Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhaofeng Wang | -- | 1691 | 2023-11-15 15:42:36 | | | |
| 2 | Lindsay Dong | Meta information modification | 1691 | 2023-11-17 02:31:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, Z.; Zhang, S. Four Effects of High-Entropy Alloys. Encyclopedia. Available online: https://encyclopedia.pub/entry/51623 (accessed on 28 February 2026).
Wang Z, Zhang S. Four Effects of High-Entropy Alloys. Encyclopedia. Available at: https://encyclopedia.pub/entry/51623. Accessed February 28, 2026.
Wang, Zhaofeng, Shuai Zhang. "Four Effects of High-Entropy Alloys" Encyclopedia, https://encyclopedia.pub/entry/51623 (accessed February 28, 2026).
Wang, Z., & Zhang, S. (2023, November 15). Four Effects of High-Entropy Alloys. In Encyclopedia. https://encyclopedia.pub/entry/51623
Wang, Zhaofeng and Shuai Zhang. "Four Effects of High-Entropy Alloys." Encyclopedia. Web. 15 November, 2023.
Copy Citation
As a new type of alloy with high hardness, high heat resistance, strong corrosion resistance, high wear resistance and fatigue resistance, high-entropy alloys are different from any existing traditional alloy. As a new alloy system, high-entropy alloys have important research value and broad application prospects. Therefore, the design, preparation and application of high-entropy alloys have become an important direction for the development of new materials.
high-entropy alloys
application
alloy materials
1. Introduction
As early as the end of the 18th century, Franz Karl Achard carried out experimental research on multicomponent alloys of five to seven elements. The innovative work was not discovered and reported until 1963 by Professor Cyril Stanley Smith, which is the earliest recorded study on high-entropy alloys. In 1993, Professor Cantor of Cambridge University first expounded the concept of multiprincipal alloys [1]. In the subsequent research work, Cantor [2] also pointed out that the traditional alloy design concept caused the lack of research on the alloy phase diagram. Under the traditional alloy design concept, the research on the endpoint or edge region of the phase diagram has been greatly developed, while the large area in the middle of the phase diagram is poorly understood, especially the phase diagram analysis of the alloy in the presence of equal moles of several or more elements. As shown in Figure 1, there is a large degree of blank area in the phase diagram of binary and ternary alloys. Therefore, it is necessary to expand and supplement the alloy phase diagram. It is generally believed that there are two ways to achieve this goal. One is to increase the alloy element content on the basis of traditional alloy design, and the other is to use an equal atom substitution method, that is, to replace individual atoms in the component with other elements. Through the above two methods, a multicomponent alloy system with closer composition can be obtained.
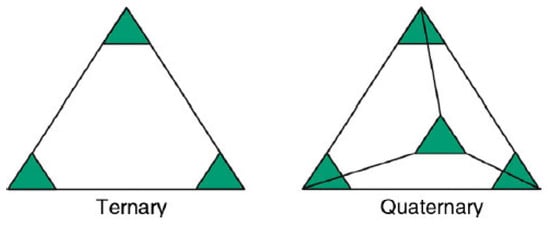
Figure 1. Schematic diagram of ternary and quaternary alloy systems [3].
Around 1995, scholars represented by Professor Yeh have also carried out a lot of basic research on multiprincipal alloy [4][5]. In 2004, Professor Yeh proposed the concept of the “high-entropy alloy” for the first time, which was expressed as: an alloy composed of five or more elements mixed in a near-equal molar ratio, with each element content (atomic ratio) greater than 5% and less than 35% [5]. On this basis, the research of multiprincipal alloys has been raised to a new height. In 2016, Lim from Singapore mentioned in an article published in Nature journal that the advent of the “high-entropy alloy” has created unprecedented broad prospects for the innovative development of materials metallurgy science, and provided new ideas and opportunities for the research and development of special property materials that can be used under extreme conditions and the upgrading and replacement of traditional materials [6].
