiling of the transcriptional response to Sclerotinia sclerotiorum and its antagonistic bacterium Bacillus amyloliquefaciens in soybean. Front. Microbiol. 2022, 13, 1025771.
- Zalila-Kolsi, I.; Sellami, S.; Tounsi, S.; Jamoussi, K. Heterologous expression and periplasmic secretion of an antifungal Bacillus amyloliquefaciens BLB 369 endo-β-1, 3-1, 4-glucanase in Escherichia coli. J. Phytopathol. 2018, 166, 28–33.
- Contesini, F.J.; Melo, R.R.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
16 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