High-entropy alloys are alloys with high-entropy values. The initial definition of high-entropy alloys is based on the mixed entropy of the alloy. In physics, entropy is a physical quantity that characterizes the degree of confusion of the system. In general, the greater the number of microscopic states corresponding to a macroscopic system, the greater the entropy of the system is.
According to the principle of Boltzmann statistical thermodynamics, the entropy of a system can be expressed by the following equation:
S = klnW
In the formula, S represents the entropy of the system, k is the Boltzmann constant, k = 1.38 × 1013 J∙K−1, and W is the thermodynamic probability, which represents the total number of microscopic states in the system. Since entropy is the macroscopic property of the system and the thermodynamic probability has microscopic characteristics, this formula organically combines the macroscopic and microscopic quantities of the system and lays the foundation of statistical thermodynamics.
According to the hypothesis of Boltzmann on the relationship between entropy change and chaos degree, the mixing entropy of n principal alloy systems can be expressed as
∆Smix =−R∑xilnxi,
where ΔSmix is the mixing entropy, R is the molar gas constant, 8.314 J/(K∙mol), and xi is the molar ratio of the i principal element in the alloy system. When the principal element atoms in the alloy system are equal molar ratio, the maximum mixing entropy is expressed as follows:
∆Smix =Rlnn
2. Four Effects of High-Entropy Alloys
2.1. High-Entropy Effect
According to the classical thermodynamic theory, the relationship between Gibbs free energy G and enthalpy H, absolute temperature T and entropy S can be expressed as follows:
G = H − TS
For a certain alloy system, the Gibbs free energy of the system before and after mixing changes as follows:
∆Gmix = ∆Hmix − T∆Smix
Here, ∆Gmix is the variation of Gibbs free energy and ∆Hmix and ∆Smix represent the mixing enthalpy and mixing entropy of the alloy system, respectively. The mixing entropy and mixing enthalpy are competitive with each other. The Gibbs free energy will descend with the decrease in mixing enthalpy and the increase in mixing entropy. High-entropy alloys are more likely to form simple solid solutions due to their high mixing entropy. The schematic diagram of the formation of solid solution promoted by high-entropy effect is shown in Figure 2.
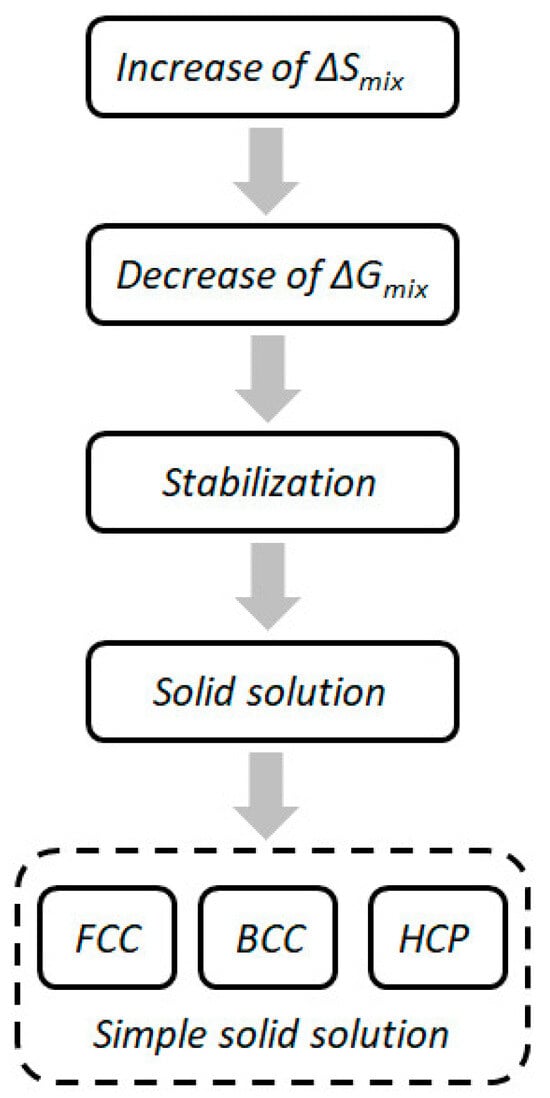
Figure 2. Schematic diagram of the formation of solid solution promoted by high-entropy effect.
The high-entropy effect of high-entropy alloys has been confirmed in many aspects [7][8][9][10][11][12][13]. The influence of high-entropy effect on the phase structure of high-entropy alloys is more intuitive. It can be seen from Figure 3 that the phases in quinary, senary, and septenary alloys remain rather simple. The major phases have simple structures such as BCC and FCC.
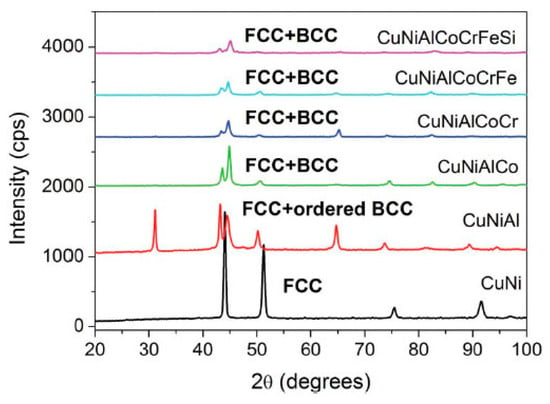
Figure 3. The effect of component increase on the XRD pattern of the alloy [14].
3.2. Lattice Distortion Effect
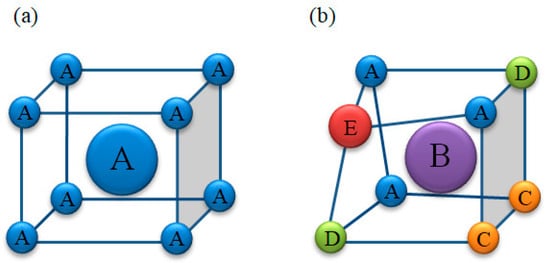
The high-entropy alloys can easily form a disordered solid solution because of the high-entropy effect, as shown in Figure 4. The atoms in the solid solution are randomly distributed. Each atom may be surrounded by different atoms. Each principal element will be different because of the structural characteristics. These differences will inevitably cause a certain degree of deviation in the lattice atom. Then, the lattice distortion is generated. This characteristic is called lattice distortion effect, which will have a remarkable impact on the microstructure and properties of high-entropy alloys.

Figure 4. Schematic diagram of the lattice distortion effect. (a) BBC no lattice distortion, one component alloy; (b) BBC servere lattice distortion, five component alloy.
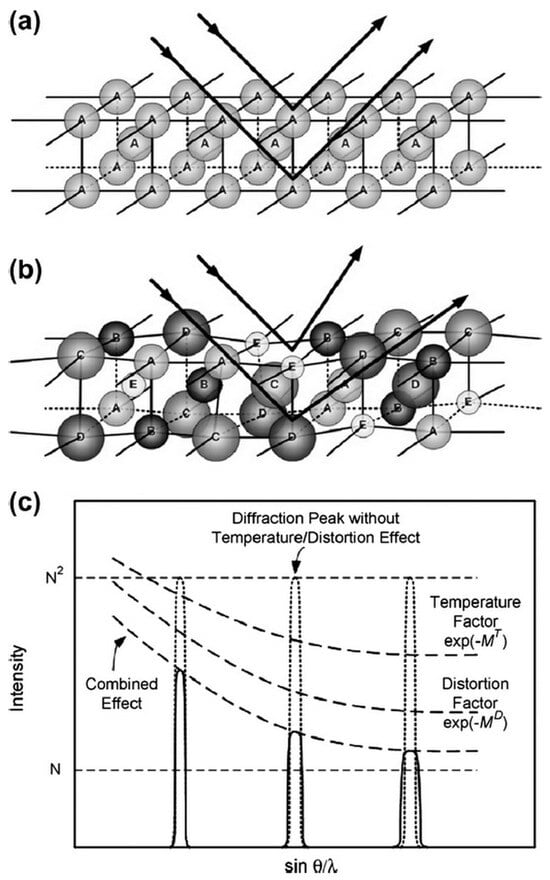
The lattice distortion can enlarge the X-ray scattering and reduce the diffraction peak intensity. When the atoms with different properties randomly occupy the crystal lattice position, the X-ray is scattered due to the serious distortion of each diffraction surface. Professor Yeh [15] studied the decrease in X-ray diffraction peak intensity in alloy systems in 2007. The results are shown in Figure 5. The intensity of the diffraction peak decreases with the enlargement of the number of alloying elements in high-entropy alloys. The degree of reduction is much larger than that of the thermal effect, which means that the internal lattice distortion of the alloy has an important effect on the change of X-ray diffraction peak intensity.

Figure 5. Schematic view of lattice distortion effect on Bragg diffraction: (a) a perfect lattice with the same atoms;(b) distorted lattices of solid solutions composed of different atoms; (c) temperature and distortion effects on the XRD intensity [15].
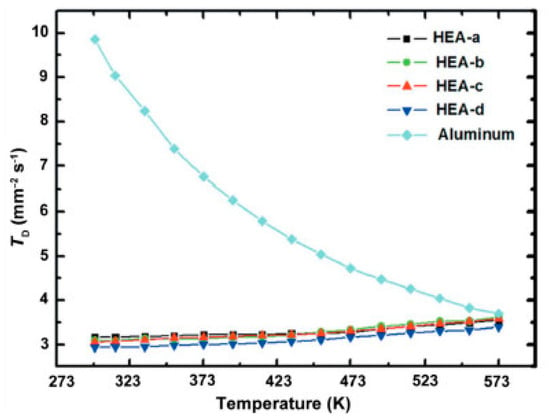
The serious lattice distortion will increase the scattering of electrons and phonons, which will reduce the electrical conductivity and thermal conductivity of the alloy. Research shows that the electrical conductivity and thermal conductivity of the alloy decrease with the increase in aluminum content, and are lower than pure aluminum [16]. It can be seen that the slope of the curve of the high-entropy alloys has a small positive value, while the slope of the curve of pure aluminum has a large negative value from Figure 6.

Figure 6. The curves of thermal diffusivity of pure aluminum and high-entropy alloys with temperature change [16].
2.3. Sluggish Diffusion Effect
Diffusion is the phenomenon that occurs when atoms migrate away from their original position, and the macroscopic flow of material is caused. In the process of phase transition, the formation of a new phase usually requires the cooperative diffusion of many atoms. In the liquid state, the principal elements of the high-entropy alloys are disordered due to the high-entropy effect. In the cooling process, the diffusion of the high-entropy alloys involves the diffusion and redistribution of the principal element atoms. The nucleation and growth of the new phase are inhibited. It can be called the sluggish diffusion effect in kinetics of the high-entropy alloys.
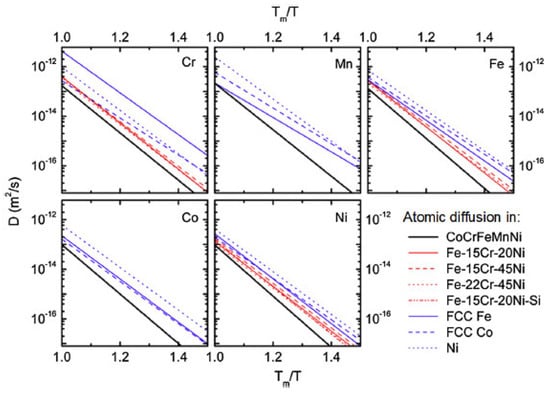
Yeh et al. [17] selected the alloy system with a single-phase face-centered cubic structure as the research object to analyze the diffusion coefficient. As shown in Figure 7, the diffusion coefficients of the five elements in high-entropy alloys are significantly smaller than those in traditional alloys.

Figure 7. The relationship between the diffusion coefficient of different materials and the temperature change [17].
The sluggish diffusion effect not only promotes the formation of supersaturated solid solution and the precipitation of nanophase, but also has a beneficial effect on the comprehensive properties of high-entropy alloys [18][19][20][21][22][23]. These beneficial effects include slowing down the phase transition rate, increasing the recrystallization temperature of the alloy, inhibiting the nucleation and growth of crystal grains, improving creep properties, etc. Therefore, the sluggish diffusion effect in kinetics is beneficial to control the properties of high-entropy alloys.
2.4. Cocktail Effect
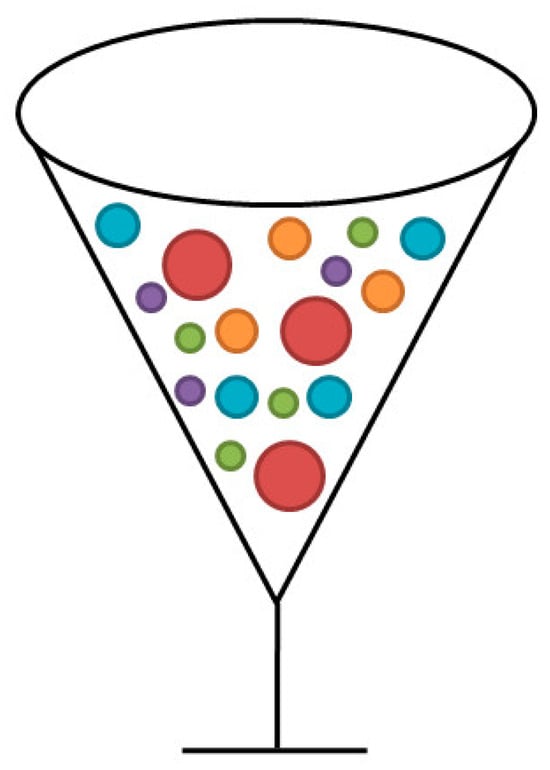
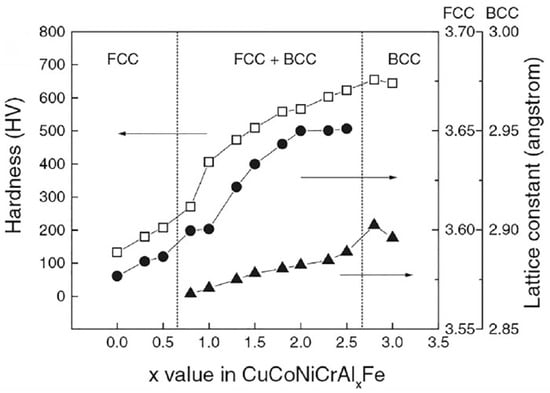
The cocktail effect was firstly proposed by Professor Ranganthan [24], as shown in Figure 8. It shows that the microstructure and properties of high-entropy alloys are determined by the various elements added, and the performance can be greatly changed by adjusting the principal component composition. Reference 7 shows that the strength of the AlxCoCrFeNiCu alloy increases with the increase in Al content, as shown in Figure 9. The phase structure of the alloy changed from FCC structure to FCC + BCC structure, and then to BCC structure. In addition, some experiments have shown that the addition of an aluminum element can reduce the density of high-entropy alloys [25]. If some refractory elements are added, the high-temperature properties can be enhanced [26]. In the alloy system, the effect of Cu contributes to the formation of an L12((Ni, Cu)3Al) phase [27]. It may be possible to obtain an unforeseeable high performance using the cocktail effect.

Figure 8. Schematic diagram of cocktail effect.

Figure 9. Comparison of hardness and lattice constant of alloy system [5].
References
- Cantor, B.; Kim, K.B.; Warren, P.J. Novel multicomponent amorphous alloys. J. Metastable Nanocrystalline Mater. 2002, 13, 27–32.
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218.
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Michael, C.G.; Karin, A.D.; Peter, K.L.; Zhao, P.L. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93.
- Yeh, J.W.; Su, J.L.; Chin, T.S.; Gan, J.Y.; Chen, S.W.; Shun, T.T.; Tsau, C.H.; Chou, S.Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-Valloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536.
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principalelements novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
- Lim, X.Z. Mixed-up metals make for stronger, tougher, stretchier alloys. Nature 2016, 533, 306–307.
- Tong, C.J.; Chen, M.R.; Chen, S.K. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36A, 1263–1271.
- Tong, C.J.; Chen, Y.L.; Chen, S.K. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36A, 881–893.
- Li, A.; Zhang, X. Thermodynamic analysis of the simple microstructure of AlCrFeNiCu high-entropy alloy with multi-principal elements. Acta Metall. Sin. 2009, 22, 219–224.
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765.
- Del, G.M.F.; Bozzolo, G.; Hugo, O.M. Determination of the transition to the high entropy regime for alloys of refractory elements. J. Alloys Compd. 2012, 534, 25–31.
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C.T. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 2012, 31, 165–171.
- Lucas, M.S.; Wilks, G.B.; Mauger, L.; Munoz, J.A.; Karapetrova, E. Absence of long-range chemical ordering in equimola FeCoCrNi. Appl. Phys. Lett. 2012, 100, 251907-1–251907-4.
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123.
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous decrease in X-ray diffraction intensities of CuNiAlCoCrFeSi alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46.
- Tsai, M.H. Physical Properties of high entropy alloys. Entropy 2013, 15, 5338–5345.
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta. Mater. 2013, 61, 4887–4897.
- Kottke, J.; Laurent-Brocq, M.; Fareed, A.; Wilde, G. Tracer diffusion in the Ni-CoCrFeMn system: Transitionfrom a dilute solid solution to a high entropy alloy. Scripta Mater. 2019, 159, 94–98.
- Chen, W.Y.; Liu, X.; Chen, Y.R.; Yeh, J.W.; Tseng, K.K.; Natesan, K. Irradiation effects in high entropy alloys and 316H stainless steel at 300 degrees C. J. Nucl. Mater. 2018, 510, 421–430.
- Wang, R.; Chen, W.M.; Zhong, J.; Zhang, L. Experimental and numerical studies on the sluggish diffusion in face centered cubic Co-Cr-Cu-Fe-Ni high-entropy alloys. J. Mater. Sci. Technol. 2018, 34, 1791–1798.
- Vaidya, M.; Trubel, S.; Murty, B.S.; Wilde, G.; Divinski, S.V. Ni tracer diffusion in CoCrFeNi and CoCrFeMnNi highentropy alloys. J. Alloys Compd. 2016, 688, 994–1001.
- Dąbrowa, J.; Kucza, W.; Cieślak, G.; Kulik, T.; Danielewski, M.; Yeh, J.W. Interdiffusion in the FCC-structured Al-Co-Cr-Fe-Ni high entropy alloys: Experimental studies and numerical simulations. J. Alloys Compd. 2016, 674, 455–462.
- Yang, M.; Liu, X.J.; Ruan, H.H.; Wu, Y.; Wang, H.; Lu, Z.P. High thermal stability and sluggish crystallization kinetics of high-entropy bulk metallic glasses. J. Appl. Phys. 2016, 119, 245112.
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406.
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Garcia, J.C. Phase prediction, microstructure and high hardness of novel light-weight high entropy alloys. J. Mater. Res. Technol. 2019, 8, 795–803.
- Huang, D.; Lu, J.; Zhuang, Y.; Tian, C.; Li, Y. The role of Nb on the high temperature oxidation behavior of CoCrFeMnNbxNi high-entropy alloys. Corros. Sci. 2019, 158, 108088.1–108088.9.
- Gwalani, B.; Choudhuri, D.; Soni, V.; Ren, Y.; Styles, M.; Hwang, J.Y.; Nam, S.J.; Ryu, H.; Hong, S.H.; Banerjee, R. Cu assisted stabilization and nucleation of L12 precipitates in Al0.3CuFeCrNi2fcc-based high entropy alloy. Acta Mater. 2017, 129, 170–182.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.9K
Revisions:
2 times
(View History)
Update Date:
17 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





